A revised model for mitochondrial dysfunction in Duchenne muscular dystrophy
Accepted: 12 September 2021
HTML: 2
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
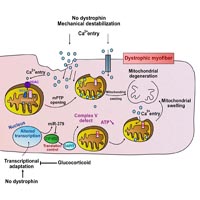
We recently identified a signaling pathway that links the upregulation of miR-379 with a mitochondrial response in dystrophic muscle. In the present commentary, we explain the significance that this pathway may have in mitochondrial dysfunction in Duchenne muscular dystrophy (DMD). We identified the upregulation of miR-379 in the serum and muscles of DMD animal models and patients. We found that miR-379 is one of very few miRNAs whose expression was normalized in DMD patients treated with glucocorticoid. We identified EIF4G2 as a miR-379 target, which may promote mitochondrial oxidative phosphorylation (OxPhos) in the skeletal muscle. We found enriched EIF4G2 expression in oxidative fibers, and identified the mitochondrial ATP synthase subunit DAPIT as a translational target of EIF4G2. The identified signaling cascade, which comprises miR-379, EIF4G2 and DAPIT, may link the glucocorticoid treatment in DMD to a recovered mitochondrial ATP synthesis rate. We propose an updated model of mitochondrial dysfunction in DMD.
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Prim. 2021;7: 13. DOI: https://doi.org/10.1038/s41572-021-00248-3
Allen DG, Whitehead NP, Froehner SC. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca 2+ , Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol Rev. 2016 Jan;96(1):253-305. DOI: https://doi.org/10.1152/physrev.00007.2015
Timpani CA, Hayes A, Rybalka E. Revisiting the dystrophin-ATP connection: How half a century of research still implicates mitochondrial dysfunction in Duchenne Muscular Dystrophy aetiology. Med Hypotheses . 2015 Dec;85(6):1021-33. Epub 2015 Sep 2. DOI: https://doi.org/10.1016/j.mehy.2015.08.015
Jeanson-Leh L, Lameth J, Krimi S, Buisset J, Amor F, Le Guiner C, Barthélémy I, Servais L, Blot S, Voit T, Israeli D. Serum profiling identifies novel muscle miRNA and cardiomyopathy-related miRNA biomarkers in Golden Retriever muscular dystrophy dogs and Duchenne muscular dystrophy patients. Am J Pathol. 2014 Nov;184(11):2885-98. Epub 2014 Sep 3. DOI: https://doi.org/10.1016/j.ajpath.2014.07.021
Vignier N, Amor F, Fogel P, Duvallet A, Poupiot J, Charrier S, Arock M, Montus M, Nelson I, Richard I, Carrier L, Servais L, Voit T, Bonne G, Israeli D. Distinctive serum miRNA profile in mouse models of striated muscular pathologies. PLoS One. 2013;8(2):e55281. Epub 2013 Feb 13. DOI: https://doi.org/10.1371/journal.pone.0055281
Amor F, Vu Hong A, Corre G, Sanson M, Suel L, Blaie S, Servais L, Voit T, Richard I, Israeli D. Cholesterol metabolism is a potential therapeutic target in Duchenne muscular dystrophy. J Cachexia Sarcopenia Muscle. 2021 Jun;12(3):677-693. Epub 2021 May 26. DOI: https://doi.org/10.1002/jcsm.12708
da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008 Jun;24(6):306-16. DOI: https://doi.org/10.1016/j.tig.2008.03.011
Tierling S, Dalbert S, Schoppenhorst S, Tsai CE, Oliger S, Ferguson-Smith AC, Paulsen M, Walter J. High-resolution map and imprinting analysis of the Gtl2-Dnchc1 domain on mouse chromosome 12. Genomics. 2006 Feb;87(2):225-35. Epub 2005 Nov 23. DOI: https://doi.org/10.1016/j.ygeno.2005.09.018
Chu C, Schwartz S, McPherson E. Paternal uniparental isodisomy for chromosome 14 in a patient with a normal 46,XY karyotype. Am J Med Genet A. 2004 Jun 1;127A(2):167-71. DOI: https://doi.org/10.1002/ajmg.a.20618
Ioannides Y, Lokulo-Sodipe K, Mackay DJ, Davies JH, Temple IK. Temple syndrome: improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J Med Genet. 2014 Aug;51(8):495-501. Epub 2014 Jun 2. DOI: https://doi.org/10.1136/jmedgenet-2014-102396
Ogata T, Kagami M. Molecular mechanisms leading to the phenotypic development in paternal and maternal uniparental disomy for chromosome 14. Clin Pediatr Endocrinol. 2008;17(4):103-11. Epub 2008 Nov 8. DOI: https://doi.org/10.1297/cpe.17.103
Stevenson DA, Brothman AR, Chen Z, Bayrak-Toydemir P, Longo N. Paternal uniparental disomy of chromosome 14: confirmation of a clinically-recognizable phenotype. Am J Med Genet A. 2004 Sep 15;130A(1):88-91. DOI: https://doi.org/10.1002/ajmg.a.30200
Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, Li W, Wu HJ, Wang L, Wang XJ, Zhou Q. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010 Jun 18;285(25):19483-90. Epub 2010 Apr 9. DOI: https://doi.org/10.1074/jbc.M110.131995
Qian P, He XC, Paulson A, Li Z, Tao F, Perry JM, Guo F, Zhao M, Zhi L, Venkatraman A, Haug JS, Parmely T, Li H, Dobrowsky RT, Ding WX, Kono T, Ferguson-Smith AC, Li L. The Dlk1-Gtl2 Locus Preserves LT-HSC Function by Inhibiting the PI3K-mTOR Pathway to Restrict Mitochondrial Metabolism. Cell Stem Cell. 2016 Feb 4;18(2):214-28. Epub 2015 Nov 25. DOI: https://doi.org/10.1016/j.stem.2015.11.001
Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh RM, Chen T, Ooi SS, Kim SY, Bestor TH, Shioda T, Park PJ, Hochedlinger K. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012 Mar 4;44(4):398-405, S1-2. DOI: https://doi.org/10.1038/ng.1110
Wüst S, Dröse S, Heidler J, Wittig I, Klockner I, Franko A, Bonke E, Günther S, Gärtner U, Boettger T, Braun T. Metabolic Maturation during Muscle Stem Cell Differentiation Is Achieved by miR-1/133a-Mediated Inhibition of the Dlk1-Dio3 Mega Gene Cluster. Cell Metab. 2018 May 1;27(5):1026-1039.e6. Epub 2018 Apr 5. DOI: https://doi.org/10.1016/j.cmet.2018.02.022
Gao YQ, Chen X, Wang P, Lu L, Zhao W, Chen C, Chen CP, Tao T, Sun J, Zheng YY, Du J, Li CJ, Gan ZJ, Gao X, Chen HQ, Zhu MS. Regulation of DLK1 by the maternally expressed miR-379/miR-544 cluster may underlie callipyge polar overdominance inheritance. Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13627-32. Epub 2015 Oct 20. DOI: https://doi.org/10.1073/pnas.1511448112
Byrne K, Colgrave ML, Vuocolo T, Pearson R, Bidwell CA, Cockett NE, Lynn DJ, Fleming-Waddell JN, Tellam RL. The imprinted retrotransposon-like gene PEG11 (RTL1) is expressed as a full-length protein in skeletal muscle from Callipyge sheep. PLoS One. 2010 Jan 8;5(1):e8638. DOI: https://doi.org/10.1371/journal.pone.0008638
Fleming-Waddell JN, Olbricht GR, Taxis TM, White JD, Vuocolo T, Craig BA, Tellam RL, Neary MK, Cockett NE, Bidwell CA. Effect of DLK1 and RTL1 but not MEG3 or MEG8 on muscle gene expression in Callipyge lambs. PLoS One. 2009 Oct 9;4(10):e7399. DOI: https://doi.org/10.1371/journal.pone.0007399
Kitazawa M, Hayashi S, Imamura M, Takeda S, Oishi Y, Kaneko-Ishino T, Ishino F. Deficiency and overexpression of Rtl1 in the mouse cause distinct muscle abnormalities related to Temple and Kagami-Ogata syndromes. Development. 2020 Sep 2;147(21):dev185918. DOI: https://doi.org/10.1242/dev.185918
Dill TL, Naya FJ. A Hearty Dose of Noncoding RNAs: The Imprinted DLK1-DIO3 Locus in Cardiac Development and Disease. J Cardiovasc Dev Dis. 2018 Jul 10;5(3):37. DOI: https://doi.org/10.3390/jcdd5030037
Eisenberg I, Eran A, Nishino I, Moggio M, Lamperti C, Amato AA, Lidov HG, Kang PB, North KN, Mitrani-Rosenbaum S, Flanigan KM, Neely LA, Whitney D, Beggs AH, Kohane IS, Kunkel LM. Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17016-21. doi: 10.1073/pnas.0708115104. Epub 2007 Oct 17. Erratum in: Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):399. DOI: https://doi.org/10.1073/pnas.0708115104
Angelini C, Peterle E. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myol. 2012 May;31(1):9-15.
Yoffe Y, David M, Kalaora R, Povodovski L, Friedlander G, Feldmesser E, Ainbinder E, Saada A, Bialik S, Kimchi A. Cap-independent translation by DAP5 controls cell fate decisions in human embryonic stem cells. Genes Dev. 2016 Sep 1;30(17):1991-2004. DOI: https://doi.org/10.1101/gad.285239.116
Sin J, Andres AM, Taylor DJ, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, Gottlieb RA. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12(2):369-80. DOI: https://doi.org/10.1080/15548627.2015.1115172
Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, Bello L, Soraru G, Pacchioni B, Bonifati MD, Lanfranchi G, Angelini C, Kesari A, Lee I, Gordish-Dressman H, Devaney JM, McDonald CM; Cooperative International Neuromuscular Research Group. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011 Jan 18;76(3):219-26. Epub 2010 Dec 22. DOI: https://doi.org/10.1212/WNL.0b013e318207afeb
Fröhlich T, Kemter E, Flenkenthaler F, Klymiuk N, Otte KA, Blutke A, Krause S, Walter MC, Wanke R, Wolf E, Arnold GJ. Progressive muscle proteome changes in a clinically relevant pig model of Duchenne muscular dystrophy. Sci Rep. 2016 Sep 16;6:33362. DOI: https://doi.org/10.1038/srep33362
He J, Ford HC, Carroll J, Douglas C, Gonzales E, Ding S, Fearnley IM, Walker JE. Assembly of the membrane domain of ATP synthase in human mitochondria. Proc Natl Acad Sci U S A. 2018 Mar 20;115(12):2988-2993. Epub 2018 Feb 12. DOI: https://doi.org/10.1073/pnas.1722086115
Siegmund SE, Grassucci R, Carter SD, Barca E, Farino ZJ, Juanola-Falgarona M, Zhang P, Tanji K, Hirano M, Schon EA, Frank J, Freyberg Z. Three-Dimensional Analysis of Mitochondrial Crista Ultrastructure in a Patient with Leigh Syndrome by In Situ Cryoelectron Tomography. iScience. 2018 Aug 31;6:83-91. Epub 2018 Jul 20. DOI: https://doi.org/10.1016/j.isci.2018.07.014
Ohsakaya S, Fujikawa M, Hisabori T, Yoshida M. Knockdown of DAPIT (diabetes-associated protein in insulin-sensitive tissue) results in loss of ATP synthase in mitochondria. 2011;286. DOI: https://doi.org/10.1074/jbc.M110.198523
Barca E, Ganetzky RD, Potluri P, Juanola-Falgarona M, Gai X, Li D, Jalas C, Hirsch Y, Emmanuele V, Tadesse S, Ziosi M, Akman HO, Chung WK, Tanji K, McCormick EM, Place E, Consugar M, Pierce EA, Hakonarson H, Wallace DC, Hirano M, Falk MJ. USMG5 Ashkenazi Jewish founder mutation impairs mitochondrial complex V dimerization and ATP synthesis. Hum Mol Genet. 2018 Oct 1;27(19):3305-3312. DOI: https://doi.org/10.1093/hmg/ddy231
Sanson M, Vu Hong A, Massourides E, Bourg N, Suel L, Amor F, Corre G, Bénit P, Barthélémy I, Blot S, Bigot A, Pinset C, Rustin P, Servais L, Voit T, Richard I, Israeli D. miR-379 links glucocorticoid treatment with mitochondrial response in Duchenne muscular dystrophy. Sci Rep. 2020 Jun 4;10(1):9139. DOI: https://doi.org/10.1038/s41598-020-66016-7
Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975 Dec;25(12):1111-20. DOI: https://doi.org/10.1212/WNL.25.12.1111
Mokri B, Engel AG. Commentary. Neurology. 1998;51:1–1. DOI: https://doi.org/10.1212/WNL.51.1.1
Wrogemann K, Pena SD. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672-4. DOI: https://doi.org/10.1016/S0140-6736(76)92781-1
Zulian A, Schiavone M, Giorgio V, Bernardi P. Forty years later: Mitochondria as therapeutic targets in muscle diseases. Pharmacol Res. 2016 Nov;113(Pt A):563-573. 09.043. Epub 2016 Sep 30. DOI: https://doi.org/10.1016/j.phrs.2016.09.043
Law ML, Cohen H, Martin AA, Angulski ABB, Metzger JM. Dysregulation of Calcium Handling in Duchenne Muscular Dystrophy-Associated Dilated Cardiomyopathy: Mechanisms and Experimental Therapeutic Strategies. J Clin Med. 2020 Feb 14;9(2):520. DOI: https://doi.org/10.3390/jcm9020520
Mareedu S, Million ED, Duan D, Babu GJ. Abnormal Calcium Handling in Duchenne Muscular Dystrophy: Mechanisms and Potential Therapies. Front Physiol. 2021 Apr 9;12:647010. DOI: https://doi.org/10.3389/fphys.2021.647010
Ljubicic V, Burt M, Jasmin BJ. The therapeutic potential of skeletal muscle plasticity in Duchenne muscular dystrophy: phenotypic modifiers as pharmacologic targets. FASEB J. 2014 Feb;28(2):548-68. Epub 2013 Nov 18. DOI: https://doi.org/10.1096/fj.13-238071
Kontro H, Hulmi JJ, Rahkila P, Kainulainen H. Cellular and tissue expression of DAPIT, a phylogenetically conserved peptide. Eur J Histochem. 2012 May 22;56(2):e18. DOI: https://doi.org/10.4081/ejh.2012.18
Nagata Y, Yamagishi M, Konno T, Nakanishi C, Asano Y, Ito S, Nakajima Y, Seguchi O, Fujino N, Kawashiri MA, Takashima S, Kitakaze M, Hayashi K. Heat Failure Phenotypes Induced by Knockdown of DAPIT in Zebrafish: A New Insight into Mechanism of Dilated Cardiomyopathy. Sci Rep. 2017 Dec 12;7(1):17417. Erratum in: Sci Rep. 2018 May 14;8(1):7768. DOI: https://doi.org/10.1038/s41598-017-17572-y
Wang Y, Luo J, Zhang H, Lu J. microRNAs in the Same Clusters Evolve to Coordinately Regulate Functionally Related Genes. Mol Biol Evol. 2016 Sep;33(9):2232-47. Epub 2016 Apr 28. DOI: https://doi.org/10.1093/molbev/msw089
Cantini L, Bertoli G, Cava C, Dubois T, Zinovyev A, Caselle M, Castiglioni I, Barillot E, Martignetti L. Identification of microRNA clusters cooperatively acting on epithelial to mesenchymal transition in triple negative breast cancer. Nucleic Acids Res. 2019 Mar 18;47(5):2205-2215. DOI: https://doi.org/10.1093/nar/gkz016
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2021.10012
https://doi.org/10.4081/ejtm.2021.10012



