Abstract
The potential energy surfaces for the formation of cytosine (Cyt) and a protonated cytosine (CytH+) from reactions of urea with cyanoacetylene (CA), cyanoacetaldehyde (CAA), or their protonated ions, CAAH+ and CAH+ with or without H2O, have been determined from quantum chemical calculation using the CBS-QB3 method. The overall activation energies of the formation of Cyt or CytH+ from urea + CA, urea + CAA and, urea + CAH+ are 127, 211, and 31 kJ mol−1, respectively, which are too high for the thermal reactions to occur in the interstellar medium (ISM). The barrierless reaction pathways have been proposed for the formation of CytH+ from urea + CAH++ H2O and urea + CAAH+. A kinetic analysis shows that the synthesis of Cyt through the formation of CytH+ from urea, CAH+, and H2O would be possible in the ISM.
Export citation and abstract BibTeX RIS
1. Introduction
Although the building blocks of life, such as amino acids, nucleobases, and lipids, have not been detected in interstellar space, their syntheses from simpler molecules detected in the interstellar medium (ISM) have been extensively studied for the last seven decades since the Miller experiment (Miller 1953). These studies include syntheses of the nucleobases (adenine, guanine, cytosine (Cyt), uracil, and thymine), the building blocks of RNA and DNA, from organic molecules as well as from small inorganic molecules. The reaction pathways to form the nucleobases have been well summarized in a recent review (Pearce & Pudritz 2015), covering studies up to 2014. Many experimental and theoretical studies have focused mainly on adenine (Oró 1960, 1961; Ferris & Hagan 1984; Orgel 2004; Glaser et al. 2007; Jung & Choe 2013).
Experimental efforts have been initiated to synthesize nucleobases, including Cyt, from smaller molecules under prebiotic terrestrial conditions. Adenine has been synthesized from HCN under basic conditions (Oró 1960, 1961). Cyt, adenine, and guanine have been formed by heating a mixture of CO, H2, and NH3 (Hayatsu et al. 1968; Yang & Oró 1971) in the presence of some catalysts. Cyt, adenine, and/or thymine have been synthesized from formamide (NH2CHO) depending on the catalyst (Saladino et al. 2001; Kumar & Sharma 2014). Cyt, adenine, guanine, and uracil have been synthesized from formamide by irradiation of high-power laser (Ferus et al. 2015). Reaction of urea with cyanoacetylene (HCCCN, CA; Ferris et al. 1968) or cyanoacetaldehyde (HCOCH2CN, CAA) (Robertson & Miller 1995; Nelson et al. 2001) has rendered Cyt and uracil in aqueous solution. Guanidine (Cleaves et al. 2006) or cyanate (Sanchez et al. 1966; Ferris et al. 1968) has been used for the formation of Cyt by reactions with CAA or CA, respectively, in an aqueous solution.
Experiments for the syntheses of nucleobases under space conditions have been performed (Carota et al. 2015). Cyt and uracil have been formed in the spark discharge experiment of the freeze-thaw 0.1 M urea solution in the presence of N2, H2, and CH4 (Menor-Salván et al. 2009). The authors suggested a reaction pathway for the formation of Cyt involving CA, CAA, and urea. Photoirridation of pyrimidine (C4H4N2) in the interstellar ice analogs has rendered Cyt, uracil, and/or thymine (Nuevo et al. 2009; Materese et al. 2013; Nuevo & Sandford 2014). Recently, ultraviolet photoirradiation to the interstellar ice analog comprising H2O, CO, NH3, and CH3OH at 10 K has generated Cyt, uracil, thymine, and adenine (Oba et al. 2019).
Theoretical investigations have been made to elucidate the mechanisms of prebiotic syntheses of nucleobases using quantum chemical calculations. Wang and Bowie (Wang & Bowie 2012) proposed mechanisms for the formation of Cyt from urea and other molecules such as CCCNH, CA, and CAA with the assistance of H2O. The reaction pathway to form Cyt from CAA and guanidine has been proposed in this laboratory (Choe 2020). Generally, however, such bimolecular reactions between neutral molecules have activation energies of several hundred kilojoules per mole. For the gas-phase bimolecular reactions to occur thermally within the timescale of chemical revolution of the typical dense interstellar clouds, the activation energy should be zero (Herbst 2001) or at most a few kilojoules per mole (Yim & Choe 2012; Jung & Choe 2013). Thus, most of the mechanisms reported previously for the reactions between neutral molecules would be more suitable in the prebiotic syntheses on Earth. To search for the pathways for the Cyt synthesis with less activation energy, theoretical mechanisms for several ion–molecule reactions or radical–molecule reactions have been proposed. The free radicals such as NH2, H, OH, CH2CHO, and/or CCH have been used as reactants for the formation of Cyt in the pathways suggested by Nguyen and coworkers (Jeilani et al. 2015, 2016; Nguyen et al. 2015). NH2 was one of reactants in the pathway for the formation of Cyt proposed by Kaur and Sharma (Kaur & Sharma 2019). However, all these reactions have activation energies of a few tens of kilojoules per mole. A barrierless reaction pathway has been proposed for the formation of Cyt, in which several species such as CA, NH, NCO−, 3H, and H+ have been used as reactants or a catalyst (Gupta et al. 2013). Most of the proposed mechanisms are for the syntheses of Cyt in the prebiotic Earth environments, and plausible mechanisms in space conditions have not been proposed to our knowledge.
There is no evidence for the prebiotic synthesis of Cyt in an extraterrestrial environment. Cyt and thymine have never been discovered in any meteorite, unlike the other three nucleobases (Pearce & Pudritz 2015). Although none of the nucleobases have been found in the ISM, their plausible prebiotic precursors, such as HCN (Snyder & Buhl 1971), cyanomethanimine (HNCHCN; Zaleski et al. 2013; Rivilla et al. 2019), formamide (Rubin et al. 1971), CA (Turner 1971; Morris et al. 1976), and urea (Jiménez-Serra et al. 2020) have been detected toward the interstellar clouds. Interestingly, Z-cyanomethanimine, recently detected toward the G+0.693−0.027 giant molecular cloud (Rivilla et al. 2019), is an intermediate proposed for the formation of adenine from HCN (Chakrabarti & Chakrabarti 2000; Jung & Choe 2013; Rivilla et al. 2019).
Urea and CA are good candidates as the reactants for the prebiotic Cyt synthesis in interstellar space because they have been found in the interstellar cloud. Urea was recently detected toward the hot core Sagittarius B2(N1) (Sgr B2(N1)) (Belloche et al. 2019) and a Giant Molecular Cloud in the center of the Milky Way (G+0.693−0.027) (Jiménez-Serra et al. 2020). CA was detected widely and abundantly in many different regions in the ISM including Sgr B2 and the Taurus molecular cloud 1 (TMC-1; Turner 1971; Morris et al. 1976). Additionally, because the molecular formula of Cyt, C4H5N3O, is the sum of those of urea (CH4N2O) and CA (HC3N), no further reactants are needed and no side products are formed. In this study, we examined the pathway for the reaction of urea with CA to form Cyt (reaction I in Figure 1). Although CAA has not been found in the ISM yet, its presence in the ISM is highly probable because it is formed easily by the association reaction between CA and H2O, an interstellar molecule (Cheung et al. 1969), and it has been mentioned by several researchers as a plausible prebiotic precursor of Cyt (Robertson & Miller 1995; Nelson et al. 2001; Menor-Salván et al. 2009; Powner et al. 2009; Jiménez-Serra et al. 2020). Therefore, we also examined the pathway for the reaction of urea with CAA to form Cyt (reaction II in Figure 1). These two reactions between neutral molecules have been investigated experimentally as mentioned above. To find pathways for the barrierless reactions, we examined the reactions of urea with a protonated CA (HCCCNH+, CAH+), CAH+ and a catalytic H2O, or a protonated CAA (HCOHCH2CN+, CAAH+) to form a protonated Cyt (CytH+) (reactions III–V in Figure 1). It should be noted that CAH+ is also an interstellar species, detected toward several clouds including TMC-1 (Kawaguchi et al. 1994) and Sgr B2 (Turner et al. 1990; Kawaguchi et al. 1994), and in the L1544 prestellar core (Quénard et al. 2017). The best potential energy surfaces (PESs) for these five reactions were determined using the quantum chemical calculation and used for the discussion for the possibility of their occurrence in the ISM.
Figure 1. Reactions for the formation of Cyt and CytH+ from urea investigated in this study. H2O in reaction IV is a catalyst.
Download figure:
Standard image High-resolution image2. Computational Methods
Quantum chemical calculation was performed with the Gaussian 16 program based on the molecular orbital theory (Frisch et al. 2016) to obtain the PESs for reactions I–V in the gas phase. The geometries of reactants, intermediates, and products were optimized at the B3LYP level (Becke 1993) of the density functional theory using the 6-311G(d,p) basis set. Transition state (TS) geometries connecting the minima were examined and checked by calculating intrinsic reaction coordinates at the B3LYP/6-311G(d,p) level. For better accuracy of energies, the complete basis set (CBS-QB3) model calculation (Montgomery et al. 1999) was performed.
3. Results and Discussion
Several reaction pathways were obtained for reactions I–V. We present only the individual lowest-energy reaction pathways. All energies presented here are the CBS-QB3 energies at 0 K, including zero-point vibrational energies. Energies of species relative to the sum of energies of individual reactants of reactions I–V, are presented in the respective potential energy diagrams. All the reactions were exoergic, indicating that they are thermodynamically favorable. The reaction energies (ΔErs) of reactions I–V were −208, −71, −411, −411, and −306 kJ mol−1, respectively.
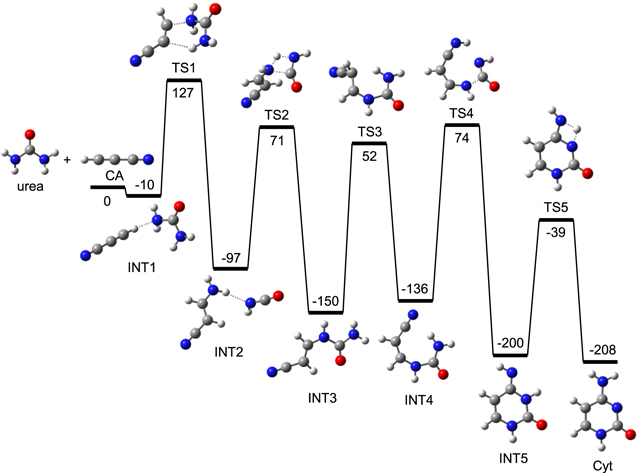
3.1. Formation of Cyt from Urea and CA (Reaction I)
To construct the backbone of Cyt by the combination of urea and CA, two covalent C–N bonds should be finally formed between each of two N atoms of urea and each of two nonneighboring C atoms of CA. Several H arrangement steps are needed to completely form Cyt. Initially, urea and CA forms a van der Waals molecule, intermediate 2 (abbreviated as INT2), through another van der Waals molecule, INT1 (Figure 2). In this step, an NH2 moiety and an H of urea move toward two C atoms of CA, and finally a C–N bond is cleaved and a new C–N bond is formed through a six-membered TS, TS1. TS1 is at 127 kJ mol−1 above the reactants, the highest in this pathway. Through a four-membered TS, TS2, INT2 isomerizes to INT3, where a C–N bond is formed. After forming a rotamer INT4, ITN3 isomerizes to a six-membered ring intermediate, INT5, where another C–N bond is formed. A subsequent H shift forms finally Cyt. As shown in Figure 2, the overall activation energy of reaction I is 127 kJ mol−1. In the study of Wang and Bowie (Wang & Bowie 2012), the overall activation Gibbs energy of the same reaction was higher than 200 kJ mol−1 without reporting the pathway data. This suggests that the present pathway is better than the previous one. The lowered activation barrier is mainly due to the relatively stable TS of the rate-limiting step, TS1, which having a six-membered structure. Compared to this, the activation barrier (168 kJ mol−1) of the step INT2 → INT3, occurring through the four-membered TS, TS2, is higher.
Figure 2. Potential energy (kJ mol−1) diagram for reaction I, derived from the CBS-QB3 calculation.
Download figure:
Standard image High-resolution image3.2. Formation of Cyt from Urea and CAA (Reaction II)
Reaction II starts through a van der Waals molecule, INT6, comprising urea and CAA (Figure 3). A covalent C–N bond is formed with an H rearrangement in the step INT6 → INT7. H2O is eliminated from INT7 through a relatively less stable four-membered TS, TS7, to form INT9. INT 9 is a conformer of INT3 shown in Figure 2, which forms another conformer INT4. The subsequent steps from INT4 to form Cyt are the same as reaction I. The overall activation energy of reaction II is 211 kJ mol−1, much higher than that of reaction I. This strongly suggests that reaction I is much more favored than reaction II assuming the same concentrations of CA and CAA. Alternatively, reaction I may occur through reaction II after the formation of CAA from CA and H2O. Horn et al. (2008) reported a theoretical PES for the reaction CA + H2O → CAA, calculated at the MP2/6-311++G(d,p) level. The overall activation energy and ΔEr were 216 and −122 kJ mol−1, respectively. Combining their data and our data for reaction II, the overall activation energy of this alternative pathway for reaction I would become at approximately 200 kJ mol−1, much higher than that (127 kJ mol−1) of the present pathway shown in Figure 2. However, the overall activation energy of 127 kJ mol−1 is too high for the reaction to occur thermally in the gas phase of the ISM.
Figure 3. Potential energy (kJ mol−1) diagram for reaction II, derived from the CBS-QB3 calculation.
Download figure:
Standard image High-resolution image3.3. Formation of CytH+ from Urea and CAH+ without and with H2O (Reactions III and IV)
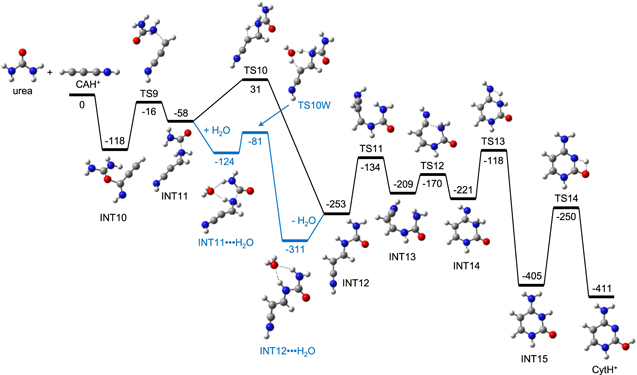
The association of urea and CAH+ forms an ion–molecule adduct, INT10, in the first step of reaction III (Figure 4). INT10 rearranges to INT11 by an O–C bond cleavage and forming a C–N bond. A subsequent H rearrangement occurs through a four-membered TS, TS10, to form INT12. TS10 is at the energy of 31 kJ mol−1 relative to the reactants, the highest among the TSs in this pathway. The next steps are similar to those from INT3 in reaction I. A cis–trans isomerization, a ring formation, and some H rearrangements are followed to form finally CytH+. All the TSs but TS10 lie below the reactants. The overall activation energy of this pathway is 31 kJ mol−1, too high for this reaction to occur thermally within the timescale of chemical revolution of the typical dense interstellar clouds.
Figure 4. Potential energy (kJ mol−1) diagrams for reactions III and IV, derived from the CBS-QB3 calculation. Reactions III and IV occur through TS10 and TS10W, respectively.
Download figure:
Standard image High-resolution imageIt is well-known that the barrier of an H rearrangement occurring through a relatively unstable four-membered TS is lowered by a catalytic H2O (Wolfe et al. 1995; Wang & Bowie 2012; Choe 2017, 2018; Lee & Choe 2017). We found that the barrier of the step INT11 → INT12 was significantly lowered when H2O was used as a catalyst. After the formation of an adduct INT11 ⋯ H2O by addition of H2O to INT11, an H is rearranged to form INT12 and H2O, through a relatively stable six-membered TS, TS10W. TS10W is at the energy of −81 kJ mol−1 relative to urea + CAH+ + H2O and at −23 kJ mol−1 relative to INT11+H2O. Then, reaction IV can occur without overall activation energy.
3.4. Formation of CytH+ from Urea and CAAH+ (Reaction V)
Urea and CAAH+ forms an ion–molecule complex, INT16, and then H2O is eliminated through a four-membered TS, TS15, to form INT18 (Figure 5). An H of INT18 shifts from C to O through a six-membered TS, TS16, to form INT19. After the formation of a rotamer of INT19, INT20, a six-membered ring intermediate, INT21, is formed, which isomerizes finally to CytH+ via an H shift. It should be noted that reaction V occurs without overall activation energy.
Figure 5. Potential energy (kJ mol−1) diagram for reaction V, derived from the CBS-QB3 calculation.
Download figure:
Standard image High-resolution image3.5. Which Reactions Are Possible in Interstellar Space?
As mentioned in the 1, the activation energy should be at most a few kilojoules per mole for a bimolecular gas-phase reaction to occur thermally in interstellar space when considering the timescale of chemical revolution of the typical dense interstellar clouds of 106 yr. Reactions IV and V satisfy this criterion. The other reactions with high overall activation energies are dismissed in the prebiotic Cyt synthesis in the interstellar gas phase. The next criterion for the possibility of the synthesis in interstellar space is the concentrations of reactants because the rate of the reaction depends on the concentrations.
Consider a bimolecular ion–molecule reaction A+ + B → P+. Its rate equation is given by
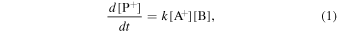
where k is the rate constant and [i] is the concentration, or density, of a species i. For a thermal reaction occurring at temperature T, k is usually estimated using the Arrhenius equation
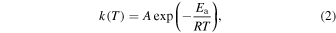
where A is the pre-exponential factor, Ea is the activation energy, and R is the gas constant. For reactions I, II, and III, k becomes extremely small because Ea ≫ RT. For a bimolecular reaction without activation energy such as reactions IV and V, k is equal to A. When [A+] ≪ [B], the above second-order equation becomes a pseudo-first-order equation as follows:
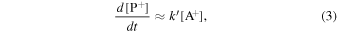
where k' = k[B].
For the estimation of the rate of reaction IV, the several steps are simplified as the following two bimolecular reaction steps.
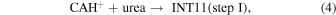
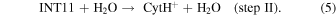
Because CAH+, urea, and H2O were all detected toward Sgr B2, the densities measured toward Sgr B2 will be used in this estimation. Consider step I first. The reported urea/H2 density ratio, [urea]/[H2], in Sgr B2(N1) is 4 × 10−8–8 × 10−8, depending on the chemical model (Belloche et al. 2019). Taking its minimum value and the reported density of H2 in Sgr B2(N1), ∼1 × 108 cm−3 (Belloche et al. 2008, 2016), the density of urea is estimated to be ∼4 cm−3. From the reported column densities of CAH+ and H2 in Sgr B2, 3.6 × 1012 and 2 × 1024 cm−2, respectively (Turner et al. 1990), [CAH+]/[H2] is estimated to be ∼2 × 10−12, and thus [CAH+]/[urea] ≈ 5 × 10−5 and the condition [CAH+] ≪ [urea] is fulfilled. It should be noted that the comparison may not be perfect because the interferometric observations of urea (Belloche et al. 2019) and the single-dish observations of CAH+ (Turner et al. 1990) are likely tracing different gas components at different spatial scales. This may trigger a new analysis of CAH+ in an interferometric observation with higher angular resolution. Using a typical value of k for an ion–molecule reaction, 1 × 10−9 cm3 s−1 (Herbst 2001), k' (=k[urea]) becomes ∼4 × 10−9 s−1. The half lifetime of CAH+,  = ln2/k', against the formation of INT11 is estimated to be ∼5 yr. Step II occurs without overall activation energy as shown in Figure 4. The reported ratio [H2O]/[H2] in Sgr B2(N) is ∼1 × 10−5 (Gensheimer et al. 1996). Then, the density of H2O is estimated to be ∼1 × 103 cm−3, much higher than urea, indicating that step II would be much faster than step I. After a similar calculation, we acquire the half lifetime of INT11 against the formation of CytH+, ∼2 × 10−2 yr. This suggests that step I, the association step of urea and CAH+, is the rate-limiting step in reaction IV. The actual rate would be slower than the present estimation because we ignored the backward reaction steps. Nevertheless, the present estimation suggests that reaction IV would occur much faster than the timescale of chemical revolution of the typical dense interstellar clouds.
= ln2/k', against the formation of INT11 is estimated to be ∼5 yr. Step II occurs without overall activation energy as shown in Figure 4. The reported ratio [H2O]/[H2] in Sgr B2(N) is ∼1 × 10−5 (Gensheimer et al. 1996). Then, the density of H2O is estimated to be ∼1 × 103 cm−3, much higher than urea, indicating that step II would be much faster than step I. After a similar calculation, we acquire the half lifetime of INT11 against the formation of CytH+, ∼2 × 10−2 yr. This suggests that step I, the association step of urea and CAH+, is the rate-limiting step in reaction IV. The actual rate would be slower than the present estimation because we ignored the backward reaction steps. Nevertheless, the present estimation suggests that reaction IV would occur much faster than the timescale of chemical revolution of the typical dense interstellar clouds.
For reaction V, the rate estimation is the same as that in step I when assuming that the reaction occurs through one step and CAAH+ is present in Sgr B2(N). However, because CAA and CAAH+ have not been found in the ISM yet, we should consider a reaction to form CAAH+ starting the species existing in the ISM. The most probable reaction is the formation of CAA from CA and H2O, followed by protonation when considering only thermal reactions. However, as mentioned in Section 3.2, the formation of CAA from CA and H2O in the ISM is highly improbable due to its activation energy being higher than 200 kJ mol−1 according to the calculation by Horn et al. (2008). Consequently, the thermal reaction to form CytH+ from CA, H2O, H+, and urea via CAAH+ would not be favored in the ISM although reaction V can occur without activation energy. One possible route for the formation of CAAH+ is the reaction initiated by photolysis of smaller interstellar species followed by thermal reactions.
CytH+ may be deprotonated to form Cyt by dissociative recombination with an electron or by proton transfer to another molecule, M
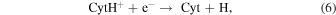
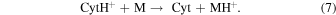
Reaction (6) is highly exoergic and hence would occur easily. Its ΔEr calculated at the CBS-QB3 level is −365 kJ mol−1 . Conversely, the occurrence of reaction (7) is much less probable because Cyt is more basic than most of abundant molecules in interstellar space. For example, reaction (7) is endoergic by 99 or 267 kJ mol−1 when M is NH3 or H2O, respectively.
4. Conclusion
Mechanisms for the reactions, urea + CA → Cyt, urea + CAA → Cyt + H2O, urea + CAH+ → CytH+, urea + CAH+ + H2O → CytH+ + H2O, and urea + CAAH+ → CytH+ + H2O, have been proposed using the CBS-QB3 calculation. Their overall activation energies are 127, 211, 31, 0, and 0 kJ mol−1, respectively. Although the formation of CytH+ from urea and CAAH+ is possible along the proposed barrierless reaction pathway, its occurrence in the ISM is doubtable at the moment because CAA or CAAH+ has not been detected in the ISM yet. A barrierless reaction pathway has been obtained for urea + CAH+ → CytH+ when assisted by a catalytic H2O. We have shown that it is possible to form Cyt from interstellar urea, CAH+, and H2O in the hot core Sgr B2 (N1) by a kinetic estimation. This possibility is further supported by the detection of the proposed precursors of Cyt in several different regions with quite different physical properties, a hot core surrounding massive protostar (Sgr B2 (N1)), a quiescent cloud without signs of star formation (G+0.693−0.02), and a low-mass prestellar core (L1544).
This study was supported by the National Institute of Supercomputing and Network/Korea Institute of Science and Technology Information, with supercomputing resources including technical support (KSC-2018-CHA-0023).