Abstract
We present a detailed theoretical exploration of the refractory compositions and volatile enrichments of planets forming in protoplanetary disks with solar-like conditions. The two cases of the Sun and WASP-12 are studied due to the availability of spectral measurements and their known planets. The distribution throughout their disks of solid compounds with a wide range of volatilities is computed by a comprehensive chemical thermodynamics code. After the calculation of refractory compounds down to the water snowline, the compositional distributions are documented for planets generated in certain locations of protoplanetary disks depending on thermodynamic conditions. These results are referred to proposed bulk compositions for solar terrestrial planets, and for the core of the hot Jupiter WASP-12b. The material left over after the formation of rocky components is collected and treated in calculations to determine the abundances of fundamental volatile molecules in the outer regions of the disks. The distributions of planetesimal volatile composition are then altered for four different cases of the carbon-to-oxygen ratios, and for oxidizing and reducing conditions, in order to adjust the best fit for the accretion zone of Jupiter and WASP-12b. We compare the Jovian results to in situ atmospheric measurements from Jupiter's atmosphere. Overall, this study proposes a holistic approach to estimate possible planetary interior and envelope compositions from hot toward cold disk zones, along with the mass of planetesimals accreted into the envelopes of gas giants.
Export citation and abstract BibTeX RIS
1. Introduction
The two most important parameters governing the overall composition of a planet are the composition of its parent star and the chemical and physical conditions of the protoplanetary disk where it formed and accreted (Guillot 2008; Eistrup et al. 2016; Mordasini et al. 2016, 2012; Cridland et al. 2017; Santos et al. 2017; Li et al. 2020b). Chemical diversity of distant condensates is, therefore, key to adapting solar system concepts such as the Kuiper Belt, snowline positions, icy satellites, and heavy-element enrichment in giant planet atmospheres to extrasolar disks, especially when considering varying abundance ratios of carbon to oxygen (Madhusudhan et al. 2011b; Madhusudhan 2012; Eistrup et al. 2016; Bitsch & Battistini 2020).
The C/O ratio is an important factor in the volatile ice composition of planetesimals, mainly determining the availability of oxygen in water formation and condensation, independent of the location of planet formation (Johnson et al. 2012; Moses et al. 2013; Cridland et al. 2017), as well as controlling the carbide and silicate distribution (Thiabaud et al. 2015). On the other hand, the C/O ratio in the atmosphere of a gas giant influences the equilibrium composition of important trace species such as CO, which can be indicators of vertical transport (Moses et al. 2013).
According to the core accretion scenario, the atmospheric C/O ratio of a giant planet might be different from that of its parent star (Mousis et al. 2011; Moses et al. 2013; Cridland et al. 2019a, 2019b), providing constraints on the composition of the circumstellar disk, location of formation, migration, and planetesimal contribution (Eistrup et al. 2018; Cridland et al. 2017; Thiabaud et al. 2015; Moses et al. 2013; Madhusudhan 2012). The expanded version of the core accretion model with the inclusion of disk evolution and migration explains the basic properties of the gas giants (Mousis et al. 2009a, 2009b, 2009c), although the best estimates for the Jovian water abundance by Li et al. (2020a) may require more radical ideas involving gaseous accretion of certain heavy elements (Bitsch & Battistini 2020; Mousis et al. 2019).
Another important parameter for the nature of chemical processes within a disk is the environmental redox state (Johnson et al. 2012), defined by what molecular forms carbon assumes in the gas phase through the circumstellar disks, resulting in a range of reducing and oxidizing conditions. Condensation of solar system disk material beyond the snowline indicates a strong correlation between these two parameters and the carbon contribution in refractory material (Johnson et al. 2012).
In this study, accordingly, we first focus on the rocky composition of objects as a function of the distance from the central star and the formation conditions. Refractory species that build up the basic constituents of terrestrial planets are examined under certain thermodynamic conditions with the assumption of chemical equilibrium.
Thermochemical equilibrium is an approach that has long been in use to calculate gas and grain chemistry inside regions of circumstellar disks with sufficiently high temperatures, showing a general agreement with solar, meteorite, and planetary observations (Bitsch & Battistini 2020; Mousis et al. 2012). Although our results for refractory compositions are consistent with our current understanding of the formation and composition of terrestrial planets, this approach has its limitation, demonstrably so for close-in gas giants because they are believed not to have formed in their current location but to have migrated inward from distant locations in their stellar systems (Lin & Papaloizou 1986; Ward 1997; Alibert et al. 2005a). Afterwards, we study the atmospheric enrichment in our own Jupiter and in the hot Jupiter detected in the WASP-12 system, connecting the compositions of the giant planets to the disk metallicity. The latter system is chosen since its stellar chemical data are measured almost fully by Fossati et al. (2010), which is rare for a host star. And Madhusudhan et al. (2011a) reported the C/O ratio in the atmosphere of WASP-12b to be at least twice that of its parent star, making it an interesting case. Since its atmospheric composition is yet to be measured directly, this study aims to provide a prediction.
The volatile accretion approach was successfully applied to the solar system (Mousis et al. 2009a, 2009b, 2009c, 2011, 2012), and it is developed further here with a greater availability of chemical species. Results of the study show consistency with the mentioned papers. The connection between the C/O ratio and issues such as the core accretion model, nebular oxidation state, and planetary enrichment are examined and discussed in the following sections.
2. Methodology
Generic planetary compositions are assumed to have two possible components: refractory, i.e., the metallic and rocky phase, and atmospheric, i.e., the volatile and gaseous phase. The treatment for the refractory part of this work holds some similarities to the studies of Bitsch & Battistini (2020), Carter-Bond et al. (2012), and Bond et al. (2010b), with an equilibrium assumption for the chemical composition of the disks, where planetesimals are formed and accrete into larger planetary embryos. The assumption of equilibrium condensation is already supported by various analyses of early chondritic meteorites (Kuchner & Seager 2005; Bond et al. 2010a, 2010b; Li et al. 2020b).
To examine the changes in types and abundances of dominating refractory species, we employ a comprehensive chemical thermodynamics code coupled with a simplified kinetics scheme to calculate gas and ice abundances depending on temperature, pressure, and elemental abundances, in a similar way to the study of Lodders & Fegley (2002).
In order to model the atmospheres of Jupiter and WASP-12b, a method is used where the remnant gas content in the disk after the formation of refractory species is taken as the initial parameter. The leftover abundances of five basic atmospheric elements, namely oxygen, carbon, sulfur, phosphorus, and nitrogen, are taken as the foundation of major volatiles in the atmospheres, which are enriched by the planetesimal-derived materials they accreted. The atmospheric compositions are modeled for four different cases: where the disk conditions are either oxidizing or reducing, and where the C/O ratio is either stellar or unity. The fractionation and estimated masses of important ices, and ice-to-rock percentages calculated under these four combinations, are documented for these two gas giants. The dependence of ice and rock enrichment on the C/O ratio and redox states is evaluated for the final expected composition of the planets.
2.1. Modeling the Disks
This work is an exploration of possible planetary compositions with specific assumptions. A quiescent disk profile is drawn in order to focus on the direct relations between chemical composition and basic disk properties, as practiced also by Bitsch & Battistini (2020), Price et al. (2020), Eistrup et al. (2018), Moriarty et al. (2014), Bond et al. (2010b), and Dodson-Robinson et al. (2009). A static model shall work fine to approximate the abundances of disk species including water and carbon gases, which are not significantly affected by disk dynamics (Price et al. 2020).
Circumstellar disks are considered to be geometrically thin, symmetric around their midplanes, and to remain in hydrostatic equilibrium. And the physical conditions are regarded as stable in their local values, as practiced also by Price et al. (2020).
Below are the disk factors that are disregarded in our study:
- 1.Stellar irradiation is not taken as an energy source, considering its negligible effect on the temperature of the midplane.
- 2.Vertical, radial, and relative motions of the gas and dust are not considered.
- 3.Modifications in midplane conditions, such as variation in mass accretion, are not considered to affect the condensation of materials throughout disks.
- 4.Viscosity, opacity, photoionization, stellar pollution, gravitational settling, turbulence, and gas giant migration are also not considered.
Nevertheless, it is possible to find various disk models in the literature that studied some of the factors dismissed in our work, as indicated in the following:
Disk evolution by Cridland et al. (2019b, 2020), Li et al. (2020b), Price et al. (2020), Eistrup et al. (2016, 2018), Alessi et al. (2017), Ali-Dib (2017), Helling et al. (2014), Moriarty et al. (2014), Mordasini et al. (2012). Viscosity by Price et al. (2020), Booth & Clarke (2018), Cridland et al. (2016), Mordasini et al. (2016), Piso et al. (2016), Wehrstedt & Gail (2003). Type I and/or type II migration by Cridland et al. (2017, 2019a), Ali-Dib (2017), Mordasini et al. (2016). Radial drift by Booth & Ilee (2019), Booth & Clarke (2018), Piso et al. (2016). Stellar evolution by Artur de la Villarmois et al. (2019), Santos et al. (2017). Ionization by Cridland et al. (2016), Eistrup et al. (2016, 2018). Radiative transfer by Cridland et al. (2017). Photoevaporation by Ali-Dib (2017). Accretion heating by Piso et al. (2016). Gravitational instability by Ilee et al. (2011). And non-equilibrium chemistry by Cridland et al. (2016).
Some of those studies are not concerned with chemical kinetics for the simplicity of physical dynamics, such as the models by Ali-Dib (2017), Mordasini et al. (2016), Ali-Dib et al. (2014), and Marboeuf et al. (2014).
2.2. Data
The Sun and WASP-12 are chosen as case studies due to the availability of chemical composition data. As summarized in Table 1, they are both G-type dwarfs and have similar physical properties.
Table 1. Basic Stellar Parameters for the Sun and WASP-12
| Parameter | Sun | WASP-12 a |
|---|---|---|
| Spectral type | G2V | G0V |
| Mass (M⊙) | 1 | 1.35 |
| Radius (R⊙) | 1 | 1.57 |
| Effective temperature (K) | 5778 | 6300 |
Note.
a Hebb et al. (2009).Download table as: ASCIITypeset image
Table 2 shows the data for their elemental number densities relative to H2 with the references. The Sun's photospheric chemical composition is fully measured and summarized by Asplund et al. (2009). The data for WASP-12 (Fossati et al. 2010) provide all elements necessary for the study except phosphorus. The elements Ca, Cr, Fe, Mg, Si, Sr, Ti, and V, each documented with their two lines, are assumed to have their average values. H and He are kept fixed to the solar values for WASP-12 as well. For the unknown phosphorus abundance of WASP-12, the value is scaled from the solar using the metallicity of the star (i.e., Fe abundance).
Table 2. Elemental Abundances for Stars Relative to H2
| Sun a | WASP-12 b | |
|---|---|---|
| C/O | 0.55 | 0.45 |
| H | 2 | 2 |
| He | 0.19 | 0.19 |
| O | 1.07 × 10−3 | 1.59 × 10−3 |
| C | 5.90 × 10−4 | 7.10 × 10−4 |
| N | 1.48 × 10−4 | 2.24 × 10−4 |
| Mg | 8.73 × 10−5 | 2.03 × 10−4 |
| Si | 7.10 × 10−5 | 1.61 × 10−4 |
| Fe | 6.93 × 10−5 | 2.18 × 10−4 |
| S | 2.89 × 10−5 | 3.32 × 10−5 |
| Al | 6.18 × 10−6 | 4.69 × 10−6 |
| Ca | 4.80 × 10−6 | 1.44 × 10−5 |
| Na | 3.81 × 10−6 | 4.69 × 10−6 |
| Ni | 3.64 × 10−6 | 4.58 × 10−6 |
| Cr | 9.57 × 10−7 | 3.65 × 10−6 |
| Mn | 5.90 × 10−7 | 7.78 × 10−7 |
| P | 5.64 × 10−7 | 6.13 × 10−7 |
| K | 2.35 × 10−7 | 1.52 × 10−6 |
| Co | 2.14 × 10−7 | 2.09 × 10−7 |
| Ti | 1.95 × 10−7 | 5.72 × 10−7 |
| Zn | 7.96 × 10−8 | 9.57 × 10−8 |
| Cu | 3.40 × 10−8 | 3.10 × 10−8 |
| V | 1.87 × 10−8 | 4.81 × 10−8 |
| Sc | 3.10 × 10−9 | 5.64 × 10−9 |
| Sr | 1.63 × 10−9 | 1.90 × 10−8 |
Notes.
a Asplund et al. (2009). b Fossati et al. (2010).Download table as: ASCIITypeset image
Since it is currently not possible to observe the chemical composition of disk midplanes (Eistrup et al. 2018), protoplanetary disks are assumed to directly reflect the chemistry of their parent stars while modeling the elemental composition of their planets, as also done by Bitsch & Battistini (2020) and Moriarty et al. (2014). Fortunately, it is possible to draw correlations between the refractory elements of the Sun's photosphere, meteorites, and rocky planets (Santos et al. 2017).
Moreover, the data for WASP-12 stand for its protostellar values as well, since detailed investigations about the compositions of exoplanetary disks are not yet available. This assumption has been employed before, at least for the solar case (Johnson et al. 2012; Alibert et al. 2005a; Bond et al. 2010a).
2.3. The Software
The solid phases, which are silicate and metal compounds and formed in the disks at equilibrium composition, are calculated with HSC Chemistry 7.1. The program has a sophisticated set of algorithms automatically utilized within the framework of a thermochemical database containing enthalpy, entropy, and heat capacity data for 25,000+ chemical compounds (Roine 2006).
This program provides reliable computations for the effects of sundry variables on a chemical system at equilibrium (Roine 2006), and documents the equilibrium composition with a minimized Gibbs free energy for the defined system (Bond et al. 2010a). Once the user sets the quantity of raw materials and system conditions, the code will calculate the abundances of products to come out according to the initial conditions and chemical reactions to take place (Roine 2006). Studies concerned with solar nebula chemistry or supernova stellar outflows, whose results were correlated with mineralogy observations of interplanetary dust particles, have used the HSC software (e.g., Bond et al. 2010a; Moriarty et al. 2014).
2.4. Refractory Phase
After being confident with the limitations and performance capabilities of the program, the largest list of species possible is constituted using all the combinations found in the code's database for the universally most abundant and important refractory-forming elements: Al, C, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, N, Na, Ni, O, P, S, Sc, Si, Sr, Ti, V, Zn, along with H and He. The input elements are entered only in the gas phase, and the system is restricted to these elements only. Inside the pool of possibilities, the alternatives listed below are included as output compounds:
- 1.Substances with the same composition, but different molecular or crystal structure (e.g., CuFeS2: both as ferrous sulfide and as chalcopyrite)
- 2.Various stoichiometric distributions for a certain species (e.g., FeSi2, FeSi2.33, FeSi2.43)
- 3.Compounds with different stoichiometric numbers (e.g., 3MgO.2SiO2.2H2O and 3MgO.4SiO2.H2O)
- 4.Organic species carrying up to three carbon atoms
- 5.Variations of compounds with an exact chemical formula (e.g., allotropic forms, organic isomers, excited states, duplets, and triplets).
The list below gives the species that are excluded due either to the numeric limitations of the code or to the conditions not considered for stellar disks, such as an ionized or aqueous environment. Liquids are excluded since they are generally found to occupy very small fields of stability in the disks, and they do not significantly affect condensation sequences (Bond et al. 2010a).
- 1.Both gaseous and aqueous ions
- 2.Radicals
- 3.Liquid compounds and species
- 4.Organic compounds containing three or more carbon atoms
- 5.Alloy combinations.
The complete list of input species, whose total number reached 1225, is given in Table A1 in the Appendix after alterations mentioned above are removed in order to avoid repetitions.
Temperature is taken within the range 100–2000 K, and pressure is kept fixed at 10−4 bar, a traditional value for solar nebula studies, because it is the characteristic total pressure at or around 1 au from the Sun (Cassen 1996; Fegley 2000; Lodders 2003; Bond et al. 2010b).
We use a solar temperature–pressure profile for WASP-12 since it is the same stellar type as the Sun. In this way, the same T–P conditions for both stars allow ready comparison of changes in chemical abundances between the two. Furthermore, as noted by Lodders & Fegley (2002), choosing a general T–P profile allows one to apply the resulting values of abundances to any other disks or bodies, and to determine the chemical compositions for any desired T–P range.
When a reaction is required to be calculated for a temperature value that is not available in the database, the code extrapolates the equation of the equilibrium constant to the required temperature. In order to avoid unexpected results, temperature values lower than 100 K are not taken into consideration.
We assume that the compounds appearing in temperature zones lower than 400 K in our results may be kinetically inhibited over the lifetime of the disk.
2.5. Volatile Phase
In the core accretion model, the rapid accretion of gases and gas-coupled solids takes place after the formation of solid cores by collisional accretion of planetesimals in disk zones beyond the water snowline (Mousis et al. 2009c). Accordingly, the material that does not get trapped in cores is free to enrich the gas in heavy elements, or to be trapped by clathration or adsorption into ice to enrich the envelopes of the giant planets at a later stage of the process.
Similar to the approach of Johnson et al. (2012), the volatile ice abundances in the envelopes of the planets are calculated taking the C, N, O, P, and S abundances remaining in the gas, following the formation of compounds consistent with mass conservation. This method seems to agree with the measured enrichments in the atmosphere of Jupiter (Alibert et al. 2005b).
Abundances of volatiles accreted into the gas giants are calculated by a FORTRAN code whose basic theory is explained briefly below, following the assumptions and formulations provided by Mousis et al. (2009c).
For the accretion disk, the turbulent model provided by Alibert et al. (2005a) is taken as a reference. Condensation leading to the formation of clathrates and pure condensates in the accretion zones of proto-giant planets is treated by assuming an initially homogenous gas phase of water consistent with the elemental stellar composition.
The actual clathration efficiency is currently unknown for the solar nebula. Therefore, among the set of trial runs from 0% to 100%, complete clathration is chosen for simplicity and assumed to be constant throughout the disk.
Hydrate, clathrate, and pure condensate stability curves, which were derived from the experimental work of Lunine & Stevenson (1985) and laboratory data of Lide (2002), are used to estimate the volatiles trapped in icy planetesimals for the temperature and pressure conditions at the distance of Jupiter.
Under these assumptions, the mass ratios of different ices can be estimated with respect to water in the planetesimals. The mass ratio of the volatile relative to that of water is given by Mousis & Gautier (2004) as
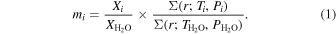
Here, X stands for the mass mixing ratio of the associated compound relative to H2 in the disk. Σ is the disk surface density at the distance r, at the clathration epoch of the associated species, and at the epoch of water condensation.
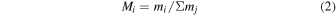
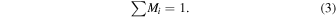
The mass fraction M i is calculated from mi , relative to the total volatile compounds involved in the formation of icy planetesimals.
Despite it being an oversimplification, it is assumed that all of the vapor ends up in the clathrate for every species, in order to follow the due implications.
The ranges of volatile abundance in planetary envelopes are estimated from the study of Mousis et al. (2011). The ices formed beyond the snowline carry an important fraction of volatiles affecting the O and C abundances by vaporization when they enter into the planetary envelopes (Madhusudhan et al. 2011b). Icy contributions are shaped by the initial elemental composition of the disk gas phase, rather than the location of their formation, or the thermodynamic conditions selected, rendering all planetesimals similar (Mousis et al. 2009b, 2012; Madhusudhan et al. 2011b). Therefore, the gas-phase composition is defined a priori, assuming that the protoplanetary disk has the stellar elemental abundances. The volatiles are then assumed to be entirely captured in the icy planetesimals (Johnson et al. 2012). While this is a simplifying assumption, it is not bad for giant planets formed beyond the amorphous-to-crystalline transition region. Mousis et al. (2020), on the other hand, provided a detailed study on the fractionation that occurs upon conversion of amorphous ice to crystalline ice.
Since the approach of the extended core scenario worked fine for the volatile enrichment in Jupiter's atmosphere (Mousis et al. 2009c, 2011), this work extrapolates that method to the hot Jupiter WASP-12b, seeking the most suitable matching of volatile abundances in its atmosphere. The abundances of species are evaluated at a fixed temperature of 300 K. This is a value higher than the condensation temperature of water in the protoplanetary disks, yet still cold enough for many volatile species to be trapped in meteorites. On the other hand, the equilibrium reactions involving refractory compounds can safely be regarded as quenched at a temperature of 300 K under stellar disk conditions. Hence, it is acceptable to assume that the leftover abundances of elements of concern would be more or less constant for the zones with temperatures lower than the quench temperature of their equilibrium reactions.
After collecting the results from the HSC code for refractory species, the ones with a molar fraction above 10−10 relative to H2 are selected. The five primal biogenic elements O, C, N, S, and P, whose initial abundances are documented in Table 2, first incorporate into solids, and then their residual abundances are determined and given in Table 3. These elemental abundances are used to compute the main volatile molecules residing in the disks before condensing or being trapped into clathrates, as described in, e.g., Mousis et al. (2011, 2012).
Table 3. Remaining Molar Fractions of Five Elements Relative to H2 Considered for Volatile Accretion after the Consumption by Refractory Phases in Stellar Disks
| Element/H2 | Sun | WASP-12 |
|---|---|---|
| O | 6.88 × 10−4 | 6.16 × 10−4 |
| C | 5.92 × 10−4 | 7.10 × 10−4 |
| N | 1.49 × 10−4 | 2.24 × 10−4 |
| P | 5.64 × 10−7 | 6.13 × 10−7 |
| S | 1.45 × 10−5 | 1.66 × 10−5 |
Download table as: ASCIITypeset image
To maintain the simplicity of the model, physical and chemical factors such as the angle and wavelength of stellar irradiation exposure, and photochemistry, which are known to influence the atmospheres (Visscher et al. 2006; Moses et al. 2011, 2013), are not taken into account.
2.6. Redox State
The alteration of the conditions, to be oxidizing or reducing, depends on the oxygen abundance. We consider the redox state of the gas phase only. Operationally, the circumstances will be oxidizing when the dominating gas in the disk is CO, and they will be reducing when the major gas is CH4.
In the oxidizing case, O, C, N, S, and P are limited to the following molecular species: H2O, CO, CO2, CH3OH, CH4, N2, NH3, H2S, and PH3 (Madhusudhan et al. 2011a, 2011b; Mousis et al. 2009a, 2009b, 2012; Eistrup et al. 2016). The ratios between carbon-bearing species are fixed to the following values: CO/CO2/CH3OH/CH4 = 70/10/2/1 (Johnson et al. 2012; Mousis et al. 2009a, 2009b, 2012; Madhusudhan et al. 2011a).
As for the nitrogen-bearing components, N2 takes the majority with the ratio N2/NH3 = 10/1. Finally, sulfur-bearing components are treated with the ratio H2S/S = 1/2 (Pasek et al. 2005; Mousis et al. 2009a, 2009b).
In the reducing case, carbon is considered to be consumed only by CH4, which leaves no carbon for CO, CO2, and CH3OH. Nitrogen gas is now the minor nitride species with a ratio N2/NH3 = 1/10. The sulfur ratio remains the same as in the oxidizing case, H2S/S = 1/2. Finally, in both cases, all phosphorus is found only in the form of PH3.
The ratios given above are compatible with thermochemical calculations for the Sun's protoplanetary disk, consistent with the measurements of the interstellar medium including both solid and gas contributors, and close to the measurements made on comets (Frerking et al. 1982; Ohishi et al. 1992; Gibb et al. 2000; Ehrenfreund & Schutte 2000; Mousis et al. 2012).
2.7. C/O Ratio
Since the planetary models depend on stellar chemical compositions, the initial C/O ratio is important to a certain extent (Bitsch & Battistini 2020; Cridland et al. 2019b; Fortney 2012), as it is in the case of the equilibrium composition of a hot Jupiter, which is known to show sensitivity to the C/O ratio (Moses et al. 2013).
Observations show that the atmospheres of such planets may have C/O ratios higher than their host stars (Madhusudhan et al. 2011b). As one possible reason for this, Mousis et al. (2009c) proposed that the formation zone within the disk might have been deprived of oxygen, and carbon-rich planetesimals were accreted into their envelopes. Therefore, the C/O ratio for both stars is converted to 1 by adjusting the oxygen abundance down to the amount of carbon. This method is preferred by Lodders (2010) as well because it does not add to the existing amount of carbon in the environment.
The leftover oxygen abundance after the formation of volatile species is calculated for both disks when the C/O ratio is unity. The initial amount of oxygen input to the software is adjusted to a value that will yield the targeted leftover oxygen abundance for the volatile compounds in each case. When the targeted oxygen abundance is reached, rocky phase compositions are calculated and documented for the Sun and WASP-12, consistent with the redox state.
For the case where C/O is 1, the O abundance is highly depleted with respect to the initial ratio defined by the solar elemental abundance, therefore oxygen is shared only among the carbonic species, leaving no space for water formation (Lodders 2010; Mousis et al. 2012). Since the elemental quantities are derived after the removal of refractory species in the disks, these C/O ratios refer to the gas-phase C/O ratios directly.
2.8. Comparison with Jovian Measurements
Following Mousis et al. (2012), once the composition of planetesimals is calculated for the oxidizing and reducing cases, the mass of heavy elements found in Jupiter's envelope is adjusted so that it can provide the best fit for volatile abundances measured by the Galileo probe, as reported by Wong et al. (2004) and Fletcher et al. (2009). When the abundances of these molecules are determined, the remaining oxygen content is left for water formation (Mousis et al. 2009c).
2.9. Enrichment Factors
Enrichment from planetesimals to the planets is considered separately for the ice and rocks. In order to estimate the mass of enrichment made in ices, the species H2O, CH4, CO, CO2, and CH3OH are taken into account. However, for the enrichment in rocks, all species that have molar fractions above 10−10 relative to H2 are considered to contribute to the bulk content of the planet. The results are provided below.
3. Refractory Phase
The refractory composition throughout the disks is documented only for the species surpassing a fraction of 5% over the total mass, in order to make the plots interpretable. The distribution of species is presented as a function of temperature. The pressure is constant at 10−4 bar for each case, and components are given as percentage fractions over a total number of 840 refractory species, within the temperature range 100–2000 K.
Generally speaking, specific temperatures associated with refractory species are found here to be slightly lower by about 50–100 K than the results given by previous studies on solar nebula chemistry, such as those from Bond et al. (2010b), Fegley (2000), and Prinn (1993). This difference is mostly observed for the species appearing at temperatures lower than 700 K. The reason might depend on the preferences of the temperature–pressure profile of the solar disk, the data source of solar chemical composition, and/or the program code used for calculations.
The temperature values attributed to species are also different in this work from the others, especially when modeling the solar terrestrial planets in the following sections. Temperatures considered are those for which the aforementioned species are found at their maximum abundances, not the maximum temperatures at which the species form, which was how it was generally done in previous studies.
For example, Moriarty et al. (2014) noted that oxygen-bearing molecules appear below 1300 K, but our results show that oxygen can safely survive up to 1730 K by combining with aluminum, and even above 1900 K if incorporated in CaTiO3 and K2TiO3.
Figure 1 depicts the dominant solid-phase species calculated with stellar elemental abundances. A zonal division between diverse refractory compounds formed out of certain elements is clearly observable throughout the disks, as noted also by Bond et al. (2010b). The disks can be imagined as zonal divisions where some certain compounds dominate inward, while others dominate outward. Toward cooler regions, the formation of compounds shifts from refractory to volatile products (Lodders 2003; Bond et al. 2010a).
Figure 1. The leading species of expected refractory composition in the disk of (a) the Sun and (b) WASP-12 on the basis of temperature. Dashed lines stand for alternative molecular combinations of species with a curve of the same color.
Download figure:
Standard image High-resolution image3.1. Magnesium-bearing Species
The dominant magnesium compound in the disks is Mg4Si6O21H12 (sepiolite). Being a magnesium silicate as well as a clay mineral, it contributes most to crustal structures formed at temperatures of up to 500 K. Below 400 K, it comprises 40%–50% of overall rocky compounds but is not available above 520 K.
Where sepiolite begins diminishing, magnesium coalesces with another silicate compound Mg2SiO4, which emerges above 400 K in two different molecular alignments of magnesium orthosilicate: forsterite and the general form, also known as 2MgO.SiO2. In the disk compositions, forsterite can reach up to 20% around 500 K, then gradually perishes around 1400 K.
MgSiO3 (magnesium metasilicate) contributes in the disks around 520 K, exhibiting a relatively stable pattern between 550 K and 1300 K. When above 1400 K, magnesium metasilicate is lost quickly, along with Mg2SiO4 isomers. These two are often regarded as the major magnesium and silicon carriers in the disks (Lodders 2003), forming the majority of mantle material for rocky planets (Alessi et al. 2017).
Apart from MgO, magnesium tends to combine with silicon, and apart from disthene (Al2SiO5), silicon tends to combine with magnesium only.
3.2. Iron-bearing Species
The formation of magnetite (Fe3O4) begins around 320 K, whereas Fegley (1988) finds it to be 400 K for the solar nebula. With higher temperatures, Fe3O4 loses its oxygen, and free iron allotropes dominate in the exact trend. Iron remains roughly stable from 700 to 1300 K, making a contribution of about 30% for the solar case and 40% in WASP-12.
Metallic iron condensation coincides with magnesium silicates, as also noted by Lodders (2003). However, she mentions this temperature interval to be as narrow as 100 K, whereas it is about 1000 K here.
Pyrrhotite (FeS; iron 0.877 sulfide) appears in the solar case only, with a maximum 7% overall up to around 580 K, as suggested also by Lodders (2003). This temperature for FeS is given as 687 K by Prinn (1993), and 710 K by Fegley (2000). Fe3O4 and FeS contribute 70%–80% of the core materials in terrestrial planets (Alessi et al. 2017).
Finally, wüstite (FeO), also known as iron II oxide, is produced with a peak around 390 K for 5% in the solar case, and between 320–400 K with a peak of 9% in the WASP-12 case.
3.3. Calcium-bearing Species
The only calcium-carrying species in cold zones is calcium triiron pentaoxide (CaFe3O5), which appears in regions colder than 350 K with a contribution of 8–10%. In the interval 1750–1500 K, variations of calcium oxides combined with aluminum and silicates appear in the forms CaAl4O7, Ca2SiO4, Ca2Al2SiO7, Ca3SiO7, and Ca3SiO5. Between 1600 and 1500 K, calcium silicates cover the entire refractory distribution in the disks along with K2TiO3 in WASP-12. For the solar case, calcium combines with titanium oxide as Ca2TiO3 above 1600 K, being the only rocky species in the range 1820–1740 K. Almost all of the calcium species mentioned here are confirmed in the study of Lodders (2003).
3.4. Aluminum-bearing Species
Aluminum oxide is observed in various forms of Al2O3. They can combine with calcium to form CaAl2O7, CaAl2O4, or Ca2Al2SiO7 between 1750 and 1500 K. One of the major calcium-aluminum oxides that appeared in the study of Lodders (2003) was hibonite (CaAl12O19), which is not included in the calculations here.
Excluding CaFe3O5, calcium and aluminum appear only in hot regions for a narrow range of 200 K, as supported also by Miyazaki & Korenaga (2020). Above 1750 K, refractory components of disks are dominated only by CrC24H36 and K2TiO3 or CaTiO3.
Miyazaki & Korenaga (2020) modeled a disk chemistry where Al, Ca, and Mg minerals drift toward inner regions from distant zones. Our study, however, shows that those minerals can naturally be produced at high temperatures without the necessity of being dragged from outer zones.
Although the order of abundances might change in two disks, and a few species might appear or disappear in one of them, the general behaviors of species are quite similar. Close abundances of elements result in similar disk refractory distributions, as also confirmed by Lodders (2010).
The majority of the silicates are already known to be the iron- and magnesium-bearing ones in the solar nebula (Léger et al. 2004). The species found to have a mass fraction of at least 5% in the disks of the Sun and WASP-12 but not mentioned in similar work by Bond et al. (2010a, 2010b) are listed in Table 4. Both studies worked with the same temperature and pressure values, therefore the only reason that Bond et al. (2010a) did not observe these species is that they did not include them in their calculations. Interestingly enough, the three species, namely Al2O3, CaTiO3, and Mg2SiO4, are not taken into consideration by Bond et al. (2010a), but they were mentioned in an earlier study by Prinn (1993), as well as here being highly refractory species that are expected to survive in hot regions of the solar disk.
Table 4. The Dominant Rocky Species Found in This Study but Not by Bond et al. (2010a)
| Al2O3 | CaAl4O7 | Mg(OH)2 |
| Al2SiO5 | CaFe3O5 | Mg2SiO4 |
| Ca2Al2SiO7 | CaSiO3 | Mg4Si6O21H12 |
| Ca2SiO4 | CrC24H36 | MgO |
| Ca3Si2O7 | FeAl2O4 | MnC2 |
| Ca3SiO5 | FeO | NaH2SiO4.7H2O |
| CaAl2O4 | K2TiO3 | SiO2 |
Download table as: ASCIITypeset image
Table 5 lists the species that appeared in the results of Bond et al. (2010a) but do not reach a mass fraction of 5% in the result here, despite being included. Accordingly, solid component distributions in the disks documented by Bond et al. (2010a) and this work differ from each other fundamentally for hot and cold extremes of the temperature regime of interest. Still, these two studies are compatible in respect of the species dominating in a bigger part of the disks, which are pyroxene (MgSiO3), olivine (Mg2SiO4), and elemental iron (Fe). This conclusion, as well as the iron peak around 1500 K, is also supported by Lodders (2003).
Table 5. The Rocky Species Found in the Results of Bond et al. (2010a) but Not in This Study
| C | FeSiO3 | MgS |
| CaMgSi2O6 | Mg3Si2O5(OH)4 | NaAlSi3O8 |
| CaS | MgAl2O4 | SiC |
| Fe2SiO4 |
Download table as: ASCIITypeset image
Another example is the study of Alessi et al. (2017), which documented planetary solid mass for five super-Earths, with the maximum mineral distributions of FeS, Fe3O4, FeAl2O4, CaMgSi2O6, MgSiO3, and Mg2SiO4. However, only the relative abundances of the last two are comparable with our results for the Earth.
Since the redox state of the disks is adjusted with the oxygen abundance, there is only one set of refractory results for neutral, oxidizing, and reducing conditions when the C/O ratio is stellar. Oxygen-bearing species increase in abundance due to the increased availability of oxygen when the circumstances are oxidizing, and endure for a broader temperature region. The influence of reducing conditions is even less important, diminishing the abundances of a few oxidized species and increasing the fraction of elemental iron, only.
4. Planetary Models
In this section, further implications from the rocky species are presented for individual planets. Bulk compositions are predicted for solar terrestrial planets. For the case of WASP-12, we present a range of possible refractory compositions for planetesimals accreted in WASP-12b.
Since many types of planets, especially terrestrial ones, are thought to be common in various extrasolar planetary systems (Bond et al. 2010a, 2010b), and since planetary bulk compositions and mineral ratios of planetesimals, meteorites, and asteroids provide a great amount of information on their places of origin, the results of this section are expected to be of use in interpreting exoplanet observations.
This approach may very well work also in the reverse direction since disk composition is correlated to the radial distance (Bond et al. 2010b). The mineral composition of a planetary body can provide a constraint on where it formed in the disk (Piso et al. 2016).
In the distributional charts of Figures 2–6 below, magnesium-bearing species are presented in green shades, iron-bearing ones in red shades, compounds with calcium in blue shades, and those with aluminum in brown shades. Other refractory species with titanium, chromium, or nickel are depicted in gray shades, and the rest of the materials with smaller fractions are collected under "others" and colored violet.
4.1. The Solar System
To present the refractory bulk composition for the solar planets, an approach is introduced on the assumption of formation in situ, because it is believed that planetary locations are important in determining their compositions (Lewis 1974; Kuchner & Seager 2005; Santos et al. 2017). This assumption is good for the terrestrial planets because the bulk of the accreted material from which they formed is probably local, and they probably formed in regions close to the Sun (Bitsch & Battistini 2020), whereas the giants are likely to have migrated before settling into their current orbits (Lin 2008). As such, the calculations are done down to 100 K only, but the giant planets fall beyond this temperature range.
First, estimated bulk compositions for the solar system's planets are collected from previous works, and the temperature value offering the closest ratios of species is taken as a reference to the birthplace temperature for the mentioned planet. Since refractory phases of the planetary bulks are not known in detail, the average value, or at least the existence of a characteristic species, is taken as a parameter to indicate the temperature of the location where it is formed and to trace the distribution of other species in its vicinity.
Among the species considered, those reaching a mass fraction down to 0.1% are documented by mass fraction and in a comparative manner between two different temperature profiles for each planet.
A planetary formation model introduced by Bond et al. (2010a) tried to reproduce the Earth, but it failed to produce the terrestrial composition. The reason was blamed on the computer code they used, for not forming the planet at the distance where the most likely composition was found. Therefore, they suggested that the Earth might have migrated by at least 0.4 au. Migration is not necessarily predicted in the evolution of inner planets (Bitsch & Battistini 2020), and this scenario is not required for the Earth according to the approach offered in this work either.
Similarly, Elser et al. (2012) applied chemical equilibrium and dynamical simulations to circumstellar disk models; however, their models could not reproduce the bulk composition of terrestrial planets.
Ronco et al. (2015) also simulated solar rocky planets, but since they documented the compositions over elemental distributions only, their results are not comparable with ours.
4.1.1. Mercury
Explanations for the inner structure of Mercury are not accurate. Given its high uncompressed density of 5.3 g cm−3, iron-based species are assumed to be dominant (Goettel 1988). The traditional assumption for Mercury's bulk composition is that it consists of 65%–70% of metallic and 25%–30% of silicate materials (Kozlovskaia & Pechernikova 1992; Strom & Sprague 2003).
Figure 1(a) shows that the maximum elemental abundance of iron is found at the temperature zone of 1420 K, with a total fraction of 55%. The refractory composition for the region where elemental iron is found at its maximum is presented in Figure 2. The next major compounds are aluminum, calcium, and magnesium silicates such as Al2SiO5, CaSiO3, MgSiO3, Mg2SiO4, MgO, as well as a small amount of elemental nickel. The sum of these silicate phases covers 41% of the overall mass after the elemental iron, nickel, and chromium. This ratio of silicates is higher than the traditional estimations of 10%–15%. It is a remarkable difference; nevertheless, dynamic processes such as evaporation and collisional stripping very likely influenced Mercury's overall composition (Bitsch & Battistini 2020; Santos et al. 2017).
Figure 2. Mercury's refractory composition calculated for the temperature where elemental iron is found at the maximum abundance.
Download figure:
Standard image High-resolution imageIn this approach, Mercury most probably formed in a temperature zone of 1400–1440 K. An increase in the temperature does not result in much difference in iron fraction, because iron is not produced above 1440 K.
4.1.2. Venus
Venus is considered to be similar in chemical composition and internal structure to the Earth in the respects of mass, mean density, and solar distance (Stolper 1980; Treiman 2009). This assumption was partially confirmed thanks to the Venera and Vega missions (Treiman 2009).
Venusian lava is measured to contain an FeO content equal to the Earth's, which is 8%, indicating the presence of a similar metallic core (Taylor 1991). K/U ratios of both planets point out a similarity for their volatile to refractory elements (Taylor 1991). The similar abundance of potassium also suggests that Venus and the Earth have comparable abundances of Fe and Mn, which leads to comparable bulk mantles and core compositions, as well as sizes (Treiman 2009).
Venusian normative constituents and the sequence of minerals aligning with pressure are also the same as those of the Earth (Stolper 1980). Therefore, our results for the Earth can be applied also to Venus.
4.1.3. Earth
The leading elements with their fractions for the entire planet are iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur (2.9%), nickel (1.8%), calcium (1.5%), and aluminum (1.4%); the core is thought to consist of 88.8% iron, 5.8% nickel, and 4.5% sulfur (Morgan & Anders 1980).
While the other major elements such as iron, oxygen, and silicon are incorporated into complex molecules with varying fractions throughout the disk, nickel does not combine with other species in detectable fractions. Therefore, its abundance is taken as a proxy for determining temperature for when the proto-Earth appeared. There are 63 nickel-bearing compounds in the calculations, but it mostly remains in elemental form, apart from 1.5% as Ni3S2 at 150–300 K. As shown in Figure 3, nickel peaks at 1460 K with a fraction of 3.9%, together with its face-centered cubic (FCC) allotrope.
Figure 3. Distribution of dominant nickel forms in the solar nebula. Dark shade: Ni (FCC), mid-shade: Ni, light shade: Ni3S2.
Download figure:
Standard image High-resolution imageFigure 4. Earth's refractory composition calculated for the temperature where elemental nickel is found at the estimated abundance.
Download figure:
Standard image High-resolution imageThe compositions for the possibilities of the maximum nickel abundance in the disk, 3.9% at 1460 K, and its traditionally estimated abundance, 1.8% at 1318 K, can be compared depending on Figure 1(a) to decide which one suggests a more realistic bulk for the Earth. For both cases, metallic iron makes up one-third of the solid phase of the planet. The next most abundant material is magnesium silicates, MgSiO3 and Mg2SiO4, where Ni is found in the desired abundance. For the maximum Ni contribution, silicates of aluminum, Al2SiO5, and calcium, Ca2SiO4, come forward.
Another important difference is that magnesium silicates comprise a fraction of only 0.2% in the case of maximum Ni. For the desired Ni fraction instead, metallic iron, nickel, chromium, and ZnCo3 have mass fractions of 31.6%, 1.8%, 0.3%, and 0.1% respectively, which can safely account for the core, as demonstrated in Figure 4. 48.7% is distributed among silicates, with 21.6% of MgSiO3, 18.8% of Mg2SiO4, 4.3% of CaSiO3, 4.0% of Al2SiO5, and 0.2% of K2TiO3. The leading oxides are SiO2 with 8.3% and MgO with 8.2%. The only carbon-bearing solid compound is MnC2 with a fraction of 0.4%. Accordingly, the composition documented for the classical nickel fraction can account also for the distribution of other compounds in the bulk of the Earth.
4.1.4. Mars
Two substances are found in the literature with bulk abundance estimations of Mars. The first one is FeO, given in a range of 15%–19.5% by different methods (Taylor 2012). Although it is not close, the maximum FeO fraction found is 7.4% at 392 K throughout the solar disk, where the composition is dominated by Mg4Si6O21H12 with 45%, metallic iron with 14%, and MgO with 11%.
The second proxy is sulfur, which is claimed to have a core fraction of 16% (Rivoldini et al. 2011). In order to determine the disk region with the maximum sulfur content, combinations of sulfur with iron and nickel are included as well, since such combinations are likely under the physical conditions of the core.
The following compounds of Fe, Ni, and S included in the calculations: Fe, FeS, Fe2S, Fe2S3, Fe7S8, Fe9S8, FeS2, S, Ni, Ni3S4, Ni6S5, Ni7S6, Ni9S8, NiS, and NiS2. The temperature where the sum of these species is at the maximum is 490 K with a bigger contribution from Fe–S molecules, and the related composition is shown in Figure 5. At that temperature, Mg2SiO4 is dominant with 33.5%, followed by metallic iron with 22.2%, then Mg4Si6O21H12 with 19.6%, and FeS with 8.6%. The rest consists of various silicates, metal oxides, and 1.5% of elemental nickel.
Figure 5. Refractory composition of Mars calculated for the temperature where FeS is found at the maximum abundance.
Download figure:
Standard image High-resolution imageSince the leading magnesium silicate is Mg2SiO4 at 490 K while it is Mg4Si6O12H21 at 392 K, and since the abundance of elemental iron is bigger in the composition of 490 K, the option based on FeS content seems more probable.
Moriarty et al. (2014) mention 550 K as the temperature of the region where terrestrial planets form, which is only close to what we offer for the formation zone of Mars, but ∼900 K lower than those of Mercury and the Earth.
4.2. The System of WASP-12
Although WASP-12 is known to harbor at least one planet, in situ approaches used for solar planets cannot be utilized for its system. This is due to the fact that its detected planet is known to be a hot Jupiter, which is thought to have migrated to its current orbital distance after forming beyond snowlines according to the core accretion scenario. As the presence of a hot Jupiter may indicate a multiplanet system, the disk of WASP-12 is worth investigating to estimate the possible refractory compositions for any other planets that might be observed in the future. Indeed, both observations (Schneider 2020) and simulations (Raymond et al. 2005) confirm the existence and formation of terrestrial planets in extrasolar systems, despite the difficulty in revealing their chemical compositions in detail (Bond et al. 2010b).
To exemplify various planetary embryos in the disk of WASP-12, temperature zones with diverse compositions are chosen so that main compositional differences can be observed easily. The general view of refractory bulks is not radically different from that of solar ones, since the fundamental parameters such as mass, size, effective temperature, and elemental abundances are close to the solar values. The plots are restricted to the compounds that reach or exceed a mass fraction of 1%. The sample refractory compositions are considered down to 300 K, and the metallic, silicate, and oxide contributions differ in each selection. Hence, all representative cases fall into the category of rocky planets. It should be noted also that the classification according to the size, and more importantly the liquid and gas contributions, are not taken into account; therefore, the fractionations stand only for solid parts of the generated planets.
Six different refractory compositions are illustrated for the disk of WASP-12 in Figure 6. According to the results, the rocky part of a planet that formed around 1854 K should consist of 92% CrC24H36 and 8% K2TiO3. This combination looks like an alternative planetary structure where CrC24H36 comprises the mantle and nearly the entire planet, whereas K2TiO3 covers the top as a thin crust. The stratification is not a mandatory expectation as both compounds can also be distributed arbitrarily.
Figure 6. Diverse refractory compositions throughout the disk of WASP-12. Pie charts show the compositions for 1854 K, 1659 K, 1562 K, 1026 K, 441 K, and 344 K respectively, from left to right and top to bottom.
Download figure:
Standard image High-resolution imageFor a planet formed at 1659 K, the entire rocky composition is shared by various oxides of calcium and/or aluminum, along with 28% K2TiO3. K2TiO3 might precipitate as the planet's central core or make up a thin mantle. However, it seems more probable to find the compounds as mixed instead of stratified, since they all have the same chemical classification.
The composition at 1562 K is another example of the mixture of oxides with aluminum and/or calcium, including also some silicates. This combination is a candidate for terrestrial planets, probably with a large mantle and rich crust structure, but lacking a metallic core. Therefore, it can be categorized as a coreless planet (Seager & Elkins-Tanton 2008). K2TiO3 is again the only oxide species bearing a metallic atom, with a ratio of 7%.
The model for 1026 K is a proper terrestrial planet with an elemental iron contribution of a mass fraction of 38% to make up the core, which may also lead to a high density (Bitsch & Battistini 2020). The rest of the planetary mass is divided among silicates of magnesium, aluminum, and calcium. This composition is compatible with the extrasolar planets of Kepler-10 and CoRoT-7b, which are known to resemble to Mercury by their iron cores making up to 65% of their bulks (Moriarty et al. 2014). Due to the presence of a metallic core, a magnetic field can also be expected if there is a motion to cause the dynamo effect.
The composition drawn for 441 K still has an iron core to cover 31% of the total mass; nevertheless, the major part consists of magnesium silicates. With a rich combination of FeS, FeO, Ca3Si2O7, and Cr2FeO4, this model is also placed in the category of terrestrial.
The iron core disappears for the composition found at 344 K. Iron is incorporated in oxide compounds such as Fe3O4, FeO, CaFe3O5, FeAl2O4, Cr2FeO4, or FeS. The dominant compound is Mg4Si6O21H12 with 42%. This combination is reminiscent of the planet Mars, as far as what is known from analyses of its crust. A planet formed at that temperature could then be imagined as a coreless terrestrial planet resembling Mars.
Most of the sample refractory compositions from the disk of WASP-12 discussed above resemble the Earth, or at least remain within the definition of a terrestrial planet. This conclusion agrees with observations in optical and ultraviolet spectra of white dwarf stars, showing that extraterrestrial planetesimals are similar to the Earth's bulk composition since their masses are dominated by terrestrial planet-building elements such as oxygen, magnesium, silicon, and iron (Santos et al. 2017; Bond et al. 2010b).
As for the interior structure, all rocky planets can be differentiated into a silicate crust and a metallic core (Schaefer et al. 2012). The models presented above can also account for the core compositions of other planet types such as gas or ice giants.
5. Volatile Phase
The results for elemental enrichment, accreted volatile content, and accretion mass concerning the two disks are documented separately. The enrichments in nitrogen, sulfur, and phosphorus are not necessarily influenced by the changes in the C/O ratio when the conditions are reducing. Their reaction to the C/O ratio under oxidizing conditions is very small as well.
5.1. Jupiter
The oxygen fraction consumed by the refractory species is calculated to be 64% for the solar nebula, leaving 36% for volatiles.
The predictions for the composition of planetesimals accreted to the envelope of Jupiter are summarized in Table 6 (left half) corresponding to the four sets of the C/O ratio and redox state. The C/O ratio of the planetary envelope is calculated as 0.7 when the disk conditions are oxidizing, and 0.4 when reducing. Oxidizing circumstances lead to a C/O ratio on Jupiter bigger than that of the Sun, and reducing circumstances to a C/O ratio lower than that of the Sun.
Table 6. The Abundances of Accreted Volatile Species, and Enrichment Factors for Basic Atmospheric Elements for Generic Jupiter and WASP-12b for Minimum and Maximum Fits
| Sun | WASP-12 | |||||||
|---|---|---|---|---|---|---|---|---|
| C/O: Solar | C/O: 1 | C/O: Stellar | C/O: 1 | |||||
| Oxidizing | Reducing | Oxidizing | Reducing | Oxidizing | Reducing | Oxidizing | Reducing | |
| C/O | 0.74 | 0.39 | 1 | 1 | 1.1 | 0.50 | 1 | 1 |
| Oxygen a | 2.7–3.2 | 4.4–6.0 | 2.0–2.5 | 1.5–2.1 | 1.5–1.9 | 2.7–3.6 | 1.6–2.0 | 1.3–1.7 |
| Carbon a | 3.7–4.3 | 3.1–4.2 | 3.7–4.6 | 2.8–3.8 | 3.6–4.5 | 3.0–4.1 | 3.6–4.5 | 3.0–3.8 |
| Nitrogen a | 2.9–3.3 | 4.5–6.2 | 2.8–3.5 | 4.5–6.2 | 2.8–3.5 | 4.6–6.2 | 2.8–3.5 | 4.9–6.2 |
| Sulfur a | 3.3–3.8 | 2.4–3.3 | 3.1–3.8 | 2.4–3.3 | 3.0–3.8 | 2.4–3.3 | 3.0–3.8 | 2.6–3.3 |
| Phosphorus b | 4.9–5.7 | 4.5–6.1 | 4.9–6.0 | 4.5–6.1 | 4.7–6.0 | 4.5–6.1 | 4.7–6.0 | 4.9–6.1 |
| M(H2O) (M⊕) | 0.8–0.9 | 11.7–15.9 | 0 | 4.1–5.5 | 0 | 10.5–14.2 | 0 | 5.3–6.6 |
| M(CO) (M⊕) | 6.1–7.1 | 0 | 5.0–6.2 | 0 | 5.5–7.0 | 0 | 5.9–7.4 | 0 |
| M(CO2) (M⊕) | 2.9–3.3 | 0 | 2.3–2.9 | 0 | 2.6–3.3 | 0 | 2.7–3.5 | 0 |
| M(CH3OH) (M⊕) | 0.5–0.6 | 0 | 0.4–0.5 | 0 | 0.4–0.6 | 0 | 0.5–0.6 | 0 |
| M(O) (M⊕) | 5.5–6.3 | 10.4–14.2 | 3.9–4.9 | 3.6–4.9 | 4.3–5.5 | 9.3–12.6 | 4.6–5.8 | 4.7–5.9 |
| M(H2O)/Mtotal | 0.1 | 0.6 | 0 | 0.4 | 0 | 0.6 | 0 | 0.4 |
| M(CO)/Mtotal | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 |
| M(CO2)/Mtotal | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 |
| M(CH3OH)/Mtotal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MIce (M⊕) | 11.6–13.5 | 17.7–24.1 | 9.9–12.4 | 9.7–13.1 | 11.6–14.7 | 17.9–24.4 | 11.9–15.0 | 13.0–16.4 |
| MRock (M⊕) | 3.9–4.5 | 7.4–10.0 | 2.8–3.5 | 2.6–3.5 | 8.6–10.9 | 18.6–25.3 | 6.0–7.6 | 19.8–25.0 |
| MIce/MRock | 3.0 | 2.4 | 3.6 | 3.8 | 1.3 | 1 | 2 | 0.7 |
Notes. Data acquired by the Galileo probe.
a Wong et al. (2004). b Fletcher et al. (2009).Download table as: ASCIITypeset image
5.1.1. Enrichment of Basic Elements
Five fundamental elements, O, C, N, S, and P, are found in higher abundances in Jupiter's envelope than in the Sun. The highest enrichment is in oxygen, being 6 times, and observed when the conditions are reducing with the solar C/O ratio. Its lowest enrichment is 1.5 times under reducing conditions, with a C/O ratio of unity. The oxygen enrichment factor is bigger when C/O is 0.55 than when it is 1, since the disk becomes oxygen-deficient as C/O increases.
The highest carbon enrichment is 4.6 when the conditions are oxidizing and the C/O ratio is 1. This value is in agreement with Mordasini et al. (2016), who mentioned it to be around 4 times. Its lowest level is 2.8 under the reducing conditions of the same C/O ratio.
5.1.2. Accretion Abundances
Water accretion is a small quantity of 0.8 M⊕ under oxidizing conditions; in reducing conditions CH4 leaves all available oxygen to H2O to comprise a mass of 12–16 M⊕. If C/O is 1, there is no water contribution for oxidizing conditions, and 4–5 M⊕ for reducing conditions.
Oxygen-bearing carbon species appear only under oxidizing conditions, comprising 6–7 M⊕ of CO, 3 M⊕ of CO2, and 0.5 M⊕ of CH3OH for the solar C/O ratio. These values decrease slightly when the C/O ratio increases to 1.
The total mass of ice accretion is naturally bigger with a lower C/O ratio (Pekmezci et al. 2019). The difference is more obvious under reducing conditions, where the ice mass is almost doubled as C/O falls to 0.55 from 1. Once the C/O ratio is 1, the redox state of the environment does not make an important difference to the ice accretion.
The dominant accretion under oxidizing circumstances is from CO and CO2, and this is not affected if the C/O ratio is doubled. For the reducing cases, H2O and CH4 dominate. When C/O is doubled, H2O loses two-thirds of its total fraction, while CH4 nearly doubles.
Rock accretion is not affected by the increase in the C/O ratio under oxidizing conditions. However, it drops to one-third under reducing conditions, in agreement with the trend noted by Cridland et al. (2019b).
5.1.3. Accreted Planetesimal Composition
The mass fractions of the planetesimals accreted to Jupiter depending on the C/O ratio and redox state are depicted in Figure 7 in a comparable manner. In the case of solar C/O ratio and oxidizing conditions, the mass distribution among volatiles is 52% CO, 25% CO2, 7% H2O, 6% N2, 4% CH3OH, 4% H2S, and others. For the case of solar C/O ratio and reducing conditions, H2O dominates with 65%, followed by CH4 with 23%, NH3 with 8%, and others. When the C/O ratio is 1 and the redox state is oxidizing, the distribution is given as follows: CO 50%, CO2 24%, CH4 9%, N2 7%, H2S 4%, and others. Finally, when the C/O ratio is 1 and conditions are reducing, H2O makes up 42%, CH4 38%, NH3 15%, and the others are less.
Figure 7. Distribution of planetesimal volatile composition in the accretion zone of Jupiter for oxidizing conditions (light shaded bars on the left) and reducing conditions (dark shaded bars on the right), and for C/O ratios of 0.55 and 1.
Download figure:
Standard image High-resolution image5.1.4. Comparison with Measurements
In the envelope of Jupiter, the dominant oxygen-carrying gas is H2O, the major carbon-bearing gas is CH4 followed by a trace amount of CO, the prime nitrogen-holder is NH3, and the main sulfur gas is H2S (Lodders & Fegley 2002; Fegley & Lodders 1994). Unlike in the solar disk, CH4 overcomes CO, and NH3 overcomes N2 in its atmosphere. This is because the accreted CO and N2 are processed into CH4 and NH3, respectively, due to the high temperature and pressure conditions provided by Jupiter's gravity (Prinn & Fegley 1981; Fegley & Lodders 1994; Bézard et al. 2002; Wong et al. 2008).
The in situ measurements collected from the Galileo probe revealed that the noble elements such as Ar, Kr, Xe, as well as C, N, and S are enriched in the atmosphere of Jupiter by about three times relative to their solar values (Madhusudhan et al. 2011a; Guillot 2008; Alibert et al. 2005b; Lodders & Fegley 1994, 2002; Hersant et al. 2001).
As noted by Mousis et al. (2012), phosphorus does not show an enrichment factor close to 3 in either of the sets considered. Varying in the range 4.5–6.1, phosphorus does not demonstrate dependence on either the C/O ratio or the redox state. Therefore, it is left out of the evaluation of the comparison between the measured and calculated values.
What is responsible for the supersolar abundances is thought to be a large quantity of planetesimal accretion into the planet from mixed clathrate hydrates, rocks, and ices that were composed of the solar disk material during its formation era, in accordance with the core accretion model (Hersant et al. 2001; Mousis et al. 2009c; Öberg et al. 2011). Nevertheless, an alternative enrichment model from the gas phase is given by Mousis et al. (2019).
The data from the Galileo probe for the 1 bar pressure zone in the atmosphere of Jupiter showed a low oxygen abundance, 30% of the solar value, contrary to expectations (Mousis et al. 2012). If low oxygen content in the Jovian atmosphere was not only a result of atmospheric dynamics and meteorological activities at the spot where the probe made the measurements, but also indicated a lack of oxygen abundance relative to carbon in the planetary bulk, then Jupiter may have formed under disk conditions where oxygen was depleted relative to carbon (Kuchner & Seager 2005; Mousis et al. 2012). Arguing against this are the results of the Juno microwave measurements, which suggest a water abundance at low northern latitudes in excess of solar (Li et al. 2020a), though this remains to be confirmed by additional data.
Since an oxygen enhancement factor bigger than 5 is ruled out by thermochemical models such as those designed by Visscher & Moses (2011) and Visscher et al. (2010), the case in which the C/O ratio is solar, the conditions are reducing, and an enrichment by 4.4 to 6.0 times is required can safely be kept out of consideration. Among three other cases, the one requiring the biggest range of oxygen enrichment is where the C/O is solar and conditions are oxidizing, with a range of 2.7 to 3.2. The oxygen enrichment range for this case is calculated to be 6.8 to 7.2 by Mousis et al. (2012), nearly two times bigger than the value found here. This is a natural result since the initial oxygen abundance is smaller here, being the quantity remaining after its consumption by rocks.
The enrichment interval of oxygen calculated by Mousis et al. (2012) for C/O ∼ 1 and the oxidizing case, 2.1–2.4 times the solar value, is close enough to our results, 2.0–2.5. Therefore, both studies suggest an average enrichment factor of 2.2 over the oxygen abundance in the solar nebula in order to maintain the compositional data measured by the Galileo probe in the atmosphere of Jupiter. This corresponds to the accretion of 3.9–4.9 M⊕ of oxygen into the planet.
Unlike the study of Mousis et al. (2012), this study also documents reducing conditions. In the case where the C/O ratio is 1 and the conditions are reducing, the necessary oxygen enrichment factor is even lower than in the previous case, from 1.5 to 2.1. The oxygen mass required to be accreted into Jupiter is then 3.6–4.9 M⊕. Therefore, consistently with the standard core accretion model, the minimum oxygen accretion required for explaining the enrichment of noble gases, carbon, nitrogen, and sulfur in the Jovian atmosphere by three times over the solar value is a C/O ratio of unity under reducing conditions, limiting oxygen accretion to only 4.3 M⊕. However, if the enrichment factor of oxygen in the envelope of Jupiter is in the middle of the range of the values in Li et al. (2020a), 2 or 3 times solar, then oxidizing conditions are preferable for both C/O cases.
The bulk oxygen fraction and the C/O ratio of Jupiter remain to be better determined with microwave measurements by Juno at other latitudes. Should the water abundance turn out to be low, it could suggest that Jupiter is a carbon-rich planet (Mordasini et al. 2016). The challenge is to produce realistic models that obtain such a low oxygen abundance in the disk, so that little water is produced. A carbon-rich model for Jupiter would be consistent with the ideas of Lodders (2003) and would even relieve the problem from Juno gravity data that the amount of heavy elements in Jupiter's envelope must be limited (Wahl et al. 2017).
5.2. WASP-12b
The model developed for Jupiter by Mousis et al. (2009c) is applied to the planet WASP-12 after the refractory formation and on the basis of the core accretion model. This method was utilized for Jupiter and Saturn before (e.g., Mousis et al. 2009c, 2012). It is then a good opportunity to test the validity of the estimation of volatile enrichment mass on extrasolar planets.
For the disk of WASP-12, the oxygen fraction consumed by the refractory species is calculated to be 39%, leaving 61% for volatiles.
The predictions for the composition of planetesimals accreted to the envelope of a generic hot Jupiter formed in the stellar disk of WASP-12, namely WASP-12b, are summarized in Table 6 (right half) corresponding to the four fits. The C/O ratio of the planetary envelope is calculated to be 1.1 when the disk conditions are oxidizing, and 0.5 when reducing. Oxidizing circumstances lead to a C/O ratio in WASP-12b bigger than in its parent star, and reducing circumstances make the C/O ratio lower than that of its parent star.
5.2.1. Enrichment of Basic Elements
Five fundamental elements are found in higher abundances in the atmosphere of WASP-12b than in the parent star. The highest enrichment in oxygen is 3.6 when the calculations are done for the stellar C/O ratio under reducing conditions. The lowest one is 1.3, still for reducing conditions, but where C/O is 1. The range of oxygen enrichment for the oxidizing case shifts from 1.5–1.9 to 1.6–2.0 where C/O changes from stellar to 1.
5.2.2. Accretion Abundances
Accretion of carbon species for the stellar C/O ratio under oxidizing conditions is as follows: 5.5–7 M⊕ of CO, 2.5–3 M⊕ of CO2, and 0.5 M⊕ of CH3OH. These values slightly increase when the C/O ratio is 1.
Total ice contribution is found to be largest for the stellar C/O ratio under reducing conditions, with an average of 21 M⊕. It is not necessarily influenced by the C/O ratio for oxidizing circumstances in the disk of WASP-12.
Reducing conditions favor a larger contribution for the rocky part of a generic WASP-12b, which can increase up to 25.3 M⊕ for the stellar C/O ratio. The lowest rock accretion is found for a C/O ratio of unity but under oxidizing conditions, which can decrease down to 6 M⊕.
5.2.3. Composition of Accreted Planetesimals
The mass fractions of the accreted volatile species for the hot Jupiter WASP-12b depending on the C/O ratio and redox state are given in Figure 8 in a comparable manner. In the case of stellar C/O ratio and oxidizing conditions, the mass distribution among volatiles is as follows: CO 48%, CO2 22%, CH4 11%, N2 9%, CH3OH 4%, H2S 4%, and others, while there is no room left for H2O.
Figure 8. Distribution of planetesimal volatile composition in the accretion zone of WASP-12b for oxidizing conditions (light shaded bars on the left) and reducing conditions (dark shaded bars on the right), and for C/O ratios of 0.45 and 1.
Download figure:
Standard image High-resolution imageFor the case of stellar C/O ratio and reducing conditions, H2O dominates with 58%, then comes CH4 with 26%, NH3 with 12%, and the others.
When the C/O ratio is 1 and the redox state is oxidizing, the distribution is given as follows: CO 49%, CO2 23%, N2 9%, CH4 8%, CH3OH 4%, H2S 4%, and others.
Finally, for unity C/O and reducing conditions, H2O comprises 41%, CH4 36%, NH3 19%, and others.
CO and CO2 are not affected if the C/O ratio is doubled under oxidizing circumstances. When C/O is doubled under reducing conditions, H2O loses some one-third of its total fraction, while CH4 increases nearly 1.5 times.
5.2.4. Comparison with Measurements
WASP-12b, a transiting hot Jupiter with an equilibrium temperature of 2600 K, has a carbon-rich dayside atmosphere detected by the thermal emission and photometric observations from Spitzer (Öberg et al. 2011; Madhusudhan et al. 2011b, 2011a; Madhusudhan 2012; Johnson et al. 2012). The C/O ratio of the atmosphere is greater than 1, whereas that of its parent star is 0.44 (Johnson et al. 2012; Madhusudhan et al. 2011a, 2011b). This difference points out an inconsistency between the planetary atmosphere and the stellar composition since the protoplanetary disk does not provide a high bulk C/O ratio, the star is not carbon-rich, and heavy-element enrichment could not be met only by accretion of icy planetesimals (Madhusudhan et al. 2011a; Johnson et al. 2012; Moses et al. 2013).
The C/O ratio in the atmosphere of WASP-12b is the result of the composition of a low abundance of H2O and high abundances of CH4 and CO relative to what could be expected by thermochemical equilibrium models with solar elemental values (Madhusudhan et al. 2011a, 2011b; Madhusudhan 2012; Moses et al. 2013). The composition of an object formed out of disk material at this equilibrium temperature should have principally been CO and H2O, with a small contribution of CH4 and CO2 (Madhusudhan et al. 2011a).
According to the core accretion model, the planet is thought to have originated from an oxygen-depleted zone of the protostellar disk (Johnson et al. 2012), beyond the snowline, and to have accreted the gas on its way toward the star (Madhusudhan et al. 2011b; Öberg et al. 2011). When the C/O ratio is 1 in the atmosphere, the majority of the oxygen is tied up in CO, leaving less than 10% for H2O formation, and CH4 exceeds the solar contribution by 10 to 100 times (Madhusudhan et al. 2011b). The calculations of Madhusudhan et al. (2011b) with stellar abundances led to a C/O ratio of 0.27 in the planetary envelope. In order to produce 1 for the planetary C/O ratio, they had to decrease the stellar oxygen entry by 40%.
However, unlike the claim by Madhusudhan et al. (2011b), the results of this work show that it is not strictly necessary for the planet WASP-12b to have formed in an oxygen-deficient zone of the protoplanetary disk in order to maintain an atmospheric C/O ratio of 1. The calculations for the stellar C/O ratio and oxidizing case are actually compatible with the measurements and models in the literature suggesting the C/O ratio to be 1 for the planet, since this case results in the planetary C/O ratio being 1.06. It should be noted, however, that the initial amount of oxygen for calculations of volatile composition is smaller here than it is in the study of Madhusudhan et al. (2011b).
As long as the conditions are oxidizing, the stellar elemental abundances naturally yield a C/O ratio of 1.06 in the envelope of the planet. The required mass of oxygen accreted is 4.3–5.4 M⊕. The oxygen accretion for the oxidizing case where the C/O ratio is 1 but not stellar is only slightly bigger, being 4.6–5.8 M⊕. In actual fact, for this C/O ratio and reducing conditions, the necessary oxygen accretion is also very close, being 4.7–5.9 M⊕. Since a reducing environment is producing a planetary C/O ratio of 0.5 with the stellar elemental abundances, this case can safely be disregarded.
The ice accretion for the case of stellar C/O and oxidizing conditions is 0.8–9.7 M⊕ according to Madhusudhan et al. (2011b) while it is calculated to be 11.6–14.7 M⊕ in this study. For the case of unity C/O and oxidizing conditions, this accretion is found to be 0.3–15.9 M⊕ by them, but it is calculated as 11.9—15.0 M⊕ here.
Madhusudhan et al. (2011b) used the same methodology as this work given by Mousis et al. (2009a, 2009b), but they calculated the predefined disk composition for a low temperature such as 30 K, whereas this study used a temperature of 300 K. Furthermore, they did not consider the oxygen sink into the silicates, unlike the planetary elementary abundances determined after the formation of rocky species here.
6. Discussion and Conclusion
Close-in giant planets are expected to have an atmospheric spectrum with a combination of H2, CH4, H2O, CO, CO2, and NH3 (Lodders & Fegley 2002; Line et al. 2010; Öberg et al. 2011; Moses et al. 2013). If strong H2O bands are detected from such a planet, in combination with weak CH4 bands, this is considered to be a sign of a C/O ratio smaller than 1 (Madhusudhan 2012).
In contrast, if CH4 is detected with strong features, especially accompanied by weak H2O bands, then that is an indication of a C/O ratio equal to or greater than 1 (Madhusudhan 2012). CO bands, however, should always appear in the spectrum, regardless of the C/O ratio (Madhusudhan 2012). The C/O ratios of a few transiting exoplanets are known, but the James Webb Space Telescope is expected to be able to more accurately determine those of many more (Madhusudhan 2012).
In agreement with the anticipation of theoretical studies, spectroscopic observations confirm the existence of extrasolar close-in giant planets whose atmospheres have an abundance of carbon exceeding that of oxygen (Madhusudhan et al. 2011b; Mousis et al. 2012; Moses et al. 2013; Pekmezci et al. 2019). A high C/O ratio leads to the formation of carbon-rich and oxygen-poor planets with diverse interior compositions (Lodders 2010; Öberg et al. 2011; Madhusudhan et al. 2011a).
For a C/O ratio lower than 1, H2O and CO2 are abundant, as well as CO, which is the major constituent regardless of the C/O ratio (Moses et al. 2013; Lodders 2010). For C/O higher than 1, CH4, HCN, and C2H2 are abundant (Moses et al. 2013), indicating a formation history in either a water-poor or a carbon-rich region (Lodders 2010; Pekmezci et al. 2019). The species that carry neither oxygen nor carbon are not necessarily affected by changes in the C/O ratio, as also supported by Moses et al. (2013).
The dominant molecules as a function of the C/O ratio and redox states calculated in this work are in agreement with the expectations and observations mentioned by previous studies. Encouraged by the results collected from every case studied here, extrasolar giant planets can be expected to have different C/O ratios from their parent stars. When the stellar elemental compositions are used, the modeled hot Jupiters exhibit atmospheric C/O ratios higher than their stars under oxidizing conditions, and vice versa under reducing conditions. If the model used here is to be proven worthy of consideration at least roughly, then the C/O ratios of the atmospheres of hot Jupiters can be generalized to be overstellar under oxidizing conditions, and substellar under reducing ones, due to gas and ice accretion into their envelopes during their formation and migration epochs (Pekmezci et al. 2019).
There are various mechanisms suggested for the difference in the C/O ratio of a gas giant and that of its protostellar disk. For instance, accretion of solids rich in carbonic content but poor in water ice, or accretion of gas rich in CO but poor in H2O, increases the carbon content in a planet (Lodders 2010; Moses et al. 2013). Diffusion and turbulence, inward drift and trapping of ices, heterogeneity throughout the disk depending on place and time, differentiation of physical and chemical characteristics, snowlines of various ices at different distances from the parent star, and many other factors can cause planets to be enriched in or deprived of carbon or oxygen at diverse levels (Lodders 2010; Moses et al. 2013).
However, even if these factors play small roles in determining the final atmospheric C/O ratio of a hot Jupiter, divergence from the stellar C/O ratio might be a natural consequence of the core accretion scenario. This might be taken into consideration for the solar system as well, since bulk oxygen contents of all of the solar giants still remain undetermined (Wong et al. 2008). The results of this study therefore may serve theoretical modeling and analyses of solar and extrasolar terrestrial planets, especially for the determination of their chemical compositions and estimation of their formation conditions. Future implications from direct measurements, especially high-quality spectral analysis for host stars, as well as from theoretical models and calculations will shed light on the credibility of the assumptions made and the self-consistent results reached in the study.
This work has been partly carried out thanks to the support of the A*MIDEX project (no ANR-11-IDEX-0001-02) funded by the "Investissements d'Avenir" French Government program, managed by the French National Research Agency (ANR).
The authors thank Dr. Torrence Johnson for important advice and suggestions that enhanced the science reported here. And Dr. Mohamad Ali-Dib was very helpful with technical details.
G. S. Pekmezci is indebted to the Institut UTINAM and Observatoire des Sciences de l'Univers de Besançon for the accommodation and research facilities provided. She also expresses her gratitude to Prof. Roberto Buonanno for his valuable explanations and mentoring, and Prof. Atila Özgüç for his continuous support.
Appendix
The complete list of input species taken into account in calculations are given in Table A1.
Table A1. The Species Taken into Account in Calculations
| 1. Elemental and Uncategorized Species | ||||
| Al | CaZn | Fe3Si | MnSi | Sc |
| Al3Ni | CaZn2 | Fe5Si3 | Na | Sc5Si3 |
| Al3Ni2 | Co | FeSi | Na3AlH6 | Si |
| Al3Ti | Co2Al9 | FeSi2 | NaAlH4 | Si2H6 |
| Al5Co2 | Co2Si | FeTi | NaH | Sr |
| AlH3 | CoAl | K | Ni | SrH2 |
| AlH4K | CoAl3 | KAlH4 | Ni2H | Ti |
| AlNi3 | CoSi | KH | Ni2Si | Ti5Si3 |
| AlTi | CoSi2 | Ni3Ti | TiCr2 | |
| C | Cr | Mg | Ni7Si13 | TiH |
| C60 | Cr3Si | Mg2Ni | NiAl | TiH2 |
| C70 | Cr5Si3 | Mg2Si | NiH | TiSi |
| Ca | CrH | Mg48Zn52 | TiSi2 | |
| Ca2Si | CrSi | MgCuAl | NiSi | V |
| CaAl2 | CrSi2 | MgH2 | NiSi2 | V3Si |
| CaAl4 | Cu | MgNi2 | NiTi | V5Si3 |
| CaH2 | Cu2Mg | MgZn2 | NiTi2 | VSi2 |
| CaMg2 | CuMg2 | Mn | P | Zn |
| CaSi | Fe | Mn3Si | P4 | ZnCo3 |
| CaSi2 | Fe2Ti | Mn5Si3 | S | |
| 2. Gases | ||||
| (AlS)2 | Ca | FeO2 | N2O5 | S6 |
| Al2 | Ca2 | FeS | N3 | S7 |
| Al2C2 | CaCoO2 | H2 | Na2(CN)2 | S8 |
| Al2O | CaH | H2CN | Na2 | Sc2 |
| Al2O2 | CaO | H2CN2 | Na2O | Sc2O |
| Al2O3 | CaS | H2CNN | Na2O2 | Sc2O2 |
| Al2S | CCN | H2O | Na2O2H2 | ScC2 |
| AlC | CH | H2O2 | Na2SO4 | ScO |
| AlC2 | CH2 | H2S | NaCN | ScO2 |
| AlC3H9 | CH2 | H2S2 | NaCu | |
| AlCuS | CH2N2 | H2SiO3 | NaH | ScS |
| AlCuS2 | CH2N4 | H2SO | NaNO2 | Si2 |
| AlH | CH3 | H2SO4 | NaNO3 | Si2C |
| AlH2 | CH3N | H3N2 | NaO | Si2H4 |
| AlH3 | CH3N2 | H3N2 | NaOH | Si2H6 |
| AlN | CH3N2CH3 | HCCN | NaOP | Si2N |
| AlO | CH3N3 | HCN | NaPO2 | Si2O2 |
| AlO2 | CH3NHNHCH3 | HCNH | NaPO3 | |
| AlP | CH4 | HN3 | NCN | Si2P |
| AlP2 | CH4N | HNC | NH | Si2P2 |
| AlS | CH4N2 | HNO | NH2 | Si3 |
| AlS2 | CH5N | HNO2 | NH2NO2 | Si4 |
| C10N | CH5N2 | HNO3 | NH2OH | SiC |
| C10N2 | CH5N3 | HO2 | NH3 | SiC2 |
| C11N | CH6N2 | HO2S | Ni(C5H5)2 | SiH |
| C2 | CHN2 | HPO | Ni(OH)2 | SiH2 |
| C2H | CN | HS | Ni2 | SiH3 |
| C2H2 | CN2 | HS2 | SiH4 | |
| C2H2N | CNN | HSO | NiH | SiN |
| C2H2N4 | CO | HSO3 | NiO | SiO |
| C2H3 | CO2 | HSOH | NiOH | SiO2 |
| C2H3N | Co2 | K2(CN)2 | NiS | SiOOH |
| C2H4 | CoH | K2 | NO | SiP |
| C2H4N4 | CoO | K2(OH)2 | NO2 | SiP2 |
| C2H5 | CoPO2 | K2O | NO3 | SiS |
| C2H5N | CoPO3 | K2O2 | O2 | SiS2 |
| C2H5N3 | COS | K2S | O3 | SN |
| C2H6 | CoS | K2SO4 | OH | SO |
| C2H6N | CP | KCN | P2 | SO2 |
| C2H7N | CP2 | KH | P2O3 | SO3 |
| C2H7N2 | Cr(CO)6 | P2O4 | SOH | |
| C2H8N2 | Cr2 | KNa | P2O5 | Sr(OH)2 |
| C2N | Cr2O | KNO2 | P2S3 | Sr2 |
| C2N2 | Cr2O2 | KNO3 | P3 | SrCoO2 |
| C2O | Cr2O3 | KO | P3O6 | SrH |
| C2P | CrH | KOH | P4 | SrO |
| C2P2 | CrN | KS | P4O10 | SrOH |
| C2S2 | CrO | Mg(OH)2 | P4O6 | SrS |
| C3 | CrO2 | Mg2 | P4O7 | Ti2 |
| C3N | CrO3 | MgH | P4O8 | TiH |
| C3O2 | CrPO2 | MgN | P4O9 | TiN |
| C4 | CrPO3 | MgO | P4S3 | TiO |
| C4N | CrS | MgOH | P4S4 | TiO2 |
| C4N2 | CrS2 | MgS | P4S5 | TiS |
| C5 | CS | MnH | P4S7 | TiS2 |
| C5N | CS2 | MnO | P5S3 | V2O3(OH)4 |
| C5N4 | Cu2 | MnO2 | PH | V4O10 |
| C6 | Cu2 | MnOH | PH2 | VN |
| C60 | Cu2S | MnS | PH3 | VO |
| C6N | CuH | N2 | PN | VO2 |
| C6N2 | CuO | N2H | PO | VS |
| C7 | CuS | N2H2 | PO2 | Zn(OH)2 |
| C70 | Fe(C5H5)2 | N2H4 | PS | ZnH |
| C7N | Fe(CO)5 | N2H4.H2O | S2 | ZnO |
| C8 | Fe2 | N2O | S2O | ZnOH |
| C8N | FeC17H16 | N2O2 | S3 | ZnS |
| C8N2 | FeH | N2O3 | S4 | |
| C9N | FeO | N2O4 | S5 | |
| 3. Carbides | ||||
| Al4C3 | Co2C | CrC24H36 | Mn15C4 | Ni(C5H5)2 |
| Al4SiC4 | Cr(C5H5)2 | Fe(C5H5)2 | Mn23C6 | Ni3C |
| AlC3H9 | Cr23C6 | Fe2C | Mn3C | SiC |
| AlC8H19 | Cr3C2 | Fe3C | Mn5C2 | SrC2 |
| AlC9H21 | Cr4C | Mg2C3 | Mn7C3 | TiC |
| CaC2 | Cr7C3 | MgC2 | MnC2 | V2C |
| Co(C5H5)2 | CrC16H20 | Mn(C5H5)2 | Na2C2 | VC |
| 4. Oxides | ||||
| (NaPO3)3 | CoTiO3 | K3PO4.7H2O | Na2S2O3 | Sr2SiO4 |
| (NH4)2SO4 | CoZnTiO4 | K4P2O7 | Na2S2O3.5H2O | Sr2TiO4 |
| (NH4)2SO4.3NH3 | Cr(CO)6 | K4P2O7.3H2O | Na2S2O7 | Sr2V2O7 |
| 12CaO.7Al2O3 | Cr2(SO4)3 | KH2PO4 | Na2SiO3 | Sr2VO4 |
| 2Ca2SiO4.CaCO3 | Cr2FeO4 | KHSO4 | Na2SiO3.5H2O | Sr3(VO4)2 |
| 2CaO.Al2O3 | Cr2O3 | KHSO5 | Na2SiO3.6H2O | Sr3Cr2O8 |
| 2CaO.Al2O3.SiO2 | Cr3O4 | KMnO4 | Na2SiO3.8H2O | Sr3Fe2O6 |
| 2CaO.Fe2O3 | Cr5O12 | KNaS2O7 | Na2SiO3.9H2O | Sr3SiO5 |
| 2CaO.SiO2 | Cr8O21 | KNO2 | Na2SO3 | Sr3Ti2O7 |
| 2CoO.SiO2 | CrO2 | KNO3 | Na2SO3.7H2O | Sr3V2O8 |
| 2CoO.TiO2 | CrO3 | KO2 | Na2SO4 | Sr4Ti3O10 |
| 2FeO.SiO2 | CrVO4 | KO3 | Na2SO4.10H2O | Sr7Fe10O22 |
| 2MnO.TiO2 | Cu(VO3)2 | KOH | Na2SO4.7H2O | SrAl2Si2O8 |
| 2Na2O.SiO2 | Cu2O | KOH.2H2O | Na2Ti6O13 | SrCO3 |
| 2NiO.SiO2 | Cu2O.Al2O3 | KOH.H2O | Na2V2O6 | SrCoO3 |
| 3Al2O3.2SiO2 | Cu2O.Fe2O3 | KPO3 | Na2V2O7 | SrCrO4 |
| 3CaO.2SiO2 | Cu2P2O7 | KVO4 | Na2ZnO2 | SrFe12O19 |
| 3CaO.Al2O3 | Cu2SO4 | Mg(NO3)2 | Na3HP2O7 | SrHPO4 |
| 3CaO.Al2O3.3SiO2 | Cu3(PO4)2 | Mg(NO3)2.2H2O | Na3PO4 | SrO |
| 3CaO.SiO2 | CuCO3 | Mg(NO3)2.6H2O | Na3PO4.12H2O | SrO.Al2O3 |
| 3CaO.V2O5 | CuCrO2 | Mg(OH)2 | Na3VO4 | SrO2 |
| 3MgO.2SiO2.2H2O | CuO | Mg(VO3)2 | Na4P2O7 | SrSiO3 |
| 3MgO.4SiO2.H2O | CuO.Al2O3 | Mg2P2O7 | Na4P2O7.10H2O | SrSO4 |
| 3Na2O.2SiO2 | CuO.Cr2O3 | Mg2SiO4 | Na4V2O7 | SrTi12O19 |
| 3SrO.Al2O3 | CuO.CuSO4 | Mg2TiO4 | Na5P3O10 | SrTiO3 |
| 3SrO.MgO.2SiO2 | CuO.Fe2O3 | Mg2V2O7 | Na5P3O10.6H2O | SrV2O6 |
| 4CaO.3TiO2 | CuSO4 | Mg3(PO4)2 | Na6P2O8 | SrVO3 |
| 4CaO.Al2O3.Fe2O3 | Fe(CO)5 | Mg3(VO4)2 | NaH2PO4 | Ti10O19 |
| 4Na2O.5TiO2 | Fe(VO3)2 | Mg3Si2O5(OH)4 | NaH2SiO4.7H2O | Ti20O39 |
| 4SrO.Al2O3 | FeO | Mg48Si34O85(OH)62 | NaH2SiO4.8H2O | Ti2O3 |
| 7MgO.8SiO2.H2O | Fe2(CO)9 | Mg4Si6O21H12 | NaH3P2O7 | Ti3O2 |
| Al2(SO4)3 | Fe2(SO4)3 | Mg4Si6O23H14 | NaHSO4 | Ti3O5 |
| Al2CO | Fe2Al4Si5O18 | Mg6Si4O10(OH)8 | NaHSO4.H2O | Ti4O7 |
| Al2O3 | Fe2O3 | Mg7Si8O22(OH)2 | NaMnO4 | Ti5O9 |
| Al2O3.2SiO2 | Fe2SiO4 | MgMn2O4 | NaNH4HPO4.4H2O | Ti6O11 |
| Al2O3.TiO2 | Fe2TiO4 | MgO | NaNO2 | Ti7O13 |
| Al2SiO5 | Fe2TiO5 | MgO2 | NaNO3 | Ti8O15 |
| Al4CO4 | Fe3(CO)12 | MgSiO3 | NaO2 | Ti9O17 |
| AlO | Fe3Al2Si3O12 | MgSO3 | NaO3 | TiNO |
| AlPO4 | Fe3O4 | MgSO3.3H2O | NaOH | TiO |
| Ca(PO3)2 | FeAl2O4 | MgSO3.6H2O | NaOH.H2O | TiO2 |
| Ca2P2O7 | FeCO3 | MgSO4 | NaPO3 | V2O3 |
| Ca2SiO4 | FeCr(VO4)2 | MgSO4.2H2O | NaV2O5 | V2O4 |
| Ca2V2O7 | FeO | MgSO4.4H2O | NaVO3 | V2O5 |
| Ca3(Al2Si2O8)3.CaCO3 | FeO | MgSO4.5H2O | NH2OH | V2O5.H2O |
| Ca3(PO4)2 | FeO.SiO2 | MgSO4.6H2O | NH4.H2PO4 | V3O5 |
| Ca3Cr2(SiO4)3 | FeO.TiO2 | MgSO4.7H2O | NH4HSO4 | V4O7 |
| Ca3Fe2Si3O12 | FePO4 | MgSO4.H2O | NH4NO2 | V5O9 |
| Ca3Si2O7 | FeSiO3 | MgTi2O5 | NH4NO3 | V6O11 |
| Ca3Si2O7.2CaCO3 | FeSO4 | MgTiO3 | NH4OH | V6O13 |
| Ca3SiO5 | FeTi2O5 | MgV2O6 | NH4VO3 | V7O13 |
| Ca3Ti2O7 | FeTiO3 | Mn(NO3)2 | Ni(NO3)2 | V8O15 |
| Ca3V2O8 | FeV2O4 | Mn(OH)2 | Ni(NO3)2.6H2O | VO |
| CaAl2SiO6 | FeVO4 | Mn(VO3)2 | Ni(OH)2 | VO2 |
| CaC2O4 | H2O | Mn2(SO4)3 | Ni(OH)3 | VOCO3 |
| CaCO3 | H2Si2O5 | Mn2O3 | Ni2O3.3H2O | VOSO4 |
| CaCrO4 | H2SiO3 | Mn2O3.3H2O | Ni2SiO4 | VOSO4.3H2O |
| CaFe(SiO3)2 | H2SO4 | Mn2SiO4 | Ni2TiO4 | VOSO4.5H2O |
| CaFe3O5 | H2SO4.2H2O | Mn3(PO4)2 | Ni3(VO4)2 | VOSO4.6H2O |
| CaFe5O7 | H2SO4.3H2O | Mn3O4 | NiMn2O4 | VOSO4.H2O |
| CaFeSiO3 | H2SO4.4H2O | MnO | NiO | Zn(NO3)2 |
| CaFeSiO4 | H2SO4.6.5H2O | MnO.OH | NiO.OH | Zn(NO3)2.2H2O |
| CaO | H2SO4.H2O | MnO.TiO2 | NiSO4 | Zn(NO3)2.4H2O |
| CaO.2Al2O3 | H3PO4 | MnO2 | NiSO4.4H2O | Zn(NO3)2.6H2O |
| CaO.6Al2O3 | H3PO4.0.5H2O | MnSiO3 | NiSO4.6H2O | Zn(OH)(NO3).H2O |
| CaO.Al2O3 | HNO3 | MnSO4 | NiSO4.7H2O | Zn(OH)2 |
| CaO.Al2O3.2SiO2 | KV2O5 | MnSO4.4H2O | NiSO4.H2O | Zn2SiO4 |
| CaO.Al2O3.SiO2 | K2H2P2O7 | MnSO4.5H2O | NiTi2O5 | Zn2TiO4 |
| CaO.Cr2O3 | K2HPO4 | MnSO4.7H2O | NiTiO3 | Zn3(OH)4(NO3)2 |
| CaO.Fe2O3 | K2Mg(SO4)2.4H2O | MnSO4.H2O | NiZnTiO4 | Zn3(PO4)2 |
| CaO.TiO2 | K2Mg(SO4)2.6H2O | MnTi2O5 | P2O5 | Zn3(VO4)2 |
| CaO.V2O5 | K2O | MnTiO2 | P4O10 | Zn5(OH)8(NO3)2.2H2O |
| CaO2 | K2O.2SiO2 | MnTiO3 | P4O8 | ZnCO3 |
| CaSiO3 | K2O.4SiO2 | N2O4 | PO2 | ZnCrO4 |
| CaSO3 | K2O.SiO2 | N2O5 | Sc(OH)3 | ZnFe2O4 |
| CaSO4 | K2O2 | Na2H2P2O7 | Sc(PO3)3 | ZnFeO4 |
| CaTiSiO5 | K2O3 | Na2HPO4 | Sc2O3 | ZnMn2O4 |
| CaV2O6 | K2O4 | Na2HPO4.12H2O | ScPO4 | ZnO |
| Co2(CO)8 | K2S2O7 | Na2HPO4.2H2O | ScPO4.2H2O | ZnO.2ZnSO4 |
| Co2TiO4 | K2S2O8 | Na2HPO4.7H2O | Si2N2O | ZnO.Al2O3 |
| Co3O4 | K2S4O6 | Na2O | SiO2 | ZnSiO3 |
| CoCO3 | K2SO3 | Na2O.2SiO2 | SO3 | ZnSO4 |
| CoO | K2SO3.H2O | Na2O.2TiO2 | Sr(NO2)2 | ZnSO4.2H2O |
| CoO.Al2O3 | K2SO4 | Na2O.3SiO2 | Sr(NO3)2 | ZnSO4.6H2O |
| CoO.Cr2O3 | K2SO4.2MgSO4 | Na2O.3TiO2 | Sr(NO3)2.4H2O | ZnSO4.7H2O |
| CoO.Fe2O3 | K2TiO3 | Na2O.TiO2 | Sr(OH)2 | ZnSO4.H2O |
| CoO.TiO2 | K2Zn3(P2O7)2.3H2O | Na2O2 | Sr2CrO4 | |
| CoSO4 | K3PO4 | Na2P2O6 | Sr2Fe2O5 | |
| 5. Phosphides | ||||
| AlP | CrP | K3P7 | Na3P7 | P4H2 |
| Ca3P2 | Cu3P | Mg3P2 | Ni2P | SiP |
| Co2P | CuP2 | Mn2P | Ni3P | Zn3P2 |
| CoP | Fe2P | Mn3P | Ni5P2 | ZnP2 |
| CoP3 | Fe3P | Mn3P2 | Ni6P5 | |
| Cr12P7 | FeP | MnP | NiP2 | |
| Cr3P | FeP2 | MnP3 | NiP3 | |
| 6. Sulfides | ||||
| Al2S3 | Fe2S | MnS2 | NiS | Ti2S |
| CaS | Fe2S3 | Na2S | P2S3 | TiS |
| Co3S4 | Fe7S8 | Na2S2 | P2S5 | TiS2 |
| Co9S8 | Fe9S8 | Na2S3 | P4S10 | TiS3 |
| CoS | FeS | Na2S4 | P4S3 | V2S3 |
| CoS2 | FeS2 | NaS | P4S5 | VS |
| Cr2S3 | K2S | NaS2 | P4S6 | VS4 |
| CrAl2S4 | K2S2 | NH4HS | P4S7 | ZnCr2S4 |
| CrS | K2S3 | Ni3S2 | Sc2S3 | ZnS |
| Cu2S | K2S4 | Ni3S4 | ScS | |
| Cu5FeS4 | K2S5 | Ni6S5 | SiS | |
| CuFeS2 | MgS | Ni7S6 | SiS2 | |
| CuS | MnS | Ni9S8 | SrS | |
| 7. Nitrides | ||||
| AlN | CuCN | KCN | NaCN | P3N5 |
| C2N4H4 | CuN3 | KN3 | NaN3 | ScN |
| Ca3N2 | Fe2N | KNH2 | NaNH2 | Si3N4 |
| CaCN2 | Fe4N | Mg3N2 | NaNH3 | Sr3N2 |
| Co3N | K3Co(CN)6 | Mn3N2 | NH4N3 | TiN |
| Cr2N | K3Fe(CN)6 | Mn4N | Ni(CN)2 | VN |
| CrN | K4Fe(CN)6 | Mn5N2 | Ni3N | Zn3N2 |











