The dynamic of nasogastric decompression after esophagectomy and its predictive value of postoperative complications
Introduction
Esophageal cancer is the eighth most common cancer in the world, especially in China (1). Surgical resection remains the gold standard for localized esophageal cancer, but esophagectomy is a complex operation with several serious postoperative complications, which may result in high morbidity and mortality (2-4). According to the previous data, anastomotic leakage and other complications increased the risk of recurrence in patients who underwent curative gastroesophageal cancer resection (5,6). Application of a nasogastric tube (NGT) is most important part in the postoperative period after esophagectomy for decompression of the gastric conduit. The purpose of postoperative NGT decompression was presumed to prevent distant of the gastric conduit and hence prevent anastomotic leak, pulmonary aspiration, and respiratory infection. However, the use of NGT was reported to have some adverse effects, including throat pain, nasal mucosal damage, sinusitis, gastritis, and epistaxis (7). Therefore, in recent years some studies have been published to support elimination of NGT decompression after gastric or esophageal surgery, especially after minimally invasive (MIE) approach (8,9). However, there is still a controversy whether the application of NGT decompression after esophagectomy is necessary. The aim of this study was to determine the dynamic of NGT decompression after esophagectomy and to evaluate its predictive value for postoperative complications.
Materials and methods
Participants
Between November 2007 and August 2013, a consecutive series of 247 patients underwent esophagectomy for esophageal or gastroesophageal junction carcinoma with curative intent were retrospectively evaluated. A total of 11 patients were excluded from this analysis: 3 patients were excluded because of application of acid-inhibitory drug for peptic ulcer; 1 patient suffered from preoperative pyothorax; 4 patients with gastric fundus invasion were performed by subtotal gastrectomy and Roux-y anastomosis; transhiatal esophagectomy in 2 cases; and hepatic metastases in 1 case. There were 191 cases with non-complication and 45 patients suffered from postoperative complications, including pulmonary infection, anastomotic leakage, disorder of gastrointestinal function recovery, anastomotic bleeding, and atrial fibrillation were included in our analysis.
Before the procedure, patients’ liver and renal function, blood-coagulate functions, tumor markers, blood and urine routine were tested. Routine examinations including upper digestive tract radiography and gastroscope were completed to definite the pathological diagnosis. And cranial MRI, abdominal ultrasound and Bone Scintigraphy were performed to confirm the absence of distant metastases. In order to assess surgery tolerance, we also performed pulmonary function and electrocardiogram pre-operatively. Esophageal cancer staging was based on the American Joint Committee on Cancer (AJCC) 2009 cancer staging (10).
Surgical approach
The following standard surgical techniques were performed: sweet in 140 patients, McKeown in 11 patients (3 MIE, 5 open, and 3 hybrid), Ivor-Lewis in 70 patients (7 MIE, 13 open, and 50 hybrid), and simple transabdominal in 15 patients. For surgical approach, at least 6-8 cm of normal tissue was resected above the proximal edge of the tumor to avoid neoplastic involvement of the resection margins. We advocate a complete resection with a 2-field lymph node dissection routinely and fewer 3-field lymphadenectomies. As for reconstruction, we preformed stomach as replacement in all patients. The anastomotic position was including: Cervical anastomosis and intra-thorax. In order to drain sufficiently, we placed the NGT into the distal remnant stomach.
Postoperative treatment
During the postoperative period, all patients were fasting and received intravenous nutrition support therapy according to the patients’ physiological requirements and biochemical indices. Meanwhile, 40 mg of omeprazole was administered intravenously per day. The NGT was kept on draining, and removed until no leakage was observed that confirmed by drinking 50 mL methylene blue, and then oral intake of liquids was initiated.
Complications
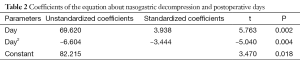
There were 45 cases suffered from postoperative complications according to the following diagnostic criteria: (I) the standard of pulmonary infection diagnosis refer to the diagnostic criteria of hospital acquired pneumonia (11); (II) anastomotic leakage was diagnosed by digestive tract radiography or methylene blue; (III) pool gastrointestinal functional recovery (i) clinical manifestations: nausea, vomiting, frequent hiccups, exhaust and defecation time obviously extended, (ii) X-ray chest radiograph: the shadow of thoracic stomach was expansive and air-fluid level can be seen in the stomach; (IV) postoperative anastomotic hemorrhage was confirmed by gastroscopy; (V) atrial fibrillation was diagnosed by electrocardiogram (Table 1).

Full table
Statistics analysis
The SPSS software package 17.0 for Windows was used for statistical analysis. The regularity of nasogastric decompression volume, stratified by postoperative days, was conducted and described by using curve estimation method. A set of equations were established and verified by fitting analysis.
The single and multiple factors regression analysis were used for patients without complication. The volume of nasogastric decompression was regarded as the dependent variable. Gender, age, height, weight, tobacco or alcohol exposure, location of the tumor, histologic type, pathological stage, operating time, mode of surgical procedures, anastomotic position and gastric conduit reconstruction were considered as independent variables. Continuous data were shown as mean ± SD or as median and interquartile range (IQR). All of the data were analyzed by Mann-Whitney U or Kruskal-Wallis H test.
After that, the factors of patients with postoperative complications were fitted into the mathematical model established previously, and the predictive value of the mathematical model was compared to the actual volume they created. P values <0.05 were considered statistically significant.
Results
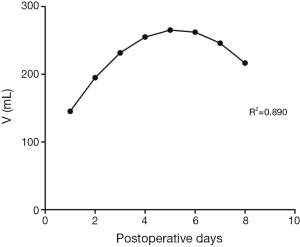
In trend analysis of 191 patients without digestive disease history, including chronic gastritis, peptic ulcer, reflux esophagitis etc., the curve estimation revealed a quadratic trend in the relationship between nasogastric decompression volume and postoperative hospitalization (R-square =0.890, P=0.004). The postoperative daily nasogastric decompression was described by Drainage (mL) =82.215 + 69.620 × days − 6.604 × days2 (the curve is shown in Figure 1). The equation has a quite good fitting degree (R2=0.890). The coefficients of that equation showed statistical significance (P<0.05) (Table 2).
Full table
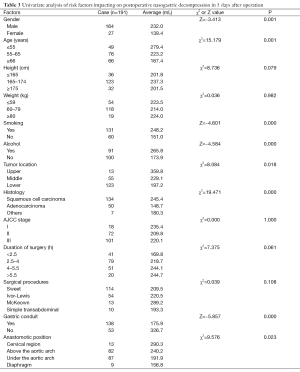
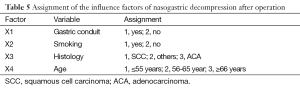
Single and multiple regression analysis were performed to find out the risk factors of postoperative nasogastric decompression of those 191 patients. The average volume of nasogastric decompression in 5 days after operation was used as dependent variable, meanwhile, gender, age, height, weight, smoking, drunk, location of the tumor, histological type, pathological staging operation time, surgical procedures, anastomotic position and gastric conduit reconstruction were considered as the independent variable. The univariate analysis showed significant differences on gender, age, smoking, drunk, location of the tumor, histological type, anastomotic position and gastric conduit reconstruction (P<0.05, Table 3). So these factors were included into the multivariate analysis. According to the results of multiple stepwise regression, gastric conduit reconstruction (β=0.410, P=0.000), smoking (β=−0.231, P=0.000), age (β=−0.193, P=0.001) and histological type (β=−0.169, P=0.006) were significantly related to the volume of postoperative nasogastric decompression (Table 4). The volume of average drainage in 5 days after surgery =262.287 + 132.873 × X1 − 72.160 × X2 − 27.904 × X3 − 36.368 × X4 (value assignment is presented in Table 5). F test was performed to evaluate the equation above (F=27.245, P=0.000).
Full table

Full table
Full table
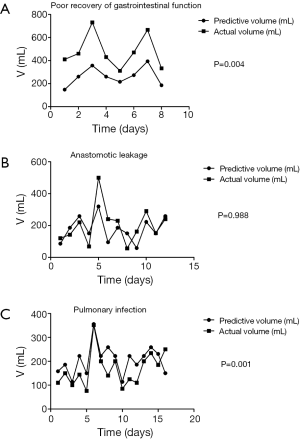
The clinical factors of patients with postoperative complications were brought into the standard equation. Compared to the predictive volume, the actual volume which created in the first 5 days after surgery of the patients with poor recovery of gastrointestinal function was significantly richer (P=0.004, Figure 2A). There was no significant difference between the actual drainage and the predictive value of standard equation in the patients with anastomotic leakage (P=0.988) (Figure 2B). Meanwhile, the nasogastric decompression of the patients with pulmonary infection in the first 5 days after operation was less than the predictive value (P=0.001, Figure 2C).
Discussion
The concept that NGT should be used after esophagectomy has been argued recently. It has been reported that using or disusing NGT decompression has no significant difference in postoperative complications, and the use of NGT decompression during MIE esophagectomy can be safely omitted (12,13). But these studies seldom comprehensively described the characteristics and influence factors of the volume of nasogastric drainage during the postoperative period. We attempt to explore the regularity and the influence factors of nasogastric decompression after esophagectomy, to assess the predictive value of nasogastric decompression for postoperative complications of esophageal carcinoma.
According to the result of 191 patients without postoperative complication, the relation between nasogastric decompression volume and postoperative hospitalization is in line with a quadratic curve showing that, after the surgery, the volume of nasogastric decompression increases firstly and decreases after reaching the peak. We considered that at the early period after the surgery (the first and the second day), stress reaction caused by operative trauma and pain can enhance the exciting level of the sympathetic nerve and depress the vagus nerve as well. The vagus nerve is the most important factors to regulate the secretion level of gastric acid (14). Therefore, transection of the vagus nerve during the esophagectomy will depress the secretion level of gastric juice. Relieved from the stress reaction, the secretion level of gastric juice recovers gradually and the nasogastric decompression volume increases. The rein creasing of gastric juice secretion indicates recovering of gastric emptying. Meanwhile, the curve of nasogastric decompression is experiencing an approximate plateau, meaning that the curve is trending up while the rate is decreasing. Increasing of gastric secretion and recovering of gastric emptying mutually restrict each other and keep balance during this period. And after 4 or 5 days the volume of nasogastric decompression reaches to the peak level, before altering to a decreasing phase. This alteration indicates that gastric emptying is in the dominated status, which happens in the same time with exhaust and defecation for most patients. In conclusion, after esophagectomy the curve of the nasogastric decompression volume may be divided into three stages: the recovery of the secretion of gastric acid during the early period, the approximate plateau caused by mutual restriction between the secretion of gastric acid and gastric emptying, and the recovery of gastric emptying during the late period. However, by reviewing the details of each patient, the data of all the 191 patients are compliant with the law that nasogastric decompression volume increases firstly and decreases lately, and the peak level appears between the fourth and the fifth day after procedure (some patients might be late). Therefore, this predictive curve can properly describe the trends of nasogastric decompression after the surgery.
According to the results of regression analysis, gastric conduit reconstruction, smoking history, age and histological type are relative factors for nasogastric decompression volume sequentially. Whether the gastric conduit is conducted or not has the most significant influence towards nasogastric decompression volume. In the previous literatures, the best replacement of esophagus after esophagectomy has been argued. Although stomach is the most popular option, the major problem of intra-thoracic stomach is discomfort and regurgitation of digestive juice caused by anatomical structure destroyed. Therefore, some foreign scholars were inclined to choose long segment jejunal interposition with microvascular augmentation or colon as the replacing organ (15). Currently, with the development of gastric conduit reconstruction, Gastric conduit has become the hotspot of esophageal cancer. However, most surgeons would agree that the stomach is the most robust and straight-forward esophageal replacement (16). Compared with whole stomach reconstruction, gastric conduit has lots of advantages, which can effectively decrease the gastric juice secretion of residual stomach, improve the blood supply, relieve the tension, and lower the occurrence rate of anastomotic leakage after surgery (17). Meanwhile, it can also relieve the discomfort of intra-thoracic stomach and oppression of heart and lungs conducted by extremely large stomach, lowering the occurrence rate of cardiopulmonary complications. Additionally, gastric conduit is reconstructed in esophageal bed, heartbeat can also, to some extent, drive peristole and accelerate gastric emptying. And this research can prove these opinions: the formation of gastric conduit reduces the amount of parietal cell on the lesser curvature, which reduces the secretion of gastric acid, and meanwhile, gastric conduit is similar with the original esophagus, which is easy for gastric emptying. The synergistic effect of all the factors makes the nasogastric decompression of those who receive gastric conduit reconstruction obviously less than that of those who not.
The nasogastric decompression of patients with long-term smoking history is richer than the patients with no smoking history. According to the past researches about the etiology of peptic ulcer, long-term smoking can increase secretion of gastric acid, weaken the function of pyloric sphincter, increase regurgitation of duodenal juice or bile and delay gastric emptying (18). All those factors can lead to the richer nasogastric decompression of the long-term heavily smoking patients.
According to this research, age influences the nasogastric decompression independently. Elder patients have lower nasogastric decompression than young patients do. It is perhaps because, as growing older, the stomach of patients will atrophy gradually. The decreasing of parietal cells reduces the secretion of basic gastric acid. And results relatively lower level of nasogastric decompression after surgery.
The major histological type of esophageal cancer is squamous carcinoma, while that most of adenocarcinoma is gastroesophageal junction carcinoma. According to the experience of clinical surgery, Gastric cardiac adenocarcinoma is closer to the stomach than esophageal squamous carcinoma. Therefore, surgery for them needs to cut more, leading to lower secretion of gastric acid. Additionally, there are some parietal cells on the gastric fundus, so cancer will probably destroy part of parietal cells, lowering the secretion of gastric acid. So the nasogastric decompression volume of adenocarcinoma is lower than esophageal squamous carcinoma’s. Therefore, combined the single factors mentioned above, the location of the tumor is also one of the effect factors. However, by analyzing multiple factors, it is removed from the regression equation. We think that dividing lower segment esophageal squamous carcinoma and gastroesophageal junction carcinoma into one group could have influence on that result of research.
Through comparison between the nasogastric decompression of patients who have complication after surgery and the predicted equation, it can be found that, during the first five days, patients whose gastrointestinal functions do not recover smoothly experience higher nasogastric decompression than normal patients will. This difference is statistically reasonable (P=0.004). For this dysfunction, gastric emptying may be delayed, which leads to the failure of automated discharge of gastric juice that need to be discharged by nasogastric decompression.
By comparing the nasogastric decompression of patients who had postoperative lungs infection with normal equation, it can be found that they have lower nasogastric decompression. This difference is statistically reasonable (P=0.001). It can be due to inadequate drainage, which leads to the inefficient discharge of gastric and digestive juice and severe gastric retention. The gastric retention will lead to expansion of thoracic stomach. Then, such expansion will press lungs and lead to pulmonary infection. On the other hand, the situation of patients with anastomotic leakage after the surgery does not show such difference with normal equation.
By comparing the drainage of patients with complications with the regression equation conducted by calculating the data of those without the complications, we can efficiently remove other influence factors towards the result. But as we all know, there are too many factors affect postoperative complications, and management after surgery is just one of all the factors. Due to the limited number of complications collected in this study, it cannot comprehensively show the influence of nasogastric decompression towards the complications. However, by this study, we can still find that nasogastric decompression after surgery is valued to predict the postoperative complications. In terms of the future studies, we will collect more data about complications as prospective study to better prove the relation between nasogastric decompression and complications.
With the improvement of surgical techniques and nutrition support, Fast-track surgery has already been the hotspot of surgery (19-22). Removing the NGT at early period of surgery or no NGT after surgery has already been attempted. We consider that the base of such attempts is that the nasogastric decompression is moderate and can be discharged by gastric emptying itself. This study can provide reference information to fast-track surgery. However, the security issues of removing the NGT at early period of surgery or no NGT after surgery still need to be approved by further large-scale random prospective studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [PubMed]
- Kinugasa S, Tachibana M, Yoshimura H, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J Surg Oncol 2004;88:71-7. [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [PubMed]
- Kofoed SC, Calatayud D, Jensen LS, et al. Intrathoracic anastomotic leakage after gastroesophageal cancer resection is associated with increased risk of recurrence. J Thorac Cardiovasc Surg 2015;150:42-8. [PubMed]
- Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. [PubMed]
- Chen H, Sonnenday CJ. Manual of Common Bedside Surgical Procedures, 2nd Edition. Baltimore: Lippincott Williams & Wilkins, 2000.
- Daryaei P, Vaghef Davari F, Mir M, et al. Omission of nasogastric tube application in postoperative care of esophagectomy. World J Surg 2009;33:773-7. [PubMed]
- Nguyen NT, Slone J, Wooldridge J, et al. Minimally invasive esophagectomy without the use of postoperative nasogastric tube decompression. Am Surg 2009;75:929-31. [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Ioanas M, Cavalcanti M, Ferrer M, et al. Hospital-acquired pneumonia: coverage and treatment adequacy of current guidelines. Eur Respir J 2003;22:876-82. [PubMed]
- Pan H, Yu Z, Zhang R, et al. Study on safety and feasibility of minimally invasive esophagectomy without the use of postoperative nasogastric tube decompression. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:920-3. [PubMed]
- Sun H, Li Y, Liu X, et al. Feasibility of "no tube no fasting" therapy in thoracolaparoscopic oesophagectomy for patients with oesophageal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:898-901. [PubMed]
- Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol 2013;29:636-41. [PubMed]
- Reece GP, Schusterman MA, Miller MJ, et al. Morbidity and functional outcome of free jejunal transfer reconstruction for circumferential defects of the pharynx and cervical esophagus. Plast Reconstr Surg 1995;96:1307-16. [PubMed]
- Kim SH, Lee KS, Shim YM, et al. Esophageal resection: indications, techniques, and radiologic assessment. Radiographics 2001;21:1119-37; discussion 1138-40. [PubMed]
- Shu YS, Sun C, Shi WP, et al. Tubular stomach or whole stomach for esophagectomy through cervico-thoraco-abdominal approach: a comparative clinical study on anastomotic leakage. Ir J Med Sci 2013;182:477-80. [PubMed]
- Garrow D, Delegge MH. Risk factors for gastrointestinal ulcer disease in the US population. Dig Dis Sci 2010;55:66-72. [PubMed]
- Shewale JB, Correa AM, Baker CM, et al. Impact of a Fast-track Esophagectomy Protocol on Esophageal Cancer Patient Outcomes and Hospital Charges. Ann Surg 2015;261:1114-23. [PubMed]
- Li Y. Fast track surgery in esophagectomy for esophageal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:865-8. [PubMed]
- Khasanov AF, Sigal EI, Trifonov VR, et al. The program of accelerated rehabilitation after esophagoplasty (fast track surgery) in esophageal cancer surgery. Khirurgiia (Mosk) 2015.37-43. [PubMed]
- Wang JY, Hong X, Chen GH, et al. Clinical application of the fast track surgery model based on preoperative nutritional risk screening in patients with esophageal cancer. Asia Pac J Clin Nutr 2015;24:206-11. [PubMed]


