Diffusion MRI on lymph node staging of gastric adenocarcinoma
Introduction
Regional lymph node evaluation is regarded as an essential step for both staging and treatment of gastric cancer (1). In cases without hematogeneous metastases, lymph node dissection based on the precise lymph node staging has been recommended as a component of radical gastrectomy which might provide the largest advantage for patients with gastric cancer. Nevertheless, an accurate preoperative assessment with regard to the extent of lymph nodes dissection remains to be a controversial topic (1,2).
As imaging technology continues to evolve, it may be expected to develop to an imaging modality with high sensitivity and specificity to assess the lymph node status of the gastric cancer (3). The reported accuracy of computed tomography (CT), endoscopic ultrasound, and conventional magnetic resonance imaging (MRI) for the N staging of gastric cancer was 43-80%, 65-87%, and 34-65%, respectively, and it has been accepted that this level of diagnostic performance was not sufficient for making a decision among treatment strategies for gastric cancer (4-7). No imaging modality has proven to be perfect with high sensitivity and specificity in the detection of lymph node metastasis in gastric cancer (2,8,9).
For conventional MRI studies, the lymph node size has been generally used as the criterion for metastasis (3). In these studies, inability to identify metastatic lymph nodes of normal size was given as an explanation for insufficient diagnostic performance of MRI. Therefore, functional MRI techniques such as diffusion weighted MRI (DW-MRI) or a combination of conventional and functional techniques have been regarded to be more accurate to identify metastatic lymph nodes in gastric cancer. However, a generally accepted algorithm has not been adopted in preoperative nodal staging of gastric cancer due to insufficient efficacy of MRI (1,2).
DW-MRI has increasingly been used to characterize various diseases and their lymph nodes including gastrointestinal cancers such as gastric or rectal cancers (1,10-15). Although the efficacy of this technique in differentiation of metastatic nodes from non-metastatic lymph nodes has been shown in patients with neck, rectal and gastric cancers, there is still lack of evidence supporting the generally accepted use of DW-MRI in nodal staging of gastric cancer (1).
In this study, we aimed to evaluate the diagnostic efficacy of DW-MRI with the utility of the apparent diffusion coefficient (ADC) measurement on detection of metastatic lymph nodes in patients with gastric cancer.
Materials and methods
The study was approved by the local Ethical Committee. Written consent was taken from all patients. The study was registered to Clinical Trials with an ID number of NCT01794026.
Patients
Between March 2013 and December 2013, all patients with a diagnosis of biopsy proven primary gastric adenocarcinoma were evaluated for radical surgical treatment. After evaluation of CT images, a total of 39 patients who were eligible for radical resection of gastric tumor with standard D1+ or D2 lymph node dissection were included in the study and underwent abdominal DW-MRI examination. Exclusion criteria were as follows: (I) improper candidates for radical surgical treatment (i.e., stage IV disease including liver metastasis or peritoneal seeding diagnosed by preoperative CT images) (n=6); (II) preoperative neoadjuvant therapy (n=4); (III) gastroesophageal junction tumor (n=2); (IV) unsatisfactory image quality of DW-MRI with obvious motion artifacts (n=2); (V) emergent admission (n=2). Therefore, a total of 23 patients with a mean age of 59.4±10.9 years were included in this study. There were 11 male and 12 female patients. Radical total and subtotal gastrectomy was performed in 10 and 13 patients, respectively. Para-aortic lymph node dissection and lymph node dissection beyond D2 was not performed. Tumors with a mean diameter of 60±18 mm were localized in lower, middle and upper part of the stomach in 9, 7 and 6 patients, respectively. In one patient, there was linitis plastic type gastric tumor. The time interval between DW-MRI examination and the surgery was 11.8±10.9 days.
The depth of tumor invasion (T) was decided according to TNM classification (16). Invasion of the mucosa and submucosa was regarded as pT1; pT2 and pT3 were defined as invasion of the muscularis propria and tumor penetration of the subserosa, respectively. N staging of the lymph nodes was performed according to N0 as no regional lymph node metastasis, N1 as metastasis in one to two regional lymph nodes, N2 as metastasis in three to six regional lymph nodes, N3a as metastasis in seven to 15 and N3b as metastasis in more than 15 regional lymph nodes. The variables with regard to the pathologic features were given in Table 1.
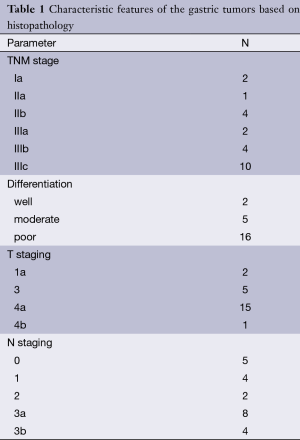
Full table
MRI and DW-MRI protocol
All MRI and DW-MRI examinations were performed using a 1.5-T MRI scanner (Magnetom Avanto, Siemens Healthcare, Erlangen/Germany) with an 18-channel body coil and high performance gradients (maximum gradient, 45 mT/m; maximum slew rate, 200 T/m/s). No specific bowel preparation or gastric distension was performed, and spasmolytics were not given. MRI examinations included a free breathing axial and coronal turbo spin-echo T1-weighted sequence (TR, 128 ms; TE, 2.4 ms; FA, 170°), and an axial and coronal fat saturated turbo spin-echo T2-weighted (TR, 1,350 ms; TE, 91 ms; FA, 70°) images. Axial and coronal spoiled gradient-echoT1-weighted (TR, 536 ms; TE, 11 ms; FA, 150°) images and axial T2-weighted fat saturated sequence [TR: 2,000 sn; TE: 92 sn; average: 1; flip angel: 160º; matrix: 256 × 256; number of slices: 30; slice thickness: 5 mm; FOV: 300 with breath hold after intravenous contrast agent administration (Meglumin gadoterat, 0.1 mmol/kg, Dotarem; Guerbet Group, France)] were obtained with body coil in the supine position. Diffusion weighted imaging consisted of an axial diffusion weighted single-shot spin-echo echoplanar sequence with a chemical shift selective fat suppression technique (TR/TE, 4,900/93) with the following parameters: matrix, 192 × 192; number of slices, 30; slice thickness, 6 mm; interslice gap, 35%; FOV, 45 cm; averages, 5; acquisition time, approximately 3 min; a generalized auto calibrating partial parallel acquisition technique PAT factor of 2; PAT mode, parallel imaging with modified sensitivity encoding. DW-MRI was performed with b-value of 50, 400 and 800 s/mm2.
Image analysis
Prior to surgery and histopathological analyses, DW-MRI of the abdomen was reviewed by one radiologist with 5 years of clinical experience in MRI. The ADC values from the healthy gastric wall, the gastric tumor and the most apparent lymph nodes were calculated. The acquired images were transferred to a workstation (Leonardo, Siemens, Erlangen, Germany) on which all the ADC maps were created automatically using standard software. To calculate and analyze the ADC maps, the ADC values (×10−3 mm2/s) were measured from the healthy gastric wall, the gastric tumor and the lymph nodes with the lowest ADC value on ADC map and highest intensity on DW-MRI (b=50, 400 and 800 s/mm2) by placing a circular region of interest (ROI) of 53 mm2 (Figures 1,2). At least three measurements were obtained and averaged.
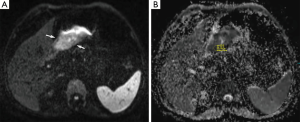
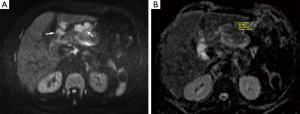
Each lymph node with short-axis diameter ≥5 mm was evaluated with enhancement pattern: homogeneous enhancement of lymph node was considered non-metastatic, whereas heterogeneous enhancement of lymph node was considered metastatic. Short axis diameter of at least 5 mm, heterogeneous signal intensity than muscle as seen on diffusion weighted imaging (b=50, 400 and 800 s/mm2) and the ADC value of <1.1×10−3 mm2/s were regarded as the standards required for radiologic diagnosis of a metastatic lymph node. Lymph nodes without these three standards were regarded as non-metastatic.
Histopathological analysis
The stomach and the surrounding fat including the omentum and the perigastric lymph nodes were resected en bloc. The lymph nodes around the anatomic landmarks of left gastric artery, common hepatic artery, celiac artery, splenic artery, and those located at the splenic hilum were resected separately. The whole specimen was fixed in formalin for 24 h.
The definition of the anatomical locations of the regional lymph nodes was based on the Japanese Classification of Gastric Carcinoma (17). Identification, counting and recording of all lymph nodes based on the grouping defined below was performed by a pathologist who was blinded to the imaging analysis (1). Grouping was performed as follows: (I) perigastric lesser curvature lymph nodes [Group Ia: right paracardial (#1), lesser curvature (#3), supra pyloric (#5)]; (II) Perigastric greater curvature lymph nodes [Group Ib: left paracardial (#2), greater curvature (#4), infra pyloric (#6)]; and (III) the lymph nodes around the left gastric artery (#7), the common hepatic artery (#8), the celiac artery (#9), the splenic artery (#11), and those located at the splenic hilum (#10) and the hepatoduodenal ligament (#12a) (Group II: #7, #8, #9, #10, #11, #12a). To achieve the most precise correlation, histopathological examination was performed in the lymph nodes 5 mm or more in diameter. The largest cut surface of each lymph node was sectioned and stained with hematoxylin and eosin (H&E). Differentiation of non-metastatic and metastatic lymph nodes was performed with the signs of metastasis including metastatic deposit, mucin production, necrosis and fibrosis using a light microscope.
Statistical analysis
Identification of the histologically metastatic lymph nodes by DW-MRI was regarded as the main outcome. All ADC values were expressed as mean ± standard deviation. The mean ADC values of the healthy gastric wall, the gastric tumor and the lymph nodes were compared with Wilcoxon Signed Ranks and Mann-Whitney tests. To find the group to cause the difference, Friedman test with post hoc Dunn multiple comparisons was used. Sensitivity, specificity, positive and negative predictive values, and overall accuracy were calculated for N staging based on the lymph nodes using the diagnostic criteria that refer to DW-MRI. The correlation of DW-MRI with histopathology was compared by a kappa agreement coefficient. SPSS 20.0 software (Chicago, Illinois, US) was used for statistical analysis. The level of significance with 0.95 confidence limits was set at P=0.05.
Results
During histopathologic analysis, a total of 1,056 lymph nodes from the 23 patients, including 180 histologically proven metastatic lymph nodes were dissected after the surgery (Table 2). Therefore, metastatic ratio of lymph nodes was calculated as 17.0%. Mean number of total and metastatic lymph nodes were counted as 46±12 and 7.8±11, respectively. As for the lymph node groups, 330 Group Ia lymph nodes (120 metastatic and 210 nonmetastatic), 487 Group Ib lymph nodes (45 metastatic and 432 nonmetastatic) and 239 Group II lymph nodes (15 metastatic and 224 nonmetastatic) were dissected.
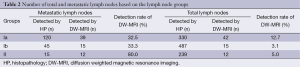
Full table
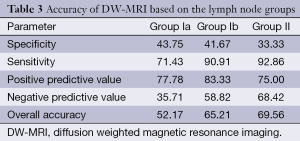
Based on the all lymph node groups, DW-MRI could identify the metastatic lymph nodes not considering their numbers in 18 out of 23 patients (77.8%). Although the rate for Group Ia was 65.2%, the rates were decreased to 30.4% and 17.4% for Groups Ib and II, respectively. However, 69 of 1,056 nodes (6.53%) in the total node regions were demonstrated by the currently used DW-MRI analysis. Of these, 66 lymph nodes (95.7%) were metastatic. Although identification rates of metastatic lymph nodes belonging in Groups Ia and Ib were 32.5% and 33.3%, respectively, DW-MRI detected metastatic lymph nodes with a rate of 80.0% in group II.
The κ agreement coefficient analysis (κ agreement coefficient values of 0.118, 0.319 and 0.291 for Group Ia, Ib and II, respectively) showed that there was no correlation between histopathology and DW-MRI with regard to the lymph node groups (P values of 0.493, 0.076 and 0.106 for Group Ia, Ib and II, respectively).
Based on the results taken from DW-MRI analysis with regard to identification of metastatic lymph nodes, the overall accuracy was found as 69.56, 65.21 and 52.17 for Groups II, Ib and Ia lymph nodes, respectively (Table 3).
Full table
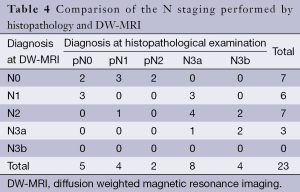
DW-MRI findings with regard to N staging included seven cases of N0, six cases of N1, seven cases of N2, three cases of N3a (Table 4). However, histopathologic examination of the lymph nodes revealed that there were five cases of N0, four cases of N1, two cases of N2, eight cases of N3a and four cases of N3b. So, there were four over staged and 16 under staged patients. Therefore, the overall accuracy of N staging based on DW-MRI was 13% (3 of 23).
Full table
Mean ADC values of the gastric tumor and the healthy gastric wall in all patients was (0.82±0.21) ×10−3 and (1.6±0.26) ×10−3 mm2/s, respectively. The lymph nodes that could be identified by DW-MRI had have mean ADC value of (0.93±0.25) ×10−3 mm2/s. Friedman test with post hoc Dunn multiple comparison and Wilcoxon Signed Ranks tests showed that the mean ADC values of the gastric tumor and the lymph nodes were significantly higher than that of the healthy gastric wall (P<0.001 for both). Mann-Whitney test revealed that there was no significant difference in ADC values between non-metastatic and metastatic lymph nodes based on the overall evaluation and groupings (Table 5), (P>0.05 for all).

Full table
Discussion
Gastric cancer is one of the most common cancers and the second leading cause of cancer-related death worldwide (2,3,10,11). Although the patients with early gastric cancer have a low risk of lymph node metastasis, it can be found in up to 20% of submucosal T1b gastric tumors (18). Thus, the extent to which lymph node dissection should be performed is still a topic of debate between Eastern and Western countries, because of the higher morbidity and mortality associated with extended lymphadenectomy. Therefore, sparing of the patients from such aggressive procedures and selection of appropriate patients for neoadjuvant chemotherapy may be possible by preoperative knowledge of the lymph node status of gastric cancer (1,3,19).
For differentiation of metastatic and non-metastatic lymph nodes, the short or long axis and short axis diameter of lymph nodes larger than at least 3 mm has been regarded as a widespread criterion used in CT and conventional MRI to predict metastases (8,19). However, there was a great controversy in consideration of diameter of the lymph nodes for metastasis. In Dai’s study, it was shown that the long axis diameter of the lymph nodes above ≥8 mm alone had has the sensitivity and specificity of 79.6% and 78.8% for metastasis, respectively (19). In Kim’s study, the range for metastatic lymph nodes has been varied from 3 mm to 3.5 cm (9). In accordance with Cheng’s study, the short axis of ≥5 mm was accepted for the minimum diameter of the lymph nodes to be evaluated (1). However, it could be possible to show lymph node positivity in 77.8% of the patients by using DWI in the present study. Although it has been reported that identification of the lymph nodes could be possible in up to 63.4% by using DW-MRI, our results could not reach to these levels (1). Strict radiologic criteria including short axis diameter of at least 5 mm, heterogeneous signal intensity than muscle as seen on diffusion weighted imaging (b=50, 400 and 800 s/mm2) and the ADC value of <1.1×10−3 mm2/s might be a causative explanation. It was also interesting that in an in vitro study of MRI to detect the lymph nodes after gastric surgery, only 49.7% of the lymph nodes could be detected (9). All these findings may remind that the N staging of gastric cancer still remains to be difficult.
In some studies, it was reported that the localization of the lymph nodes might be important for the identification by imaging techniques (19). Although the identification of the metastatic lymph nodes in Group II was 80%; the number of the lymph nodes was too small to conclude. Additionally, the overall accuracy of DW-MRI varied from 52.17% to 69.56% for different lymph node groups. Although Joo et al. has reported the accuracy for the N staging and the sensitivity for identification of any metastasis as 76.6% and 86.7%, the specificity for identification of any metastasis has been found as 58.8% (20). Although our results were lower than the other reported rates, parameters with regard to the technique or compliance of the patients might be important for this problem (15,20). Use of butylscopolamine bromide or glucagon to minimize peristaltic movements causing motion and distortion artifacts and distention of the stomach with water to increase the imaging quality on the gastric wall has been recommended. Several modifications or applications with regard to motion correction and respiratory-triggering have been also proposed (1,15,20-22). Such maneuvers were not planned in the present study. Increased spatial resolution of 3T scanners is believed to be another important issue (20). So, all these parameters should be considered for evaluation and comparison of the results of the current study. Therefore, it should be kept in mind that DW-MRI without these features may be insufficient consistently to distinguish non-metastatic and metastatic lymph (8,19).
The utility of DW-MRI to distinguish metastatic lymph nodes from benign nodes in breast cancer, head and neck cancer, and rectal cancer has been widely investigated (1,11,23,24). It has been also thought that ADC measurements can be valuable in differentiating benign and malignant lesions of the stomach. In the evaluation of the gastric wall thickening, use of DW-MRI was shown to be beneficial in the diagnosis of malignant gastric lesions (10,11,25-27). In this study, significantly lower values of ADC of the gastric tumors in comparison to the healthy gastric wall were also detected. There are also some studies in which use of DW-MRI with ADC measurement might be useful to differentiate metastatic lymph nodes from non-metastatic ones with regard to the gastric tumors (1). In Cheng’s study, it was thought that DW-MRI might provide great potential to differentiate metastatic lymph nodes by using both ADC measurement and the morphological features (1). Shinya et al. showed the efficacy of DW-MRI to detect lymph node metastasis in comparison to CT (27). However, the results in the present study could not support the benefit of DW-MRI by using ADC measurement in differentiation of metastatic lymph nodes from non-metastatic ones. Therefore, it may be concluded that use of DW-MRI with ADC measurement can be beneficial to diagnose only gastric wall lesions in accordance with the literature focusing on T stage of the gastric adenocarcinoma (26,28).
It has been reported that there was a significant amount of overlapping between ADC values of benign and malignant lesions; which was thought to be caused by desmoplastic reaction in the stroma of malignant tissues. New blood vessels, necrosis, severe inflammation and fibrotic stroma within metastatic lymph nodes may also account for the pattern of heterogeneous enhancement leading to this overlapping (1,8,12). Artifacts caused by physiologic motions like cardiac pulsation and respiration, bulk motion and intestinal peristalsis, and presence of various parenchymal gas interfaces also make it difficult to perform DW-MRI in the abdomen. Following advanced fast imaging sequences and application of several motion correction methods in MRI, it is expected that these artifacts may be eliminated and, DW-MRI may be used in the evaluation of abdominal organs with great success (11,22,29). In the present study, it could not be possible to reach high accuracy for DW-MRI to detect and differentiate metastatic lymph nodes in gastric cancer patients without use of such maneuvers. Therefore, it is logical to use these correction methods during evaluation of abdominal organs with DW-MRI (22,29).
We could not define cutoff ADC values for differentiation of non-metastatic and metastatic lymph nodes in this study because of the small number of the patients. Cheng et al. used 1.39×10−3 mm2/s as a cutoff value of ADC with sensitivity of 85.7% and specificity of 79.4% for metastatic lymph nodes (1). However, we compared ADC values of the gastric tumor and the healthy gastric wall of the each patient. It was shown that there was a statistically significant difference between ADC values of the gastric tumor and the healthy gastric wall. In contrary to the present study, it was thought that measurement of ADC values in the lymph nodes were useful to differentiate the metastatic lymph nodes (1). Therefore, mean ADC values of non-metastatic and metastatic lymph nodes and their comparisons with the healthy gastric wall and the gastric tumor may be useful as a reference value for radiologists in evaluation of gastric wall thickening and comparison of non-metastatic and metastatic lymph nodes.
It was though that the significant difference between ADC values of benign and malignant gastric lesions at b-value of 600 and 1,000 gradients helped to distinguish benign and malignant gastric lesions, and to increase the sensitivity to diffusion, to improve bowel suppression, and to decrease signal-to noise ratio and artifacts (30). Although b-values from 0 to 1,000 were used in several studies, it is generally offered to use b-value between intermediate (b-value of 600) and high (b-value of 1,000) level of diffusion gradients, which is less affected from capillary perfusion and reflects mostly diffusion of water molecules (1,10,11,20,26). Therefore, b-values of 50, 400 and 800 were preferred in the present study.
The small number of the patients caused by the strict exclusion criteria and lack of comparison of CT with DW-MRI were the leading limitative factors. Although it has been previously planned to compare both CT and DW-MRI, CT images with variations in scanning protocols between our hospital and the other imaging centers, comparison of DW-MRI with CT and histopathology was not used. Due to the great difference between the lymph nodes identified in DW-MRI and histopathology, one-to-one pathologic-to-radiologic correlation on each lymph node was not performed in our study. It might be also thought that review of DW-MRI by one radiologist was another limitative factor for the reliability of the results. However, DW-MRI has yielded only overall diagnostic accuracy from 52.17% to 69.56% and lower values of specificity in comparison to that of sensitivity. Therefore, presence of one radiologist to review the images could not be regarded as a limitative factor. Lack of motion correction maneuvers to increase the accuracy of DW-MRI may be another limiting parameter for the results in the present study.
In conclusion, although DW-MRI has had higher accuracy to detect the lymph nodes localized around the anatomic landmarks of left gastric artery, common hepatic artery, celiac artery, splenic artery, and those located at the splenic hilum and the hepatoduodenal ligament, there was no significant correlation between histopathology and DW-MRI with regard to the lymph node groups. However, DW-MRI has shown metastatic lymph nodes in 77.8% of the patients. Besides the presence of statistically significant difference between ADC values of the gastric tumor, the lymph nodes and the healthy gastric wall, differentiation of metastatic and non-metastatic lymph nodes by ADC values has not been shown. Therefore, DW-MRI cannot be used as the first choice imaging technique for a strict N staging of gastric cancer because of its low accuracy to detect or to differentiate metastatic and non-metastatic lymph nodes based on their total numbers.
Acknowledgements
Authors’ contributions: M Hasbahceci designed the overall study with contributions from the others, performed surgical procedures, collected and analyzed the data, and wrote the article; A Akcakaya designed the study, performed the surgical procedures and co-wrote the paper; N Memmi performed the surgical procedures; G Cipe performed the surgical procedures analyzed the data with M Hasbahceci; I Turkmen evaluated MRI and DW-MRI images, co-write for Materials and Methods section; P Yildiz and DS Arici evaluated histopathological analysis of the gastric specimens; M Muslumanoglu performed the surgical procedures, discussed and edited the paper.
Disclosure: The authors declare no conflict of interest.
References
- Cheng J, Wang Y, Deng J, McCarthy RJ, Wang G, Wang H, Ye Y. Discrimination of metastatic lymph nodes in patients with gastric carcinoma using diffusion-weighted imaging. J Magn Reson Imaging 2013;37:1436-44. [PubMed]
- Tokuhara T, Tanigawa N, Matsuki M, Nomura E, Mabuchi H, Lee SW, Tatsumi Y, Nishimura H, Yoshinaka R, Kurisu Y, Narabayashi I. Evaluation of lymph node metastases in gastric cancer using magnetic resonance imaging with ultrasmall superparamagnetic iron oxide (USPIO): diagnostic performance in post-contrast images using new diagnostic criteria. Gastric Cancer 2008;11:194-200. [PubMed]
- Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009;12:6-22. [PubMed]
- Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol 2000;174:1551-7. [PubMed]
- Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska-Guttmejer A, Butruk E. Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy 2004;36:617-23. [PubMed]
- Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, Lee MS, Kim BS. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc 2004;59:619-26. [PubMed]
- Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, Wu MT, Liu GC. Gastric cancer: preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology 2007;242:472-82. [PubMed]
- Zhou ZG, Liu F, Jiao LC, Wang ZL, Zhang XP, Wang XD, Luo XZ. An evidential reasoning based model for diagnosis of lymph node metastasis in gastric cancer. BMC Med Inform Decis Mak 2013;13:123. [PubMed]
- Kim IY, Kim SW, Shin HC, Lee MS, Jeong DJ, Kim CJ, Kim YT. MRI of gastric carcinoma: results of T and N-staging in an in vitro study. World J Gastroenterol 2009;15:3992-8. [PubMed]
- Onur MR, Ozturk F, Aygun C, Poyraz AK, Ogur E. Role of the apparent diffusion coefficient in the differential diagnosis of gastric wall thickening. J Magn Reson Imaging 2012;36:672-7. [PubMed]
- Avcu S, Arslan H, Unal O, Kotan C, Izmirli M. The role of diffusion-weighted MR imaging and ADC values in the diagnosis of gastric tumors. JBR-BTR 2012;95:1-5. [PubMed]
- Bakir B, Bakan S, Tunaci M, Bakir VL, Iyibozkurt AC, Berkman S, Bengisu E, Salmaslioglu A. Diffusion-weighted imaging of solid or predominantly solid gynaecological adnexial masses: is it useful in the differential diagnosis? Br J Radiol 2011;84:600-11. [PubMed]
- Weber MA, Bender K, von Gall CC, Stange A, Grünberg K, Ott K, Haberkorn U, Kauczor HU, Zechmann C. Assessment of diffusion-weighted MRI and 18F-fluoro-deoxyglucose PET/CT in monitoring early response to neoadjuvant chemotherapy in adenocarcinoma of the esophagogastric junction. J Gastrointestin Liver Dis 2013;22:45-52. [PubMed]
- Cho EY, Kim SH, Yoon JH, Lee Y, Lim YJ, Kim SJ, Baek HJ, Eun CK. Apparent diffusion coefficient for discriminating metastatic from non-metastatic lymph nodes in primary rectal cancer. Eur J Radiol 2013;82:e662-8. [PubMed]
- Vandecaveye V, De Keyzer F, Vander Poorten V, Dirix P, Verbeken E, Nuyts S, Hermans R. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology 2009;251:134-46. [PubMed]
- Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2010;116:5592-8. [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [PubMed]
- Dai CL, Yang ZG, Xue LP, Li YM. Application value of multi-slice spiral computed tomography for imaging determination of metastatic lymph nodes of gastric cancer. World J Gastroenterol 2013;19:5732-7. [PubMed]
- Joo I, Lee JM, Kim JH, Shin CI, Han JK, Choi BI. Prospective comparison of 3T MRI with diffusion-weighted imaging and MDCT for the preoperative TNM staging of gastric cancer. J Magn Reson Imaging 2015;41:814-21. [PubMed]
- Liau J, Lee J, Schroeder ME, Sirlin CB, Bydder M. Cardiac motion in diffusion-weighted MRI of the liver: artifact and a method of correction. J Magn Reson Imaging 2012;35:318-27. [PubMed]
- Mazaheri Y, Do RK, Shukla-Dave A, Deasy JO, Lu Y, Akin O. Motion correction of multi-b-value diffusion-weighted imaging in the liver. Acad Radiol 2012;19:1573-80. [PubMed]
- Caivano R, Rabasco P, Lotumolo A, D' Antuono F, Zandolino A, Villonio A, Macarini L, Guglielmi G, Salvatore M, Cammarota A. Gastric cancer: The role of diffusion weighted imaging in the preoperative staging. Cancer Invest 2014;32:184-90. [PubMed]
- Akduman EI, Momtahen AJ, Balci NC, Mahajann N, Havlioglu N, Wolverson MK. Comparison between malignant and benign abdominal lymph nodes on diffusion-weighted imaging. Acad Radiol 2008;15:641-6. [PubMed]
- Sun YS, Cui Y, Tang L, Qi LP, Wang N, Zhang XY, Cao K, Zhang XP. Early evaluation of cancer response by a new functional biomarker: apparent diffusion coefficient. AJR Am J Roentgenol 2011;197:W23-9. [PubMed]
- Liu S, He J, Guan W, Li Q, Yu H, Zhou Z, Bao S, Zhou Z. Added value of diffusion-weighted MR imaging to T2-weighted and dynamic contrast-enhanced MR imaging in T staging of gastric cancer. Clin Imaging 2014;38:122-8. [PubMed]
- Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The usefulness of diffusion-weighted imaging (DWI) for the detection of gastric cancer. Hepatogastroenterology 2007;54:1378-81. [PubMed]
- Jang KM, Kim SH, Lee SJ, Lee MW, Choi D, Kim KM. Upper abdominal gadoxetic acid-enhanced and diffusion-weighted MRI for the detection of gastric cancer: Comparison with two-dimensional multidetector row CT. Clin Radiol 2014;69:827-35. [PubMed]
- Benner T, van der Kouwe AJ, Sorensen AG. Diffusion imaging with prospective motion correction and reacquisition. Magn Reson Med 2011;66:154-67. [PubMed]
- Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol 2009;193:461-70. [PubMed]


