Carbohydrate intake and nonalcoholic fatty liver disease: fructose as a weapon of mass destruction
Introduction
The earliest stage of nonalcoholic fatty liver disease (NAFLD) is hepatic steatosis, which is defined by hepatic triglyceride concentration exceeding 55 mg/g liver (5.5%) (1). NAFLD can progress to nonalcoholic steatohepatitis (NASH), characterized by the signs of hepatocyte injury and hepatic inflammation with collagen deposition. Approximately 10-29% of patients with NASH will develop cirrhosis within 10 years (2). NAFLD is an independent and a stronger predictor of cardiovascular disease than peripheral or visceral fat mass (3,4). NAFLD prevalence is 15% in non-obese patients, but increases in obese [body mass index (BMI) =30.0-39.9 kg/m2] and extremely obese (BMI ≥40.0 kg/m2) patients to 65% and 85%, respectively (5).
In addition to genetic susceptibility, environmental factors play important roles in the development of NAFLD & NASH (6-8). The rapid rise in NAFLD prevalence supports the role of environmental factors. It was reported that overconsumption of high fructose corn syrup (HFCS) in the soft-drink and pre-packaged foods were linked to the rise in the prevalence of obesity and associated with NAFLD. Ingested carbohydrates are a major stimulus for hepatic de novo lipogenesis (DNL), and more likely to directly contribute to NAFLD than dietary fat intake.
Pathophysiology of NAFLD
Obesity is associated with low-grade chronic inflammation (9). This chronic inflammation is a link between obesity and insulin resistance. Insülin resistance plays a central role in NAFLD pathogenesis.
Normally, insulin binds α-subunits of its receptor on adipocytes and hepatocytes leading to autophosphorylation of β-subunits and activates tyrosine kinase (10). The autophosphorylated receptor activates insulin receptor substrate (IRS)-1, IRS-2, Src homology collagen (Shc), and APS [adaptor protein with a pleckstrin homology (PH) and Src homology 2 (SH2) domain] which activate downstream components of the insulin signaling pathways. In both skeletal muscle and adipose tissue, these insulin-mediated signaling cascades induce the translocation of glucose transporters (GLUT). IRS-1 was linked to glucose homeostasis while IRS-2 was linked to the lipogenesis with the regulation of lipogenic enzymes sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase.
In obese, increased production of TNF-α and plasma free fatty acids are major stimuli of Ser 307 phosphorylation of IRS-1 (11). Inhibition of IRS-1 due to the phosphorylation of its Ser 307 residues also requires the activation of both c-Jun N-terminal kinase (JNK) and inhibitor κB kinase β (IKK-β). Both TNF-α and free fatty acids induce JNK and IKK-β activation. TNF-α stimulates phosphorylation of Ser residues of both IRS-1 and IRS-2 in hepatocytes and Ser residues of IRS-1 in muscles. JNK is one of the stress related kinases and plays an important role in the development of insulin resistance. Activated JNK induces Ser 307 phosphorylation of IRS-1, disturbs insulin downstream signaling, and subsequently causes insulin resistance. Protein kinase C theta (PKCθ) and IKK-β are two pro-inflammatory kinases involved in insulin downstream signaling that are activated by lipid metabolites. IKK-β phosphorylates the inhibitor of nuclear factor kappa B (NF-κB). NF-κB has both apoptotic and anti-apoptotic effects.
The hepatocyte mitochondria are the main site of β-oxidation of free fatty acids (12-15). The electrons removed from free fatty acids during β-oxidation, eventually leading to ATP synthesis. Depletion of the energy (ATP) stores increases the susceptibility of hepatocytes to various injuries.
Carbohydrates: glucose, fructose and HFCS
Fructose is a monosaccharide (16,17). It is a sweet tasting sugar and found naturally in fruits and some vegetables. Fructose is sweeter than either glucose or sucrose. Before the development of the worldwide sugar industry, fructose was limited in the human diet. Honey, dates, raisins, molasses, and figs have a content of >10% of this sugar. A fructose content of 5-10% by weight is found in grapes, raw apples, apple juice, persimmons, and blueberries.
Today, the principal sources of fructose in the American diet are HFCS (18-20). Industrially, HFCS are frequently found in soft drinks and pre-packaged foods. The most common form of HFCS is HFCS 55, which has 55% fructose compared to sucrose which is 50% fructose. Foods and drinks are made with HFCS 55. A study showed that certain popular sodas and other beverages contain a fructose content approaching 65% of sugars. Moreover, HFCS can be made to have any proportion of fructose, as high as 90%. It was recently reported that more than 50% of preschool children consume some calorie-sweetened beverages.
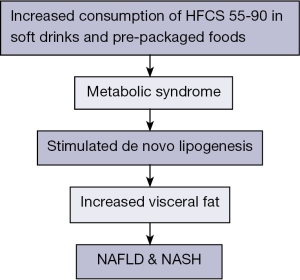
Several meta-analyses suggested that the consumption of sugar-sweetened beverages is related to the risk of metabolic syndrome; increased triglycerides (TG) levels, stimulated DNL and increased visceral fat (20,21) (Figure 1). Another study compared milk, diet cola, a sugar-sweetened cola, and water. The study showed that the sugar-sweetened beverage increased liver and visceral fat over the 6 months of beverage intake by consuming two 16-ounce sugar-containing beverages per day for 6 months (22).
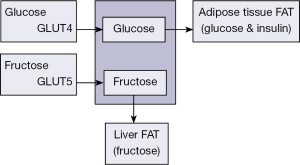
Fructose is an intermediary in the metabolism of glucose (17-20). But, it differs in several ways from glucose. Fructose is poorly absorbed from the gastrointestinal tract by a different mechanism than that for glucose (Figure 2). Most cells have only low amounts of the glucose transporter type-5 (GLUT-5) transporter, which transports fructose into cells. Glucose is transported into cells by GLUT-4, an insulin-dependent transport system. Fructose is almost entirely cleared by the liver. Hepatic metabolism of fructose stimulates lipogenesis. These events are independent of insulin exertion and phosphofructokinase regulation step. High fructose intake is associated with increased plasma TGs by an up-regulation of hepatic DNL and TGs secretion, and a decreased clearance of very low density lipoprotein triglyceride (VLDL-TG). Fructose phosphorylation in the liver consumes ATP, consequently the accumulated ADP serves as substrate for uric acid formation. These events facilitate hepatic oxidative damage and lipid peroxidation.
Aeberli et al. conducted a 4-week randomized cross-over study with a 4-week wash-out between each diet in nine healthy young men comparing four different soft drinks with levels of fructose, glucose, and sucrose that are closer to normal intake (23). This is a randomized crossover comparison of four beverages with two levels of fructose, glucose, and sucrose (50% fructose). The investigators examined insulin sensitivity of the liver and the whole body by the hyperinsulinemic-euglycemic clamp technique. They showed that compared with the high-glucose beverage, the low-fructose beverage impaired hepatic insulin sensitivity, but not whole-body insulin sensitivity. In addition, they found that total and low density lipoprotein (LDL) cholesterol levels were increased by fructose relative to glucose. Free fatty acids were also increased in the fructose beverage groups. This study adds to the information about the role of fructose either from sucrose (ordinary table sugar) or from high-fructose corn syrup in initiating liver dysfunction and the metabolic syndrome.
Cohen and Schall also reported that sucrose increased TG following a meal but glucose had no effects on lipids (24).
Fructose as a main source of hepatic DNL in NAFLD
Increased DNL (increased hepatocellular carbohydrate is converted to fat) is a significant contributor to increased hepatic triglyceride content in NAFLD (25,26). Recent techniques such as isotope methodologies, multiple-stable-isotope approach and gas chromatography/mass spectrometry showed that relative contribution of three fatty acid sources to the accumulated fat in NAFLD as adipose tissue, DNL and dietary carbohydrates. Twenty-six percentage of the liver fat arises from DNL and 15% from the diet in patients with NAFLD.
Fructose can induce NAFLD by its ability to act as an upregulated substrate for DNL and by bypassing the major rate-limiting step of glycolysis at phosphofructokinase. Continuous fructose ingestion may cause a metabolic burden on the liver through the induction of fructokinase and fatty acid synthase (27).
Evidence support fructose as a weapon of mass destruction in NAFLD
Animal studies
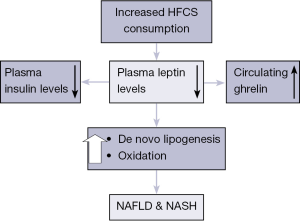
Fructose ingestion can rapidly cause fatty liver in animals with the development of leptin resistance (28-30). It was reported that consumption of high-fructose meals reduced 24-hour plasma insulin and leptin concentrations, increased postprandial fasting and not suppress circulating ghrelin (Figure 3). Our group previously demonstrated that male C57BL/6 mice fed relevant amounts of a high-fructose corn syrup equivalent (drinking water containing 55% fructose) for 16 weeks developed severe hepatic steatosis associated with necroinflammatory changes (31).
Ackerman et al. showed that rats given fructose-enriched diet increased hepatic TG and cholesterol amounts (32).Fructose fed rodent at supraphysiological doses under isocaloric (~60% energy) or hypercaloric (+30% excess energy) conditions induces steatosis and steatohepatitis by DNL; fructose accounts for 60-70% of fatty acids in this study (33).
Nagai et al. demonstrated that the transcriptional factor peroxisome proliferator-activated receptor gamma coactivator-1 beta (PGC-1 β) plays a crucial role in the pathogenesis of fructose-induced insulin resistance in Sprague-Dawley rats (34). Armutcu et al. reported that male Wistar albino rats provided with drinking water containing 10% fructose for 10 d developed macrovesicular and microvesicular steatosis without inflammation in the liver (35).
Lipocalin-2 (LCN-2) is a 25-kDa secretory glycoprotein initially identified in human neutrophils and it is abundantly present in the circulation (36). It was demonstrated that the liver is the main source of serum LCN-2 which plays a key role in the acute-phase response, regulation of immune responses, and apoptosis. A recently published study investigated LCN-2 expression and its role in rat models fed by high fructose (1). In this study, fatty liver was triggered in male Sprague-Dawley rats fed either with liquid Lieber-DeCarli (LDC) or LDC +70% cal fructose (L-HFr) diet for 4 or 8 weeks. Both LDC-fed and L-HFr-fed rat showed fatty liver, histologically. In the liver, the transcription of inducible nitric oxide synthase (iNOS), and TNF-α was significantly up-regulated at week 4. Hepatic LCN-2 expression was 90-fold at week 4 and 507-fold at week 8 higher in L-HFr-subjected ratsvs.control (P<0.001). Additionally, fasting leptin and TG were elevated in the L-HFr regimen. Moreover, protein expression of hepatic LCN-2, CD14, phospho-MAPK, caspase-9, cytochrome-c and 4-hydroxynonenal were increased in the L-HFr group. The localization of LCN-2 was predominantly restricted to MPO+ granulocytes in the liver. This study showed that fructose diet up-regulates hepatic LCN-2 expression, which correlates with the increased indicators of oxidative stress and mitochondrial dysfunction. The level of fasting blood uric acid was significantly elevated in L-HFr-treated rats. Hepatic GLUT-5 (fructose transporter) gene expression was also significantly elevated in L-HFr fed rats, which was correlated with the accumulated fat in the liver.
A very low-carbohydrate diet causes weight loss and increased hepatic and myocardial fatty acid oxidation in wild-type mice, compared with mice maintained on standard chow diets rich in polysaccharides (37). A recent study revealed that C57BL/6J mice over 12-week fed with very low-carbohydrate, low-protein, and high-fat ketogenic diet led to hepatic fat accumulation, systemic glucose intolerance, hepatic endoplasmic reticulum stress, steatosis, cellular injury, and macrophage accumulation (38). However, animals remain lean and insulin-induced hepatic Akt phosphorylation and whole-body insulin responsiveness was not impaired. The ketogenic diets provoked weight loss in rodents. However, long-term maintenance on a ketogenic diet stimulated the development of NAFLD and systemic glucose intolerance in mice (37,38).
Human studies
Small cross-sectional and retrospective case–control studies showed an association between fructose-containing sugar intake and NAFLD (39-41). A meta-analysis showed a triglyceride-raising effect of fructose (39). A recently published human study investigated whether there is a relation between spontaneous carbohydrate intake and NAFLD (41). They found that hepatic steatosis was related to the energy and carbohydrate intakes. The role of dietary carbohydrates was detectable in the range of usual carbohydrate intake: 32% to 58% calories.
A systematic review and meta-analysis of controlled feeding trials investigated effect of fructose on markers of NAFLD (42). They found seven isocaloric trials, in which fructose was exchanged isocalorically for other carbohydrates, and six hypercaloric trials, in which the diet was supplemented with excess energy (+21-35% energy) from high-dose fructose (+104-220 g/day). Although there was no effect of fructose in isocaloric trials, fructose in hypercaloric trials increased both hepatic lipid [standardized mean differences (SMD) =0.45; 95% confidence interval (CI): 0.18-0.72] and alanine aminotransferase (ALT) [mean difference (MD) =4.94 U/L; 95% CI: 0.03-9.85]. They concluded that isocaloric exchange of fructose for other carbohydrates does not induce NAFLD. Fructose providing excess energy and raises hepatic lipid amount and serum ALT. Moreover, this study concluded that finding of a lack of effect of fructose on NAFLD markers in isocaloric trials. Energy represented an important confounding factor in the effect of fructose in this meta-analysis. Main limitation of this meta-analysis was that few trials were available for inclusion and most of them were small and short (≤4 weeks).
Ryan et al. reported a post hoc analysis of 52 obese, insulin resistant adults in a weight loss program (43). These patients were randomized to receive either a low carbohydrate diet (40% carbonhydrate/40% fat) or a low fat diet (60% carbohydrate/25% fat) for 16 weeks. Both groups lost a significant amount of weight over the trial period. Serum ALT levels decreased twice in the low carbohydrate diet compared to the low fat diet. Insulin resistance levels were also shown to decrease in both groups with no significant differences between them. The authors concluded that low carbohydrate diets are more beneficial than low fat diets at reducing ALT levels. de Luis et al. reported that a 3-month intervention of hypocaloric diet (either low fat or low carbohydrate) in obese patients improved biochemical parameters, BMI and circumference (44).
Rodríguez-Hernández et al. demonstrated effect of low fat and low carbohydrate diet on liver transaminases (45). This trial included 54 women, with ultrasonographically diagnosed NAFLD, and randomly assigned them to either a low fat (25% protein, 10% fat, 54% carbohydrate) or low carbohydrate (27% protein, 28% fat, 45% carbohydrate) diet for a period of 6 months. At the end of the trial, those on the low carbohydrate diet lost 5.7% of their body weight and those in the low fat group 5.5%, a non-significant result. ALT and AST levels were decreased in both groups without significant difference.
In another study by Haufe et al. demonstrated in a total of 102 patients including both male and female, over a 6-month period diet therapy with low carbohydrate (90 g of carbohydrate and 0.8 g protein per kg weight, 30% fat) and low fat (20% fat, 0.8 g of protein per kg, the remainder carbohydrate) (46). This study results were also similar to Rodríguez-Hernández et al. study results (45). In addition, intrahepatic fat content also not showed statistical difference, 47% decreased in the low carbohydrate group and 42% decreased hepatic fat content in the low fat group.
Sevastianova et al. demonstrated 16 subjects (BMI =30.6±1.2) for 3 weeks induced on high carbohydrate diet (>1,000 Kcal) showed >10-fold greater relative change in liver fat (27%) than in body weight (2%) and increased liver fat positively correlated with DNL (47). Furthermore, consequent hypocaloric diet for 6 months led to decrease in body weight as well as reduced liver fat to normal. This study suggests that human fatty liver accumulates fat during carbohydrate overfeeding and support a role for DNL in the pathogenesis of NAFLD.
Low-carbohydrate diets have been shown to promote weight loss, decrease intrahepatic triglyceride content, and improve metabolic parameters of patients with obesity (48). A meta-analysis investigated the long-term (6 or more months) effects of low-carbohydrate diets (≤45% of energy from carbohydrates) versus low-fat diets (≤30% of energy from fat) on metabolic risk factors by randomized controlled trials (48). Totally 2,788 participants met the predetermined eligibility criteria (from January 1, 1966 to June 20, 2011) and were included in the analyses. Both low-carbohydrate and low-fat diets lowered weight and improved metabolic risk factors. Compared with participants on low-fat diets, persons on low-carbohydrate diets experienced a slightly but statistically significantly lower reduction in total cholesterol, and LDL cholesterol, but a greater increase in high density lipoprotein cholesterol and a greater decrease in TG.
Abdelmalek et al. studied 341 adult NAFLD patients (49). They evaluated whether increased fructose consumption correlates merely with the development of NAFLD or promote the transition from NAFLD to NASH and more advanced stages of liver damage. Fructose consumption was estimated based on reporting (frequency × amount) of kool, fruit juices, and non-dietary soda intake, expressed as servings per week. The authors found that increased fructose consumption was univariately associated with decreased age (P<0.0001), male gender (P<0.0001), hypertriglyceridemia (P<0.04), low HDL cholesterol (P<0.0001), decreased serum glucose (P<0.001), increased calorie intake (P<0.0001) and hyperuricemia (P<0.0001). After controlling for age, gender, BMI, and total calorie intake, daily fructose consumption was associated with lower steatosis grade and higher fibrosis stage (P<0.05 for each). In older adults (age >48 years), daily fructose consumption was associated with increased hepatic inflammation (P<0.05) and hepatocyte ballooning (P=0.05). Abdelmalek et al. concluded that daily fructose ingestion is associated with reduced hepatic steatosis but increased fibrosis.
Aeberli et al. investigated the relation between fructose ingestion and LDL particle size in children (50). They showed that greater total and central adiposity are associated with smaller LDL particle size and lower HDL cholesterol in school-age children. Overweight children consume more fructose from sweets and sweetened drinks than do normal-weight children, and higher fructose intake predicts smaller LDL particle size.
Conclusions
HFCS-containing beverages are associated with the development of NAFLD by hepatic DNL. Epidemiological studies linked HFCS consumption to the severity of fibrosis in patients with NAFLD, too. Recently, animal studies showed that excessive consumption of HFCS-55 increases hepatic Glut5 gene expression and TNF-alpha levels, gut-derived endotoxemia, endoplasmic reticulum stress, hepatic lipid peroxidation and apoptotic activity. The lipogenic and proinflammatory effects of fructose appear to be due to transient ATP depletion. Fructose can also raise intracellular and serum uric acid levels. Large prospective studies that evaluated the relationship between fructose and NAFLD are needed.
Key points
- The rapid rise in NAFLD prevalence supports the role of environmental factors.
- Overconsumption of HFCS in the soft-drink is linked to the rise in the prevalence of obesity and associated with NAFLD.
- Ingested carbohydrates are a major stimulus for hepatic DNL, and more likely to directly contribute to NAFLD than dietary fat intake.
- Fructose phosphorylation in the liver consumes ATP, consequently the accumulated ADP serves as substrate for uric acid formation.
- The lipogenic and proinflammatory effects of fructose appear to be due to transient ATP depletion.
Acknowledgements
This article is dedicated to the Tuscany and Aegean people, the region of grape. All authors contributed equally during the preparation of this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462-8. [PubMed]
- Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis 2010;28:162-8. [PubMed]
- Vanwagner LB, Bhave M, Te HS, et al. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012;56:1741-50. [PubMed]
- Sung KC, Ryan MC, Wilson AM. The severity of nonalcoholic fatty liver disease is associated with increased cardiovascular risk in a large cohort of non-obese Asian subjects. Atherosclerosis 2009;203:581-6. [PubMed]
- Sinn DH, Gwak GY, Park HN, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol 2012;107:561-7. [PubMed]
- Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013;10:307-18. [PubMed]
- Mittal S, Sada YH, El-Serag HB, et al. Temporal Trends of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma in the Veteran Affairs Population. Clin Gastroenterol Hepatol 2015;13:594-601. [PubMed]
- Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384-91. [PubMed]
- Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab 2014;3:664-75. [PubMed]
- Basaranoglu M, Basaranoglu G. Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol 2011;17:4055-62. [PubMed]
- Maximos M, Bril F, Portillo Sanchez P, et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology 2015;61:153-60. [PubMed]
- Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol 2013;19:1158-65. [PubMed]
- Petrie JL, Patman GL, Sinha I, et al. The rate of production of uric acid by hepatocytes is a sensitive index of compromised cell ATP homeostasis. Am J Physiol Endocrinol Metab 2013;305:E1255-65. [PubMed]
- Koliaki C, Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol 2013;379:35-42. [PubMed]
- Basaranoglu M, Kayacetin S, Yilmaz N, et al. Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2010;16:2223-6. [PubMed]
- Sapp V, Gaffney L. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology 2014;60:1581-92. [PubMed]
- Moeller SM, Fryhofer SA, Osbahr AJ 3rd, et al. The effects of high fructose syrup. J Am Coll Nutr 2009;28:619-26. [PubMed]
- Basaranoglu M, Basaranoglu G, Sabuncu T, et al. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol 2013;19:1166-72. [PubMed]
- Bursać BN, Vasiljević AD, Nestorović NM, et al. High-fructose diet leads to visceral adiposity and hypothalamic leptin resistance in male rats--do glucocorticoids play a role? J Nutr Biochem 2014;25:446-55. [PubMed]
- Tetri LH, Basaranoglu M, Brunt EM, et al. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol 2008;295:G987-95. [PubMed]
- Ferder L, Ferder MD, Inserra F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr Hypertens Rep 2010;12:105-12. [PubMed]
- Maersk M, Belza A, Holst JJ, et al. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. Eur J Clin Nutr 2012;66:523-9. [PubMed]
- Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150-6. [PubMed]
- Cohen JC, Schall R. Reassessing the effects of simple carbohydrates on the serum triglyceride responses to fat meals. Am J Clin Nutr 1988;48:1031-4. [PubMed]
- Ameer F, Scandiuzzi L, Hasnain S, et al. De novo lipogenesis in health and disease. Metabolism 2014;63:895-902. [PubMed]
- Chung M, Ma J, Patel K, et al. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:833-49. [PubMed]
- Chong MF, Fielding BA, Frayn KN. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc 2007;66:52-9. [PubMed]
- Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology 2000;119:1340-7. [PubMed]
- Nair S, Cope K, Risby TH, et al. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol 2001;96:1200-4. [PubMed]
- Spruss A, Kanuri G, Wagnerberger S, et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009;50:1094-104. [PubMed]
- Neuschwander-Tetri BA, Ford DA, Acharya S, et al. Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools. Lipids 2012;47:941-50. [PubMed]
- Ackerman Z, Oron-Herman M, Grozovski M, et al. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 2005;45:1012-8. [PubMed]
- Nunes PM, Wright AJ, Veltien A, et al. Dietary lipids do not contribute to the higher hepatic triglyceride levels of fructose- compared to glucose-fed mice. FASEB J 2014;28:1988-97. [PubMed]
- Nagai Y, Yonemitsu S, Erion DM, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 2009;9:252-64. [PubMed]
- Armutcu F, Coskun O, Gürel A, et al. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin Biochem 2005;38:540-7. [PubMed]
- Alwahsh SM, Xu M, Seyhan HA, et al. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J Gastroenterol 2014;20:1807-21. [PubMed]
- Handa K, Inukai K, Onuma H, et al. Long-term low carbohydrate diet leads to deleterious metabolic manifestations in diabetic mice. PLoS One 2014;9:e104948. [PubMed]
- Garbow JR, Doherty JM, Schugar RC, et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol 2011;300:G956-67. [PubMed]
- David Wang D, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014;232:125-33. [PubMed]
- Madero M, Arriaga JC, Jalal D, et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism 2011;60:1551-9. [PubMed]
- Gonzalez C, de Ledinghen V, Vergniol J, et al. Hepatic Steatosis, Carbohydrate Intake, and Food Quotient in Patients with NAFLD. Int J Endocrinol 2013;2013:428542.
- Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2014;68:416-23. [PubMed]
- Ryan MC, Abbasi F, Lamendola C, et al. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care 2007;30:1075-80. [PubMed]
- de Luis DA, Aller R, Izaola O, et al. Effect of rs6923761 gene variant of glucagon-like peptide 1 receptor on metabolic response and weight loss after a 3-month intervention with a hypocaloric diet. J Endocrinol Invest 2014;37:935-9. [PubMed]
- Rodríguez-Hernández H, Cervantes-Huerta M, Rodríguez-Moran M, et al. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol 2011;10:486-92. [PubMed]
- Haufe S, Engeli S, Kast P, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011;53:1504-14. [PubMed]
- Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012;96:727-34. [PubMed]
- Hu T, Mills KT, Yao L, et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 2012;176:S44-54. [PubMed]
- Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1961-71. [PubMed]
- Aeberli I, Zimmermann MB, Molinari L, et al. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr 2007;86:1174-8. [PubMed]


