Abstract
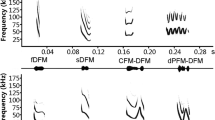
There is an apparent allometric relationship between peak frequency of echolocation and body size in rhinophilids. However, some rhinolophids deviate from this rule. To date this variation has been explained as a result of partitioning of communication channels. An alternative hypothesis that food resource partitioning results in this divergence in expected frequencies was tested by comparing prey selection between Rhinolophus macrotis Blyth, 1844 and Rhinolophus lepidus Blyth, 1844 in Yunnan Province, China. These two sympatric species are morphologically similar but acoustically divergent: R. macrotis has an echolocation frequency significantly lower than that predicted by the allometric relationship, whereas that of R. lepidus agreed with expectations. Prey selection experiments, conducted in a flight tent, indicated that the dominant prey taxa of R. macrotis were Lasiocampidae, Arctiidae and Noctuidae, whilst that of R. lepidus were Arctiidae, Noctuidae and Ichneumonidae. R. macrotis ate more earless moths and fewer eared moths than R. lepidus, and R. macrotis fed on larger prey in general and captured a wider size range than that captured by R. lepidus. These results confirmed the existence of finely tuned trophic niche differentiation and suggested that food resource partitioning is one of the factors leading to lower peak frequency of calls in R. macrotis.
Similar content being viewed by others
References
Acharya L. and Fenton M. B. 1992. Echolocation behaviour of vespertilionid bats (Lasiurus cinereus and Lasiurus borealis) attacking airborne targets including arctiid moths. Canadian Journal of Zoology 70: 1292–1298. DOI: 10.1138/z92-180
Aguirre L. F., Herrel A., van Damme R. and Matthysen E. 2002. Ecomorphological analysis of trophic niche partitioning in a tropical savannah bat community. Proceedings of the Royal Society B: Biological Sciences 269: 1271–1278. DOI: 10.1098/rspb.2002.2011
Alldredge J. R. and Ratti J. T. 1992. Further comparison of some statistical techniques for analysis of resource selection. Journal of Wildlife Management 56: 1–9.
Barclay R. M. R. and Brigham R. M. 1991. Prey detection, dietary niche breadth, and body size in bats: Why are aerial insectivorous bats so small? The American Naturalist 137: 693–703. DOI: 10.1086/285188
Bogdanowicz W., Fenton M. B. and Daleszczyk K. 1999. The relationships between echolocation calls, morphology and diet in insectivorous bats. Journal of Zoology, London 247: 381–393. DOI: 10.1017/S0952836999003106
Bumrungsri S., Leelapaibul W. and Racey P. A. 2007. Resource partitioning in sympatric Cynopterus bats in lowland tropical rain forest, Thailand. Biotropica 39: 241–248. DOI:10.1111/j.1744-7429.2006.00245.x
Burles D. W., Brigham R. M., Ring R. A. and Reimchen T. E. 2008. Diet of two insectivorous bats, Myotis lucifugus and Myotis keenii, in relation to arthropod abundance in a temperate Pacific Northwest rainforest environment. Canadian Journal of Zoology 86: 1367–1375. DOI: 10.1139/Z08-125
Byers C. R., Steinhorst R. K. and Krausman P. R. 1984. Clarification of a technique for analysis of utilizationavailability data. The Journal of Wildlife Management 48: 1050–1053.
Campbell P., Schneider C. J., Zubaid A., Adnan A. M. and Kunz T. H. 2007. Morphological and ecological correlates of coexistence in Malaysian fruit bats (Chiroptera: Pteropodidae). Journal of Mammalogy 88: 105–118. (DOI: 10.1644/06-MAMM-A-160R1.1
Connell J. H. 1980. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35: 131–138.
Csorba G., Ujhelyi P. and Thomas N. (eds) 2003. Horseshoe bats of the world (Chiroptera: Rhinolophidae). Alana Books, Shropshire: 1–160.
Dingle H., Blakley N. R. and Miller E. R. 1980. Variation in body size and flight performance in milkweed bugs (Oncopeltus). Evolution 34: 371–385.
Fenton M. B. 2004. Aerial-feeding bats: Getting the most out of echolocation. [In: Echolocation in bats and dolphins. J. A. Thomas, C. F. Moss and M. Vater, eds]. University of Chicago Press, Chicago: 350–355.
Fenton M. B. and Fullard J. H. 1979. The influence of moth hearing on bat echolocation strategies. Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology 132: 77–86. DOI: 10.1007/BF00617734
Fenton M. B., Portfors C. V., Rautenbach I. L. and Waterman J. M. 1998. Compromises: sound frequencies used in echolocation by aerial-feeding bats. Canadian Journal of Zoology 76: 1174–1182. DOI: 10.1139/cjz-76-6-1174
Fox J. W. 2004. Modelling the joint effects of predator and prey diversity on total prey biomass. Journal of Animal Ecology 73: 88–96. (DOI: 10.1111/j.1365-2656.2004.00784.x
Fullard J. H. 1987. Sensory ecology and neuroethology of moths and bats: Interactions in a global perspective. [In: Insect defenses: adaptive mechanisms and strategies of prey and predators. M. B. Fenton, P. A. Racey and J. M. V. Rayner, eds]. Cambridge University, Camridge: 244–272.
Fullard J. H., Jackson M. E., Jacobs D. S., Pavey C. R. and Burwell C. J. 2008. Surviving cave bats: auditory and behavioural defences in the Australian noctuid moth, Speiredonia spectans. Journal of Experimental Biology 211: 3808–3815. DOI: 10.1242/jeb.023978
Gannon W. L. and Rácz G. R. 2006. Character displacement and ecomorphological analysis of two long-eared Myotis (M. auriculus and M. evotis). Journal of Mammalogy 87: 171–179. DOI: 10.1644/05-MAMM-A-140R1.1
Griffin D. R., Webster F. A. and Michael C. R. 1960. The echolocation of flying insects by bats. Animal Behaviour 8: 141–154. DOI: 10.1016/0003-3472(60)90022-1
Guillén A., Francis C. M. and Ricklefs R. E. 2003. Phylogeny and biogeography of the horseshoe bats. [In: Horseshoe bats of the World. G. Csorba, P. Ujhelyi and N. Thomas, eds]. Alana Books, Shrewsbury, UK: xii-xxiv.
Heller K. G. and Helversen O. 1989. Resource partitioning of sonar frequency bands in rhinolophoid bats. Oecologia 80: 178–186. DOI: 10.1007/BF00380148
Hickey M. B. C., Acharya L. and Shannon P. 1996. Resource partitioning by two species of vespertilionid bats (Lasiurus cinereus and Lasiurus borealis) feeding around street lights. Journal of Mammalogy 77: 325–334.
Houston R. D., Boonman A. M. and Jones G. 2001. Do echolocation signal parameters restrict bats’ choice of prey. [In: Echolocation in bats and dolphins. J. Thomas, C. F. Moss and M. Vater, eds]. University of Chicago Press, Chicago: 339–345.
Jacobs D. S. 2000. Community level support for the allotonic frequency hypothesis. Acta Chiropterologica 2: 197–207.
Jacobs D. S., Barclay R. M. R. and Walker M. H. 2007. The allometry of echolocation call frequencies of insectivorous bats: why do some species deviate from the pattern? Oecologia 152: 583–594. DOI: 10.1007/s00442-007-0679-1
Jacobs D. S., Ratcliffe J. M. and Fullard J. H. 2008. Beware of bats, beware of birds: the auditory responses of eared moths to bat and bird predation. Behavioral Ecology 19: 1333- 1342. DOI: 10.1093/beheco/arn071
Jiang T. L., Feng J., Sun K. P. and Wang J. 2008. Coexistence of two sympatric and morphologically similar bat species Rhinolophus affinis and Rhinolophus pearsoni. Progress in Natural Science 18: 523–532. DOI: 10.1016/j.pnsc.2007.12.005
Jin L. R., Feng J., Sun K. P., Liu Y, Wu L, Li Z. X. and Zhang X. C. 2005. Foraging strategies in the greater horseshoe bat (Rhinolophus ferrumequinum) on Lepidoptera in summer. Chinese Science Bulletin 50: 1477–1482. DOI: 10.1360/982004-831
Jones G. 2008. Sensory ecology: Echolocation calls are used for communication. Current Biology 18: 34–35. DOI: 10.1016/j.cub.2007.10.056
Jones G. and Barlow K. E. 2004. Cryptic species of echolocating bats. [In: Echolocation in bats and dolphins. J. Thomas, C. F. Moss and M. Vater, eds]. University of Chicago Press, Chicago: 345–349.
Kingston T., Jones G., Zubaid A. and Kunz T. H. 2000. Resource partitioning in rhinolophoid bats revisited. Oecologia 124: 332–342. DOI: 10.1007/PL00008866
Kingston T. and Rossiter S. J. 2004. Harmonic-hopping in Wallacea’s bats. Nature 429: 654–657. DOI: 10.1038/nature02487
Neuweiler G. 1989. Foraging ecology and audition in echolocating bats. Trends in Ecology & Evolution 4: 160–166. DOI: 10.1016/0169-5347(89)90120-1
Nijhout H. F. and Emlen D. J. 1998. Competition among body parts in the development and evolution of insect morphology. Proceedings of the National Academy of Sciences 95: 3685–3689.
Norberg U. M. and Rayner J. M. V. 1987. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 316: 335–427. DOI: 10.1098/rstb.1987.0030
Novick A. 1977. Acoustic orientation. [In: Biology of bats. A. Wimsatt, ed]. Academic Press, New York: 74–289.
Pavey C. R. and Burwell C. J. 1998. Bat predation on eared moths: a test of the allotonic frequency hypothesis. Oikos 81: 143–151.
Russo D., Mucedda M., Bello M., Biscardi S., Pidinchedda E. and Jones G. 2007. Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): a case of character displacement? Journal of Biogeography 34: 2129–2138. DOI: 10.1111/j.1365-2699.2007.01762.x
Schnitzler H. U. and Kalko E. K. V. 2001. Echolocation by insect-eating bats. BioScience 51: 557–569. DOI: 10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2
Schnitzler H. U., Moss C. F. and Denzinger A. 2003. From spatial orientation to food acquisition in echolocating bats. Trends in Ecology & Evolution 18: 386–394. DOI: 10.1016/S0169-5347(03)00185-X
Schoeman C. M. and Jacobs D. S. 2003. Support for the allotonic frequency hypothesis in an insectivorous bat community. Oecologia 134: 154–162. (DOI: 10.1007/s00442-002-1107-1
Scoble M. J. (eds) 1992. The Lepidoptera: Form, Function and Diversity. Oxford University Press, New York: 1–404.
Siemers B. M., Beedholm K., Dietz C., Dietz I. and Ivanova T. 2005. Is species identity, sex, age or individual quality conveyed by echolocation call frequency in European horseshoe bats? Acta Chiropterologica 7: 259–274. DOI: 10.3161/1733-5329(2005)7[259:ISISAO]2.0.CO;2
Siemers B. M. and Gütinger R. 2006. Prey conspicuousness can explain apparent prey selectivity. Current Biology 16: 157–159. DOI: 10.1016/j.cub.2006.02.056
Siemers B. M. and Schnitzler H. U. 2004. Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429: 657–663. DOI: 10.1038/nature02547
Siemers B. M. and Swift S. M. 2006. Differences in sensory ecology contribute to resource partitioning in the bats Myotis bechsteinii and Myotis nattereri (Chiroptera: Vespertilionidae). Behavioral Ecology and Sociobiology 59: 373–380. DOI: 10.1007/s00265-005-0060-5
Sokal R. R. and Rohlf F. J. (eds) 1995. Biometry: the principles and practice of statistics in biological research. 3rd. W. H. Freeman and company, New York: 1–797.
Surlykke A. and Kalko E. K. V. 2008. Echolocating bats cry out loud to detect their prey. PLoS ONE 3: e2036. DOI: 10.1371/journal.pone.0002036
Svenning J. C. 1999. Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. Journal of Ecology 87: 55–65. DOI: 10.1046/j.1365-2745.1999.00329.x
Swift S. and Racey P. 2002. Gleaning as a foraging strategy in Natterer’s bat Myotis nattereri. Behavioral Ecology and Sociobiology 52: 408–416. DOI: 10.1007/s00265-002-0531-x
Thabah A., Rossiter S. J., Kingston T., Zhang S., Parsons S., Mya K. M., Akbar Z. and Jones G. 2006. Genetic divergence and echolocation call frequency in cryptic species of Hipposideros larvatus sl (Chiroptera: Hipposideridae) from the Indo-Malayan region. Biological Journal of the Linnean Society 88: 119–130. DOI: 10.1111/j.1095-8312.2006.00602.x
Tilman D. 1982. Resource competition and community structure. Princeton University Press, Princeton: 1–296.
Wickman P. O. and Karlsson B. 1989. Abdomen size, body size and the reproductive effort of insects. Oikos 56: 209–214.
Xue R. D. and Ali A. 1994. Relationship between wing length and fecundity of a pestiferous midge, Glyptotendipes paripes (Diptera: Chironomidae). Journal of the American Mosquito Control Association 10: 29–34.
Zhang L., Jones G., Rossiter S., Ades G., Liang B. and Zhang S. 2005. Diet of flat-headed bats, Tylonycteris pachypus and T. robustula, in Guangxi, South China. Journal of Mammalogy 86: 61–66.
Zheng L. Y. and Gui H. (eds) 1999. [Insect classification]. Vol. 1, 2. Nanking Normal Univetrsity Press, Nanjing: 1–1070. [In Chinese] DOI: 10.1644/1545-1542(2005)086〈0061:DOFBTP〉2.0.CO;2
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate editor was Joseph F. Merritt.
Rights and permissions
About this article
Cite this article
Shi, Lm., Feng, J., Liu, Y. et al. Is food resource partitioning responsible for deviation of echolocation call frequencies from allometry in Rhinolophus macrotis?. Acta Theriol 54, 371–382 (2009). https://doi.org/10.4098/j.at.0001-7051.099.2008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.4098/j.at.0001-7051.099.2008