Summary
Mesenteric afferent nerves convey information from the gastrointestinal tract towards the brain regarding normal homeostasis as well as pathophysiology. Gastrointestinal afferent nerve activity can be assessed by mounting isolated intestinal segments with attached afferent nerves into an organ bath, isolating the nerve, and assessing basal as well as stimulated activity.
Abstract
Afferent nerves not only convey information concerning normal physiology, but also signal disturbed homeostasis and pathophysiological processes of the different organ systems from the periphery towards the central nervous system. As such, the increased activity or 'sensitization' of mesenteric afferent nerves has been allocated an important role in the pathophysiology of visceral hypersensitivity and abdominal pain syndromes.
Mesenteric afferent nerve activity can be measured in vitro in an isolated intestinal segment that is mounted in a purpose-built organ bath and from which the splanchnic nerve is isolated, allowing researchers to directly assess nerve activity adjacent to the gastrointestinal segment. Activity can be recorded at baseline in standardized conditions, during distension of the segment or following the addition of pharmacological compounds delivered intraluminally or serosally. This technique allows the researcher to easily study the effect of drugs targeting the peripheral nervous system in control specimens; besides, it provides crucial information on how neuronal activity is altered during disease. It should be noted however that measuring afferent neuronal firing activity only constitutes one relay station in the complex neuronal signaling cascade, and researchers should bear in mind not to overlook neuronal activity at other levels (e.g., dorsal root ganglia, spinal cord or central nervous system) in order to fully elucidate the complex neuronal physiology in health and disease.
Commonly used applications include the study of neuronal activity in response to the administration of lipopolysaccharide, and the study of afferent nerve activity in animal models of irritable bowel syndrome. In a more translational approach, the isolated mouse intestinal segment can be exposed to colonic supernatants from IBS patients. Furthermore, a modification of this technique has been recently shown to be applicable in human colonic specimens.
Introduction
Sensory signaling and pain perception is a complex process that results from an intricate interplay between afferent nerves, spinal neurons, ascending and descending facilitatory and inhibitory pathways and several different brain regions. As such, changes at one or more of these levels may result in altered sensory signaling and visceral pain in disease states. To study all these different aspects of sensory signaling multiple techniques have been developed ranging from single cell experiments (e.g., calcium imaging on neurons) to whole animal models (e.g., behavioral responses such as the visceromotor response). The technique described in this paper allows researchers to specifically assess afferent nerve activity in vitro from an isolated segment of small bowel or colon in rodents. In short, an isolated gastrointestinal segment (usually jejunum or colon) is mounted in a purpose-built recording chamber perfused with a physiological Krebs solution. The splanchnic nerve is dissected free and connected to an electrode allowing registration of afferent neuronal activity in splanchnic or pelvic afferent nerves. Nerve activity can be recorded basally or in response to increasing intraluminal pressures and/or pharmacological compounds that can be applied either directly into the recording chamber (serosally), or via the intraluminal perfusate (mucosally) to assess their effect on afferent discharge 1-6. Of note, splanchnic nerves also contain efferent fibers and viscerofugal afferents in addition to the sensory afferents. One of the major advantages of ex vivo splanchnic nerve recording is the fact that researchers can quantify nerve activity without modulation or input from the central nervous system, allowing one to study the direct effect of locally applied compounds on nerve activity. Furthermore, monitoring of vital parameters, as is necessary using the in vivo approach (see below), is no longer relevant. In vitro splanchnic recording is finally much less time-consuming than its in vivo counterpart.
Afferent neuronal activity in response to other stimuli, such as mucosal stroking, probing using von Frey hairs or stretching of the segment, can be studied in a modified experimental setup in which the intestinal tissue is pinned down and opened longitudinally (which is in contrast to our setup using an intact segment), as was described in a previous issue 7,8. In addition, only recently, a technique was described to study colonic afferent nerve activation in the colonic wall itself via calcium imaging, again using a pinned down, longitudinally opened segment 9.
An alternative version of this in vivo technique consists out of measuring neuronal activation near the afferent's entry into the spinal cord. In short, the sedated animal is placed in the prone position, exposing the lumbosacral spinal cord to which the afferent nerve of interest projects by means of laminectomy, constructing a paraffin-filled well using the skin of the incision and draping the dorsal rootlet over a platinum bipolar electrode 10,11. This technique furthermore allows researchers to characterize fibers based upon their conduction velocity, and distinguish unmyelinated C-fibers from thinly myelinated Aδ-fibers. Furthermore, dorsal rootlets exclusively contain sensory afferent fibers, in contrast to the mixed afferent and efferent splanchnic nerves mentioned previously.
Recording afferent nerve discharge in vitro from isolated gut segments can also be done using human specimens, as two research groups independently published first-in-man manuscripts recording colonic afferent nerve activity in human resection specimens 12,13. The implementation of this technique could result in a more readily translation of murine data to the humane state, and could allow researchers to easily identify drugs targeting the sensitized sensory nerve. The clinical importance of characterizing the afferent nerve activity, as well as the discovery of new therapeutic reagents that target exorbitant afferent nerve activity, has been elaborately discussed by many experts in the field 14-19.
The aforementioned in vitro technique complements the more commonly known in vivo measurement of afferent nerve activity. During in vivo neuronal activity measurement, nerve activity can be measured directly in the sedated animal during which the segment of interest is identified and subsequently intubated, and a liquid paraffin-filled well is constructed using the abdominal wall and skin of the rodent 20. The afferent nerve of interest is then identified, sectioned and placed on a bipolar platinum electrode, allowing neuronal activity measurement. This technique allows the researcher to modulate afferent nerve activity in living albeit sedated animals; as such, one can study neuronal activity responding to interferences such as luminal distension or the intravenous administration of a compound.
Translational research nowadays mainly focuses on the application of human-derived supernatants (e.g., from colonic biopsies, cultivated peripheral blood mononuclear cells, etc.) on jejunal and/or colonic mouse afferents 21,22. Researchers can directly apply supernatants either into the organ bath or into the intraluminal solution that perfuses the bowel segment, so that differential effects of serosal versus mucosal application can be studied on afferent nerve discharge. As such, it was shown that colonic mucosal biopsy supernatans from patients with irritable bowel syndrome can cause hypersensitivity in mouse colonic afferents, guinea pig submucous neurons and mouse dorsal root ganglion neurons 21,23,24.
Finally, recording neuronal activity is not restricted to the mesenteric and/or pelvic neurons innervating the gastrointestinal tract. Others have demonstrated that nerve recordings can be performed in afferents supplying the knee joint 25, whereas others have characterized bladder afferent nerve activity as well 26-28, and demonstrated that pelvic afferents from the bladder as well the gastrointestinal tract converge, possibly resulting in neuronal crosstalk 29.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
All animal experiments described below were approved by the Committee for Medical Ethics and the use of Experimental Animals at the University of Antwerp (file number 2012-42).
1. Tissue Preparation of Jejunal and Colonic Afferent Nerves
- Preparation of the jejunal afferent nerve
- Perform rodent euthanasia of the adolescent or adult rodent that has been approved prior to the experiment by the local Ethical Committee (e.g., terminal sedation followed by cardiac puncture, cervical dislocation, etc.).
NOTE: We used cervical dislocation to sacrifice the animals thus resulting in experiments without the need for further anesthesia or post-surgical care as tissues are further processed in vitro.
NOTE: Age has been shown to attenuate mesenteric afferent mechanosensory functions 30, we therefore advise researchers to adhere to a specific age-group for the duration of a single experiment. - Place the sacrificed laboratory animal in the supine position and perform an abdominal midline incision through the skin and abdominal muscle layer using a scalpel, extending from the xyphoid process until the pubic bone.
- Bathe the abdominal cavity with cold Krebs solution in order to prevent the intra-abdominal tissues from drying out (Krebs composition: 120.03 mM NaCl, 6.22 mM KCl, 1.57 mM NaH2PO4, 15.43 mM NaHCO3, 1.21 mM MgSO4, 11.52 mM D-glucose and 1.52 mM CaCl2).
- Rapidly excise the entire jejunum using sharp scissors by excising approximately 20 cm of the small bowel starting immediately distally of the duodenojejunal flexure, taking care not to damage surrounding structures and keeping the bowel's mesentery, which contains jejunal blood vessels and afferent nerves, intact.
NOTE: For the mere jejunal dissection in the abdominal cavity, one does not need to use a stereomicroscope, as this can be easily visualized with the naked eye. - Place the excised jejunum in ice-cold Krebs solution and keep on ice, while oxygenating the Krebs solution continuously with carbogen (95% O2, 5% CO2).
- Cut the jejunum with sharp scissors in approximately 3-cm long loops. Observe the mesenteric bundle containing the vessels and splanchnic nerve somewhere near the center of the respective loop.
- Flush each segment with Krebs solution using a blunt catheter to remove luminal contents and chyme as these contain digestive enzymes that will accelerate the deterioration of the tissue sample in vitro.
NOTE: Take care not to damage the lumen of the loop during flushing, as the destruction of the villi will result in the release of mediators that can alter the afferent nerve activity. - Identify the segment to measure the afferent nerve activity (e.g., the first jejunal segment distally of the ligament of Treitz or the duodenojejunal flexure), and place this in a purpose-built recording chamber coated with a silicone elastomer layer.
NOTE: The beginning of the jejunum is anatomically defined as the part of the small bowel where the ligament of Treitz crosses the small bowel, also called the duodenojejunal flexure.
NOTE: Cover the bottom of the recording chamber with a thin silicone elastomer layer well in advance before the start of the experiment. The preparation of this elastomer layer should be performed according to the manufacturer's instructions 1. - Perfuse the chamber constantly with warm, carbogenated Krebs solution at a rate of 10 ml/min and keep the Krebs' temperature in the recording chamber constant at 34 °C.
- Mount the jejunal segment in the organ bath so that the oral end is connected to the syringe driver providing luminal flow and the aboral end connects to the outflow. Slightly stretch the segment but take care not to exert excessive tension. Attach both ends firmly using 4/0 silk ligatures to the in- and outflow ports.
- Attach the syringe driver to the oral end, and perfuse the jejunal segment intraluminally with Krebs solution (non-oxygenated, ambient temperature) at a rate of 10 ml/hr.
- Pin the mesentery of the mounted intestinal segment flat against the silicone elastomer bottom layer, using insect pins. Stretch the mesentery out in order to optimize visualization of the mesenteric bundle; do not exert strain on the bundle or the jejunum.
- Perform a test ramp distension (vide infra) by closing the output port until the intraluminal pressure of the intestinal segment reaches 60 mmHg, in order to verify that no intraluminal Krebs solution is leaking from the mounted segment. Observe a smooth rise in intraluminal pressure without interruptions.
- Observe small contractions of the segment (peristaltic waves) during the initial distension phase. If required, block peristaltic activity by adding 1 µM of the L-type calcium channel blocker nifedipine to the Krebs solution.
NOTE: Adding 1 µM of atropine to the Krebs solution in addition to nifedipine, will completely paralyze the intestinal segment. We however have no personal experience with the use and effect of atropine on afferent nerve recording. - Under a stereomicroscope, gently start to peel off the fat tissue from the mesentery by gently tugging it with two small tweezers, taking care not to damage the vessels and afferent nerve that lie buried in the fat tissue.
- Start at a remote distance from the jejunum, and expose both blood vessels in the mesenteric bundle.
- Observe the afferent jejunal nerve in between both vessels as a thin, white thread encapsulated in adipose tissue. Only dissect more proximally towards the jejunum by gently peeling the fat tissue away using tweezers when the initial identification of both mesenteric vessels and/or the afferent nerve is difficult.
- Dissect the jejunal mesenteric nerve of the segment free over a distance of several millimeters, by removing the adipose tissue adherent to the nerve.
- Transect the nerve using sharp tissue scissors. If required, peel off the remaining fat and connective tissue, as well as the epineuronal sheath by gently tugging it with the small tweezers.
- Using a micromanipulator, lower the tip of the suction electrode, connected to a syringe with plunger, into the organ bath; then, by manipulating the plunger, gently aspirate some Krebs solution from the organ bath so that the tip of the electrode is submerged in the Krebs solution (Figure 1). Ensure that the Krebs solution covers the wire electrode inside the suction electrode.
NOTE: Prepare the borosilicate glass suction capillary that contains the wire electrode prior to the start of the experiment using a pipette puller. - Position the tip of the suction electrode immediately next to the transected afferent nerve strand and draw the transected nerve strand into the capillary over its entire length.
- Maneuver the tip of the electrode towards some adipose tissue and aspirate this into the glass capillary while firmly aspirating with the plunger, thereby mechanically 'sealing' the nerve in the capillary from the contents of the organ bath.
- Verify the recording of afferent nerve activity using the data acquisition system, e.g., by performing a ramp distension-induced increase in afferent firing (vide infra). Following the isolation of the nerve into the suction electrode, stabilize the preparation for 15 min in order to obtain a steady state spontaneous afferent nerve activity before performing the actual experiments.
- To perform ramp distension, distend the intestinal segment by closing the output port, leading to gradual rise in pressure in the intestinal segment (up to 60 mmHg). Only perform the desired experimental protocol when three consecutive ramp distensions (with a 15 min interval) yield a reproducible multi-unit discharge (Figure 2).
- Perform rodent euthanasia of the adolescent or adult rodent that has been approved prior to the experiment by the local Ethical Committee (e.g., terminal sedation followed by cardiac puncture, cervical dislocation, etc.).
- Preparation of the lumbar splanchnic afferent (colonic afferent nerve)
NOTE: The dissection of colonic afferent nerves requires a more detailed dissection. Deviations from the former 'jejunal' protocol are listed below:- Euthanize the animal by means of a humane method, place it in the supine position, perform a midline laparotomy and excessively pour ice-cold Krebs solution in the abdominal cavity. NOTE: Krebs composition: 118 mM NaCl, 4.75 mM KCl, 1 mM NaH2PO4, 22 NaHCO3, 1.2 mM MgSO4, 11 mM D-glucose and 2.5 mM CaCl2, 3 µM indomethacin.
NOTE: Note the altered composition of the Krebs solution; indomethacin is added to the solution in order to prevent alterations of afferent nerve activity by prostaglandins. - Discard the adipose tissue, bladder and internal genitalia, and gently shift the small bowel to one side in the abdominal cavity. Perform an extended prelevation of the distal part of the colon with intact mesenteric nerves from the abdomen.
NOTE: Important landmarks that may be included in this dissection to aid further preparation of the tissue include the abdominal aorta and vena cava, the left kidney and the pelvic floor musculature.
NOTE: During the dissection, take care not to exert traction on the connecting tissue between colon and the abdominal aorta, as this region contains the lumbar splanchnic nerves. - Transfer the tissue segment into the silicone elastomer lined recording chamber. Use the left renal artery, which originates from the abdominal aorta, as a starting point. Follow the abdominal aorta in the aboral direction, encounter the superior mesenteric artery together with the celiac and superior mesenteric ganglion. Finally, arrive at the connecting tissue between the distal part of the colon and the aorta, wherein the nerve of interest is located.
- Identify the inferior mesenteric artery originating from the abdominal aorta. The lumbar splanchnic afferent nerve can be identified at the base of the inferior mesenteric artery; it runs parallel to the artery (Figure 3).
- Following the transection of the nerve, gently peel off the surrounding connective tissue sheath and tease the nerve into several fine threads. Make sure to keep a safe distance from the colon.
- Draw one of these single threads into the suction electrode, 'seal' the capillary with surrounding adipose tissue as previously described and perform the desired experimental protocol.
- Discard any superfluous tissue that is present in the organ bath (e.g., kidneys, abdominal vessels, muscle tissue), as these may disturb the afferent signal.
NOTE: Nifedipine (1µM) can be added to the Krebs solution in case the afferent nerve signal is disturbed by spontaneous intestinal movement due to smooth muscle cell contractions.
NOTE: Drugs can be administered at three different sites: 1) serosally by dissolving the desired compound into the Krebs that perfuses the recording chamber, 2) directly into the organ bath while temporarily stopping the perfusion or 3) intraluminally by dissolving the compound of interest into the Krebs solution in the syringe drive. Desensitization of the afferent nerve can occur when cumulative dosages of a compound are administered too fast consecutively.
- Euthanize the animal by means of a humane method, place it in the supine position, perform a midline laparotomy and excessively pour ice-cold Krebs solution in the abdominal cavity. NOTE: Krebs composition: 118 mM NaCl, 4.75 mM KCl, 1 mM NaH2PO4, 22 NaHCO3, 1.2 mM MgSO4, 11 mM D-glucose and 2.5 mM CaCl2, 3 µM indomethacin.

Figure 1: Schematic Overview of the Purpose-built Recording Chamber and Suction Electrode. Detailed overview of the technical setup with the suction electrode and the recording chamber in place. Please click here to view a larger version of this figure.

Figure 2: Representative Tracing of the In Vitro Recording of Jejunal Afferent Nerve Activity. Typical recording of jejunal multi-unit afferent nerve activity (imp.sec-1) (upper panel) at baseline and in response to 2 ramp distensions up until 60 mmHg (lower panel), and the subsequent identification (wavemark analysis) of different single-units in the nerve signal (third panel). Please click here to view a larger version of this figure.
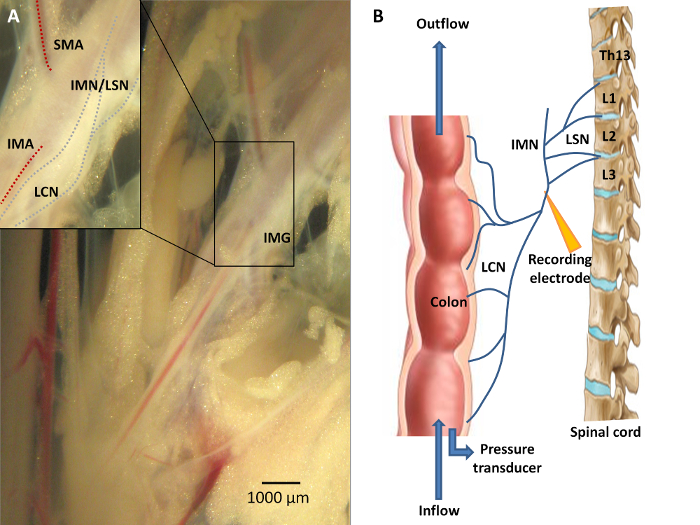
Figure 3: Neuroanatomy of the Colon. A) Sensory information from the colon is conveyed via the lumbar colonic nerves (LCN) towards the central nervous system, with the LCN running in close proximity to the inferior mesenteric artery (IMA). A portion of the fibers from this lumbar colonic nerve will course along the intermesenteric nerve (IMN) to form the lumbar splanchnic nerves (LSN). The inferior mesenteric ganglion (IMG) is located at the origin of the IMA from the abdominal aorta. Recording distally of the IMG is mandatory should researchers wish to study viscerofugal afferent nerve activity. B) A schematic overview of the experimental set-up. Afferent recording of the LCN is performed in an organ both by means of a suction electrode connected to the data acquisition system. Ramp distension can be performed upon closure of the outlet port while continuing the inflow of Krebs solution. Please click here to view a larger version of this figure.
2. Data Acquisition
- Record the nerve activity via a suction electrode connected to a headstage. Amplify (gain 10k) and filter the signal (bandpass 500 - 5,000 Hz) 20.
NOTE: A 50/60 Hz Noise Eliminator should be included in the experimental setup in order to remove 50/60 Hz electrical interference noise signals. The signal is automatically digitized and sampled at 20 kHz for analysis.
3. Analysis5,20
- Report the afferent nerve discharge as the overall number of impulses/second for the entire nerve or use specialized software to perform further analysis, as multi-unit recordings of overall nerve activity contain action potentials of different shape, amplitude and width, corresponding to different nerve units in each afferent fiber (Figure 2).
- Match individual wavemarks to predefined templates, allowing discrimination between single-units. Before allocation of a spike to a certain wave-mark, a signal-to-noise ratio of >2:1 should be enforced.
NOTE: During wavemark analysis, we construct a new template when at least 10 similar spikes are identified by the analysis software. No template is constructed for shapes rarer than 1 in 50 spikes. A spike is matched to a template using principle component analysis when at least 80% of its points are identical to the template spike. Template matching is operator-dependent, and should always be performed by the same researcher in a blinded manner. - Calculate the action potential responses by subtracting the spontaneous activity at baseline (intraluminal pressure of 0 mmHg) from the response during distension at fixed time points (5 mmHg of increment from 0 to 20 mmHg intraluminal pressure, increments of 10 mmHg from 20 mmHg onwards). Measure baseline afferent discharge using a bin size of 10 sec.
- Use the single-unit analysis to classify fibers based upon their discharge profile during ramp distension (Figure 4). In addition, single-unit analysis can be utilized to study the chemosensitive profile of different types of units, as not all types of units will display the same altered firing activity in response to a drug or compound.
- Classify fibers as low threshold fibers ('LT', typically exert increased discharge at the lower distension pressures), high threshold fibers ('HT', exert increased discharge at the highest distension pressures), wide dynamic range fibers ('WDR', demonstrate increased firing during the entire ramp distension) or mechanically insensitive afferent ('MIA', nerve fibers that typically do not respond to ramp distensions) 5;20.
- Express the nerve firing response at 20 mmHg as the percentage of the maximum firing response during distension (LT%) as it reflects the extent of firing at low distension pressure.
NOTE: Therefore, LT fibers are characterized by an LT% > 55%, whereas HT are defined by a value of <15%. Values for WDR units range between 15 and 55% (20). A MIA displays spontaneous afferent firing that is unaffected by distensions.

Figure 4: Schematic Representation of the Different Afferent Fiber Units Based on Their Mechanosensitive Profile. Units are classified based upon the percentage (LT%) of their firing rate at 20 mmHg distension pressure compared to the maximum firing response during distension. Low threshold fibers (upper left panel) predominantly display an increased nerve activity at low distension pressures, resulting in an LT% of over 55%. High threshold units (upper right panel) on the contrary only display an increase in firing rate at noxious pressures (%LT < 15). Wide dynamic range fibers (lower left panel) display a gradual increase in nerve activity during the entire distension (%LT ranging between 15 and 55), whereas mechanically insensitive fibers (lower right panel) do not respond to increasing distension pressures. LT%: (afferent firing at 20 mmHg / maximal afferent firing) Please click here to view a larger version of this figure.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
Jejunal afferent nerve activity was measured at baseline and in response to ramp distension in 9 eight-week old male OF-1 mice. Animals were housed in groups in standardized conditions (6 animals per cage, 20 - 22 °C, humidity 40 - 50%, 12 hr light-dark cycle) with unlimited access to tap water and regular chow. Jejunal segments of mice displayed irregular spontaneous afferent nerve discharge at baseline at an intraluminal pressure of 0 mmHg (mean spontaneous activity 11.47 ± 3.31 imp/sec).
The jejunal afferent nerve activity increased upon performing ramp distensions up until 60 mmHg. Typically, the increase in afferent nerve activity following the rise in intraluminal pressure is characterized by a biphasic response (Figure 5), consisting of an initial rapid increase in firing activity up until the intraluminal pressure reaches 20 mmHg, which can mainly be attributed to the increased firing rate of low threshold fibers. This is then followed by a plateau phase, after which a second increase in firing activity can be observed from 40 mmHg onwards, representing the activation of predominantly high threshold fibers.
Based upon their waveforms, single-units can be discriminated in each multi-unit recording and classified in one of the aforementioned four categories. In 9 mice, we discriminated 40 different units (4.44 ± 1.01 units/jejunal afferent nerve), with the LT units being the most prevalent ones, followed by WDR and HT fibers (Figure 6). The firing activity of the different units in response to ramp distension can be observed in Figure 7.

Figure 5: Mesenteric Afferent Nerve Discharge (imp.sec-1) in Wild-type Mice during Ramp Distension. Mesenteric multi-unit afferent nerve discharge (imp/sec-1) in wild-type mice during ramp distension for the whole nerve. Values represent mean afferent discharge ± s.e.m., n = 9 mice. imp.sec-1: impulses per second. Please click here to view a larger version of this figure.

Figure 6: Single Unit Distribution of 40 Units Identified in Jejunal Afferent Nerves from 9 Wild-type Mice. HT: high threshold fiber, LT: low threshold fiber, MIA: mechanically insensitive fiber, WDR: wide dynamic range fiber. Please click here to view a larger version of this figure.

Figure 7: Pressure-response Curves for the Different Types of Subunits in Wild-type Mice. The single-unit afferent nerve discharge (imp.sec-1) from the four different units that can be identified, in wild-type mice during ramp distensions. A low threshold fiber (LT, upper left figure) is characterized by an initial rapid increase in firing activity during distensions, whilst the high threshold fibers (HT, lower left figure) only display increased firing during noxious intraluminal pressures. Wide dynamic range fibers (WDR, upper right figure) show a steady increase in firing activity during the entire distension, and mechano-insensitive afferent fibers (MIA, lower right figure) do not respond to increasing intraluminal pressures. Values represent mean afferent discharge ± s.e.m. imp.sec-1: impulses per second. Please click here to view a larger version of this figure.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
The protocol in this paper describes a reproducible laboratory technique to study mesenteric afferent nerve activity in rodents as used by our group and others 3,4,7,8,12,20,21,31. Critical steps within the protocol include the rapid isolation of the tissue, the aspiration of the nerve strand into the suction electrode and the adequate 'sealing' of the glass capillary from the organ bath by aspirating surrounding adipose tissue into the capillary. The aperture of the glass capillary should be precisely determined: an aperture that is too small will complicate the aspiration of the nerve strand into the electrode, whereas a too wide aperture will hinder the 'sealing' of the capillary with adipose tissue, resulting in redundant background noise that will hamper the analysis (low signal-to-noise recordings). To allow for reliable single-unit classification, splanchnic afferents should be divided in different strands in order to reduce the number of units in the recording. Typically, we would suggest aiming to have a maximum of 4 - 5 units in each recording. Researchers therefore ought to adjust the aperture based upon the fiber of interest, and the lab animal that is applied.
Another critical point encompasses the sufficient grounding of the experimental setup. The suction electrode and recording chamber should be adequately grounded and covered by a Faraday cage in order to minimize interfering electrical fields that impede the analysis of neuronal activity, whereas all other equipment including the recording apparatuses, syringe driver et cetera should be installed outside the cage.
By recording afferent nerve activity in close proximity to the jejunum or colon, one can isolate the first part of the afferent signal transduction chain and easily study the contribution and alterations that occur at the sole afferent level without interference from the central nervous system. One of the limitations of this technique is the fact that in vitro observations cannot always be effortless extrapolated to the in vivo setting, as the in vitro setup only incorporates one relay station in the complex nerve signaling cascade. As such, a broader picture must be made incorporating all other stations, such as the dorsal root ganglia, central nervous system (e.g., functional brain imaging) and descending (inhibitory) efferent pathways.
Another advantage of this method constitutes the rather simple technical procedure, as one no longer has to monitor the wellbeing of the lab animal that provides the gastrointestinal specimen. On the other hand is the in vitro measurement of neuronal activity not suitable for elucidating the effect of a systemically administered drug on afferent nerve discharge, but researchers can theoretically overcome this obstacle by systemically administering the drug of interest to the animal, followed by the ex vivo in vitro recording of afferent nerve activity. However, one should be attentive to the fact that any drug present in the recording chamber will be diluted due to the bath perfusion during the dissection and subsequent recordings. Finally, performing in vitro splanchnic nerve recordings using genetically engineered animals could allow researchers to fully elucidate the role of different channels and receptors expressed on afferent fibers.
Researchers attempting to implement this technique must also bear in mind that the identification and isolation of the mesenteric afferent and pelvic afferents obviously requires knowledge of basic anatomy and technical training, and researchers ought to be acquainted with the basic principles of neuronal electrophysiology.
The in vitro setting furthermore allows researchers to easily identify possible pharmacological targets, and provides insight on the physiological role of neuronal activity as well as altered sensory signaling in several disease processes.
In case of jejunal afferent measurements, several tissue segments of a single animal can be studied simultaneously, a feature that is rather difficult using an in vivo setup. Researchers however should cautiously interpret results obtained from different segments, as regional differences could bias results. Therefore we would recommend to consistently measure afferent nerve activity from the same site (e.g., first segment distally from the ligament of Treitz or the duodenojejunal flexure).
Typical current and future applications of this technique comprise the screening of pharmacological compounds that can alter sensitization of mesenteric afferents during pathologies that are characterized by visceral hypersensitivity and pain. As already mentioned before, the target of these compounds can be encountered somewhere along the intricate nervous system ranging from the enteric intrinsic nervous system to the brain; as such, characterizing and modulating afferent nerve activity contributes to the broader picture that also encompasses the calcium imaging of the intrinsic enteric nerves and dorsal root ganglia, the measurement of the visceromotor response as an indicator of visceral hypersensitivity in vivo, and functional brain imaging, among others.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
SN performed the experiments described above, performed the data analysis and drafted the manuscript. AD and JDM implemented the technique at our research facilities and aided in the data analysis. HC aided in performing the experiments. WJ, CK and DG assisted in implementing the afferent measurement technique in our lab, the data analysis and interpretation of the results. SF, JDM and BDW designed the study. All authors critically read and approved the final manuscript. SN is an aspirant of the Fund for Scientific Research (FWO), Flanders (11G7415N). This work was supported financially by the FWO (G028615N and G034113N).
Materials
| Name | Company | Catalog Number | Comments |
| sodium chloride (NaCl) | VWR Chemicals | 27,810,295 | compound Krebs solution |
| potassium chloride (KCl) | Acros organics | 196770010 | compound Krebs solution |
| sodium dihydrogen phosphate (NaH2PO4) | VWR Chemicals | 1,063,461,000 | compound Krebs solution |
| sodium bicarbonate (NaHCO3) | Merck | 1,063,291,000 | compound Krebs solution |
| magnesium sulfate (MgSO4) | Merck | 1,058,861,000 | compound Krebs solution |
| calcium chloride (CaCl2) | Merck | 23,811,000 | compound Krebs solution |
| D-glucose | VWR Chemicals | 1011175P | compound Krebs solution |
| Distilled water | compound Krebs solution | ||
| PVC tubing | Scientific Laboratory Supplies | The intestinal segment should be mounted over PVC tubing | |
| Silicone tubing | Scientific Laboratory Supplies | The rest of the tubing, ideally silicone-based - more easily dislodging of debris in the tubing | |
| Silk thread | Pearsall Limited | 10B15S220 | Attachment of the segment over the PVC tubing |
| Syringe driver | Harvard Apparatus | 55-2222 | Intraluminal infusion of Krebs |
| Binocular - including 10X magnification in oculair | Zeiss STEMI 2000 | Optimal visualization for the dissection of the afferent nerve | |
| Homeothermic Blanket Control Unit | Harvard Apparatus | 507214 | Heating of the organ chamber |
| Custom made organ bath with Sylgard covered bottom | |||
| Spike2 software | Recording and analysis of the data | ||
| Insect pins, 500 pieces, stainless steel, diameter 0.2 mm | Austerlitz insect pins minutiens | Dissection of the afferent nerve | |
| Tweezer Dumont #5 inox 11 cm | World Precision Instrument | 500341 | Dissection of the afferent nerve |
| Scissors, spring, 14 cm | World Precision Instrument | 15905 | Dissection of the afferent nerve |
| DB digitimer | NL 108T2/10 | pressure transducer | |
| Micromanipulator | Narishige | M-3333 | 3D manipulation of the suction electrode |
| Micromanipulator | X-4 rotating block | 3D manipulation of the suction electrode | |
| Micromanipulator | GJ-8 magnetic stand | 3D manipulation of the suction electrode | |
| LightSource | Euromex Microscopes Holland EK-1 | Optimal visualization for the dissection of the afferent nerve | |
| CED 1401 Recording Apparatus | Recording of afferent nerve activity | ||
| Humbug 50/60 Hz Noise Eliminator | Quest Scientific Instruments | Elimination of background noise | |
| Infusion Pump | Gibson Minipuls 2 | Infusion of the organ chamber in which the segment is mounted | |
| Microelectrode Holder Half Cells 1.5 mm | World Precision Instrument | MEH2SW | Suction electrode for isolation of the afferent fiber |
| Borosilicate Glass Capillaries, 300 pc; 1.5/0.84 OD/ID | World Precision Instrument | 1B150-4 | Capillary for the isolation of the afferent nerve |
References
- Donovan, J., Grundy, D. Endocannabinoid modulation of jejunal afferent responses to LPS. Neurogastroenterol Motil. 24 (10), 956-e465 (2012).
- Gregersen, H., Jiang, W., Liao, D., Grundy, D. Evidence for stress-dependent mechanoreceptors linking intestinal biomechanics and sensory signal transduction. Exp Physiol. 98 (1), 123-133 (2013).
- Keating, C., et al. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J Physiol. 586 (18), 4517-4530 (2008).
- Liu, C. Y., Jiang, W., Muller, M. H., Grundy, D., Kreis, M. E. Sensitization of mesenteric afferents to chemical and mechanical stimuli following systemic bacterial lipopolysaccharide. Neurogastroenterol Motil. 17 (1), 89-101 (2005).
- Deiteren, A., et al. Mechanisms contributing to visceral hypersensitivity: focus on splanchnic afferent nerve signaling. Neurogastroenterol Motil. 27 (12), 1709-1720 (2015).
- Nullens, S., et al. The effect of prolonged CLP-induced sepsis on mesenteric afferent nerve activity in mice. Neurogastroenterol Motil. 27 (Suppl 2), 22 (2015).
- Brierley, S. M., Jones, R. C. III, Gebhart, G. F., Blackshaw, L. A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 127 (1), 166-178 (2004).
- Feng, B., Gebhart, G. F. In vitro functional characterization of mouse colorectal afferent endings. J Vis Exp. (95), e52310 (2015).
- Travis, L., Spencer, N. J. Imaging stretch-activated firing of spinal afferent nerve endings in mouse colon. Front Neurosci. 7, 179 (2013).
- De Schepper, H. U., et al. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am J Physiol Gastrointest Liver Physiol. 294 (1), G245-G253 (2008).
- Sengupta, J. N., Gebhart, G. F. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 71 (6), 2046-2060 (1994).
- Jiang, W., et al. First-in-man': characterising the mechanosensitivity of human colonic afferents. Gut. 60 (2), 281-282 (2011).
- Peiris, M., et al. Human visceral afferent recordings: preliminary report. Gut. 60 (2), 204-208 (2011).
- Brookes, S. J., Spencer, N. J., Costa, M., Zagorodnyuk, V. P. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 10 (5), 286-296 (2013).
- Bulmer, D. C., Grundy, D. Achieving translation in models of visceral pain. Curr Opin Pharmacol. 11 (6), 575-581 (2011).
- De Winter, B. Y., De Man, J. G. Interplay between inflammation, immune system and neuronal pathways: effect on gastrointestinal motility. World J Gastroenterol. 16 (44), 5523-5535 (2010).
- Akbar, A., Yiangou, Y., Facer, P., Walters, J. R., Anand, P., Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 57 (7), 923-929 (2008).
- De Schepper, H. U., et al. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J Physiol. 586 (21), 5247-5258 (2008).
- Vermeulen, W., et al. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol. 698 (1-3), 404-412 (2013).
- Booth, C. E., Shaw, J., Hicks, G. A., Kirkup, A. J., Winchester, W., Grundy, D. Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterol Motil. 20 (2), 149-158 (2008).
- Hughes, P. A., et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut. 62 (10), 1456-1465 (2013).
- Hughes, P. A., et al. Immune derived opioidergic inhibition of viscerosensory afferents is decreased in Irritable Bowel Syndrome patients. Brain Behav Immun. 42, 191-203 (2014).
- Buhner, S., et al. Neuronal activation by mucosal biopsy supernatants from irritable bowel syndrome patients is linked to visceral sensitivity. Exp Physiol. 99 (10), 1299-1311 (2014).
- Wouters, M. M., et al. Histamine Receptor H1-mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. , (2016).
- Brenn, D., Richter, F., Schaible, H. G. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 56 (1), 351-359 (2007).
- Christianson, J. A., Liang, R., Ustinova, E. E., Davis, B. M., Fraser, M. O., Pezzone, M. A. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 128 (3), 235-243 (2007).
- Daly, D. M., Chess-Williams, R., Chapple, C., Grundy, D. The inhibitory role of acetylcholine and muscarinic receptors in bladder afferent activity. Eur Urol. 58 (1), 22-28 (2010).
- Minagawa, T., Wyndaele, M., Aizawa, N., Igawa, Y., Wyndaele, J. J. Mechanisms of pelvic organ cross-talk: 2. Impact of colorectal distention on afferent nerve activity of the rat bladder. J Urol. 190 (3), 1123-1130 (2013).
- Wyndaele, M., et al. Mechanisms of pelvic organ crosstalk: 1. Peripheral modulation of bladder inhibition by colorectal distention in rats. J Urol. 190 (2), 765-771 (2013).
- Keating, C., Nocchi, L., Yu, Y., Donovan, J., Grundy, D. Ageing and gastrointestinal sensory function: Altered colonic mechanosensory and chemosensory function in the aged mouse. J Physiol. , (2015).
- Valdez-Morales, E. E., et al. Sensitization of Peripheral Sensory Nerves by Mediators From Colonic Biopsies of Diarrhea-Predominant Irritable Bowel Syndrome Patients: A Role for PAR2. Am J Gastroenterol. 108 (10), 1634-1643 (2013).



