Very late outcomes of drug-eluting stents coated with biodegradable polymers: insights from the 5-year follow-up of the randomized PAINT trial
Introduction
Drug-eluting stents (DES) are a milestone development in interventional cardiology, due to their ability to reduce the risk of restenosis and repeat revascularization after percutaneous coronary intervention (1). However, in the long run, beyond the so-called “restenosis-period“, clinical studies have raised concerns about the very safety of DES (2,3). Delayed stent thrombosis (ST) and neo-atherosclerosis have been reported to occur in patients treated with early DES formulations, potentially related to impaired reendothelialization and hypersensitivity response to polymer carriers (4-7).
Biodegradable polymers (BP) have been developed as an answer to this concern, essentially enabling for a DES to become a bare metal stent within months. Putatively, this would permit a more physiological vascular healing response after stenting.
Until the present time, few studies have examined the very long-term outcomes after implantation of BP-DES (8,9). The “Percutaneous intervention with biodegradable-polymer based paclitaxel-eluting or sirolimus-eluting versus bare stents for de novo coronary lesions”—PAINT randomized trial, was designed to evaluate the safety and efficacy of two DES metallic formulations coated with BP carriers (10-12). The present report presents the 5-year clinical follow-up of patients included in the PAINT trial.
Methods
The PAINT trial was a prospective, multicenter randomized controlled trial in which patients were allocated for percutaneous coronary intervention of de novo native coronary lesions with one of the following: the control Millennium Matrix® bare metal stent (BMS), the Infinnium® paclitaxel-eluting stent (PES), or the Supralimus® sirolimus-eluting stent (SES) (all by Sahajanand Medical Technologies Pvt. Ltd., India) in a 1:2:2 ratio, respectively. All study stents were built with the same 316L stainless metallic platform and delivery system. The DES differed by the drug (paclitaxel or sirolimus respectively) but had the same drug carrier (thickness 4-5 µm), which was composed of a blend of BPs that included Poly L-Lactide, 50/50 Poly DL-Lactide-co-Glycolide, 75/25 Poly L-Lactide-co-Caprolactone, and Polyvinyl Pyrrolidone. Both DES formulations release approximately 50% of the drug content in the first 9-11 days, 90% in 38 days and 100% in 48 days. The polymeric matrix ultimately biodegraded as water and carbon dioxide and complete polymer degradation occurs within seven months. The study protocol, 1- and 3-year clinical follow-ups of the PAINT trial has been published previously elsewhere (10-12). The study complies with the Declaration of Helsinki, was approved by the ethics committee at each participating center, and written informed consent was obtained from all the patients.
The primary end-point of this sub-study was defined as the composite of the major cardiac adverse events (MACE) cardiac death, myocardial infarction (MI) or ischemia-driven target vessel revascularization (TVR) at 5 years. Adverse events, including ST according to Academic Resource Consortium, were also analyzed.
Statistical analysis was performed using STATA version 12 (College Station, TX, USA). The Kaplan-Meier method was utilized to estimate the incidence of clinical adverse events. The outcomes of each arm were compared using the log-rank test of equality.
Results
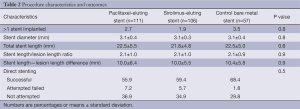
The final study population included 274 patients (57 in the bare stent, 111 in the paclitaxel, and 106 in the sirolimus groups), enrolled between April 2006 and August 2008. Follow-up through 5 years was completed in 89.8% of patients, including 87.7% of patients receiving BMS, 91,0% of patients receiving PES and 88.7% of patients receiving SES. Median and interquartile follow-up of surviving patients was 5.0 (range, 4.9-5.0) years. There were no significant differences between baseline demographic, clinical, angiographic, and procedural characteristics which are presented in Tables 1,2.

Full table
Full table
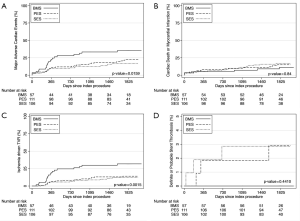
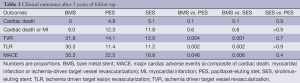
At the end of the follow-up, the rates of MACE were different among the groups: 35.3%, 22.5% and 16.9% for BMS, PES and SES, respectively (P=0.046 for PES compared to BMS and P=0.006 for SES compared to BMS). The occurrence of the primary end-point was mainly driven by TVR: 31.8%, 14.1% and 12.2% for BMS, PES and SES, respectively (P=0.004 for PES compared to BMS and P=0.006 compared to SES). The composite of cardiac death and MI did not differ among the groups (Figure 1 and Table 3).
Full table
The absolute difference in the risk of MACE increased over time when comparing the DES groups and the BMS group; at 1 year, the difference in the incidence of MACE between the pooled DES population and BMS-treated patients was 11.3%, while the difference was 15.5% by the end of the fifth year of follow-up (Figure 1).
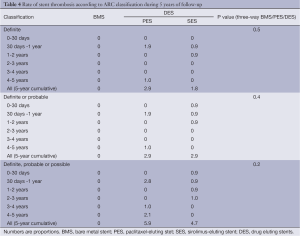
The incidence of ST was null for BMS during the entire follow-up. There were no definite or probable stent thromboses in the SES group after the second year, while one patient (1.0%) presented with a definite ST episode in the PES group between 4 and 5 years (Table 4). To exemplify, this last patient was a 63-year-old woman with insulin dependent diabetes, hypertension, hypercholesterolemia, and who smoked. She was treated for stable angina with a PES to the distal right coronary artery. It had a minimal luminal diameter (MLD) of 0.89 mm, which after stenting had increased to 2.21 mm. On follow-up angiography a moderate restenosis was observed with an MLD of 1.34 mm. She used clopidogrel for a year, and therefore had been off dual anti-platelet therapy when after her fourth year (4.3 years) of follow-up she presented with an ST segment elevation MI. A definitive ST was diagnosed, which was treated with primary PCI with adequate resolution of the event.
Full table
Discussion
Previous findings from the PAINT trial have shown that, compared to bare metal stents, both PES and SES with BPs reduced 9-month late luminal loss and TVR during the first 3 years of follow-up (11,12). The present study builds on previous results. At 5 years, both DES groups preserved the reduction of combined major adverse cardiac events, mainly by decreased rates of TVR.
The so-called late catch-up phenomenon (i.e., delayed and progressive luminal loss long after DES implantation) has been previously described by others (13). The present findings do not support the occurrence of clinically relevant late catch-up in our cohort. The anti-restenosis properties of both DES formulations appeared to resist the effect of time. After the first year, the risk of repeat revascularization remained rather stable in patients treated with either SES or PES. In fact, the absolute difference in the risk of adverse events increased over time between the DES-treated patients and the BMS group.
Very late (after the first year) ST was uncommon for both DES formulations, yielding an annualized rate of definite or probable ST of approximately 0.25%. In fact, no patient in the SES group had a thrombotic event after the second year. It is noteworthy, however, that an episode of definite ST still occurred at 4 years in the PES group.
DES comparisons in this study are particularly revealing since the only difference between the formulations is the drug used (sirolimus versus paclitaxel), as both DES are built with the same BP mounted on the same metal platform. In spite of the significantly lower 9-month angiographic late loss of SES compared to PES (in-segment late loss 0.15±0.42 vs. 0.35±0.42 mm respectively, P<0.01) (11), this did not translate into reduced TVR or reduced MACE at 5 years of follow-up. Conversely, the higher neointimal growth in the paclitaxel group was not associated with a clearly improved safety profile in the long run. As a matter of fact, the only stent thrombotic event after the second year occurred in the PES group.
Thus far, few studies have reported the incidence of very-late thrombosis in long-term follow-up of BP DES. The relatively small sample size of the present study limits its ability to evaluate in-depth such uncommon events. Indeed, the rarity of very-late ST practically confines well-powered safety analysis to meta-analyses and large populational studies. Nevertheless, our findings are in line with previous reports showing a low risk of very late ST after BP-DES implantation (14,15).
Our results cannot be directly extrapolated to other BP-DES formulations, which might markedly differ in their bioengineering construct. For instance, the stents tested in our study were built with conformable coating (i.e., the entire strut surface), instead of the abluminal coating (i.e., restricted to the strut surface in contact with the vessel wall) used in some of the current DES (14,16,17). Different DES may not have the same drug load, kinetics of drug release and polymer degradation, biopolymer composition, or metallic platform, which may all influence in final DES performance.
Conclusions
The tested biodegradable-polymer coated stents releasing either paclitaxel or sirolimus, compared with same bare metal platform, sustained their effectiveness in reducing combined major adverse cardiac events and re-intervention without an increase in ST during five years of follow-up. The direct comparison between the DES groups shows that despite superior angiographic end-points for the sirolimus stents, both groups maintain similar event curves throughout the 5-year follow-up.
Acknowledgements
Disclosure: Sahajanand Medical Technologies Pvt. (India) and CMS Medical (Brazil) provided the study stents and the funds to cover the expenses with the angiographic core laboratory.
References
- Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 2007;370:937-48. [PubMed]
- Serruys PW, Daemen J. Are drug-eluting stents associated with a higher rate of late thrombosis than bare metal stents? Late stent thrombosis: a nuisance in both bare metal and drug-eluting stents. Circulation 2007;115:1433-9; discussion 1439. [PubMed]
- Marchini JF, Manica A, Croce K. Stent thrombosis: understanding and managing a critical problem. Curr Treat Options Cardiovasc Med 2012;14:91-107. [PubMed]
- Liu HT, Li F, Wang WY, et al. Rapamycin inhibits re-endothelialization after percutaneous coronary intervention by impeding the proliferation and migration of endothelial cells and inducing apoptosis of endothelial progenitor cells. Tex Heart Inst J 2010;37:194-201. [PubMed]
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193-202. [PubMed]
- Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 2004;109:701-5. [PubMed]
- Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol 2006;47:175-81. [PubMed]
- Serruys P, Buszman P, Linke A, et al. TCT-44 LEADERS: 5-Year Follow-Up from a Prospective, Randomized Trial of Biolimus A9-Eluting Stents with a Biodegradable Polymer vs. Sirolimus-Eluting Stents with a Durable Polymer- Final report of the LEADERS study. J Am Coll Cardiol 2012;60:B13-4.
- Costa R, Mueller R, Abizaid A, et al. TCT-215: STEALTH I: 5-year Follow-up from a Prospective Randomized Study of Biolimus A9TM-Eluting Stent with a Biodegradable Polymer Coating vs a Bare Metal Stent. J Am Coll Cardiol 2011;58:B58.
- Lemos PA, Moulin B, Perin MA, et al. Rationale and design for the PAINT randomized trial. Arq Bras Cardiol 2009;93:547-53. [PubMed]
- Lemos PA, Moulin B, Perin MA, et al. Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-year results of the PAINT trial. Catheter Cardiovasc Interv 2009;74:665-73. [PubMed]
- Lemos PA, Moulin B, Perin MA, et al. Late clinical outcomes after implantation of drug-eluting stents coated with biodegradable polymers: 3-year follow-up of the PAINT randomised trial. EuroIntervention 2012;8:117-9. [PubMed]
- Natsuaki M, Morimoto T, Furukawa Y, et al. Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: long-term (5-7 years) follow-up of the Coronary Revascularization Demonstrating Outcome study-Kyoto registry Cohort-2. Circ Cardiovasc Interv 2014;7:168-79. [PubMed]
- Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv 2013;6:777-89. [PubMed]
- Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 2012;33:1214-22. [PubMed]
- Ribeiro EE, Campos CM, Ribeiro HB, et al. First-in-man randomised comparison of a novel sirolimus-eluting stent with abluminal biodegradable polymer and thin-strut cobalt-chromium alloy: INSPIRON-I trial. EuroIntervention 2014;9:1380-4. [PubMed]
- Meredith IT, Verheye S, Weissman NJ, et al. Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention 2013;9:308-15. [PubMed]


