Abstract
Female zebra finches Taeniopygia guttata will copy the novel foraging choice of males. The degree to which they do so, however, can vary considerably. Among-individual differences in social learning and their underlying neural pathways have received relatively little attention and remain poorly understood. Here, then, we allowed female zebra finches to observe live-streamed male demonstrators feeding from one of two novel-coloured feeders (social information acquisition phase). After this social information acquisition phase, we tested from which feeder the females preferred to feed to determine whether they copied the feeder choice of the male demonstrator (social learning test phase). We then examined the brains of these females for immediate early gene activity (c-fos) in the neural social behaviour network for the time during which they were observing the male feeding. Of the 12 regions and sub-regions in the brain examined we found only one weak correlation: greater copying was associated with lower activity in the bed nucleus of the stria terminalis, BSTmv. Future work should perhaps focus on neural activity that occurs during the stage in which there is evidence that animals have copied a demonstrator (i.e., social learning test phase in the current experiment) rather than during the period in which those animals observe that demonstrator (i.e., social information acquisition phase in the current experiment). What is clear is that the considerable emphasis on examining the behavioural component of social learning has not yet been accompanied by neural analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Learning from observing or interacting with other individuals or the products of other individuals’ behaviour is considered to be ‘social’ learning (Heyes, 1994) and may be quicker and less effortful than gathering information on one’s own (sometimes called in contrast ‘asocial’ learning, which hereafter we refer to as ‘learning’; Laland, 2004). There is now a large body of observational and experimental work chronicling the prevalence of social learning across taxa (e.g., fish – Duffy et al., 2009; insects – Leadbeater & Chittka, 2007; mammals – Carter et al., 2014; birds – Aplin et al., 2015; reptiles – Kis et al., 2015) and behavioural context (e.g., foraging – Slagsvold & Wiebe, 2011; tool-use – Auersperg et al., 2014; song learning – Belzner et al., 2009; predators – Griffin, 2004; nest building – Guillette et al., 2016). There is also theory predicting under what conditions, called when-strategies, and from whom, called who-strategies, animals should copy (i.e., socially learn; Rendell et al., 2011; Galef, 2009; Laland, 2004). For instance, when-strategies can include state-based conditions such as ‘copy if uncertain’, whereby animals with no prior or relevant information should copy knowledgeable individuals (demonstrators) such as when first-time nest-building birds copy the nest-material choice of experienced birds (Guillette et al., 2016). When-strategies also include frequency-dependent conditions such as ‘copy the majority’, as seen when naïve nine-spine sticklebacks were more likely to copy the foraging decisions of larger groups of demonstrators (Pike & Laland, 2010). Alternatively, copying strategies may depend on the identity of the demonstrator (so-called who-strategies), which include ‘copy dominant individuals’ rather than subordinates or ‘copy familiar individuals’ (e.g., zebra finches naïve to building copy material choices of familiar individuals; Guillette et al., 2016).
However, while these strategies often describe the patterns of social learning by groups of naïve individuals, the degree to which naïve individuals learn from a demonstrator has received relatively little attention (Mesoudi et al., 2016; Watson et al., 2018). And there can be considerable variation in the degree to which naïve observers learn socially, as seen from data collated across 12 years of experiments conducted on the same group of chimpanzees: half of the variation in social learning scores across 16 different tests was attributed to the individual identity of the observer. Furthermore, individual identity accounted for the most variation of all traits measured (sex, age, rearing history, experimental history, and type of task; Watson et al., 2018). In chimpanzees, at least then, individuals vary considerably in the degree to which they learn from conspecifics. Moreover, there is mounting evidence that individuals are consistent in their propensity to learn socially (Mesoudi et al., 2016).
Given this considerable, but thus far little understood, variation in the degree to which naïve individuals learn from demonstrators, it seemed plausible that determining the neural bases of social learning would help to explain why there is among-individual variation in social learning. Because female zebra finches (Taeniopygia guttata) vary in the degree to which they copy foraging decisions of males (Guillette & Healy, 2014, 2017), here we coupled this behavioural paradigm with quantifying immediate early gene (IEG) immunoreactivity in the brain using the expression of c-fos protein as a marker to test whether individual variation in social learning is related to neural activity in the social behaviour neural network.
Much is known about the processing of social information in the social behaviour network, a highly conserved area across vertebrate taxa, and social learning may rely on the procession of social information (Johnson & Young, 2015, 2017; O’Connell & Hofmann, 2011; Olsson et al., 2020). We selected the brain regions belonging to the social behaviour network as potential targets underpinning variation in social learning for two main reasons. First, social information processing may affect attention, motivation and aggression that can filter the information observers learn from demonstrators. For example, rodents raised in social isolation are less likely to learn from demonstrators than are conspecifics that are raised normally (Yusufishaq & Rosenkranz, 2013). Second, in estrildid finches, the neural and neuroendocrine activity in particular regions of the social behaviour network co-vary according to (i) species differences in sociality (measured as average group size) and (ii) exposure to social stimuli (Goodson, 2005, 2008; Goodson et al., 2005; Goodson & Wang, 2006). The particular regions we investigated were the bed nucleus of the stria terminalis (BSTl/mv/md), which is involved in social arousal, approach and avoidance, and the septal complex (LScv/cvl/r and MS), which mediates affiliative behaviour, social recognition and non-sexual contact (Goodson, 2005; Goodson et al., 2006). Moreover, these two regions, the BST and the LS, also belong to the mesolimbic reward system, where the salience of external stimuli is assessed (O’Connell & Hofmann, 2011), which seems relevant to social learning.
Although we did not have specific predictions, we quantified expression of c-fos in the remaining three regions that comprise the neural social behaviour network: the anterior and ventromedial hypothalamus (AH and VMH, respectively, which are implicated in agonistic challenges, handling and restraint stress; Goodson & Evans, 2004), and the medial preoptic area (POM; which plays a role in aggression, reproduction and parental care; O’Connell & Hofmann, 2011).
We also quantified IEG activity in the hippocampus because of its role in learning (O’Connell & Hofmann, 2011) and, as a control region, in the anterior ventral mesopallium (AMV), which is part of the anterior motor pathway. We expected social learning to be linked to IEG activity in the former, but not the latter, brain region. To assess whether IEG activity was a reflection of other behaviours, we scored both social (vocalizations) and non-social behaviours (scratches, hops, drinks, preen, pecks).
In this experiment we gave female observers 30 min to watch live-streamed male demonstrators eat from one, but not a second, novel-coloured feeder. In the following 60 min we measured the degree to which the females copied their demonstrator’s choice of coloured feeder. To do this each female was offered both novel-coloured feeders and we scored the proportion of food she ate from the feeder that matched the colour of the demonstrator’s feeder. As we immediately sacrificed each female and fixed the brain in paraformaldehyde to stop protein synthesis, the immediate early gene activity we quantified reflected the neuronal activity that occurred when the female observed the male demonstrator’s feeder choice. If social learning is dependent on the processing of information in the brain regions in the social behaviour network, then we expect to see correlations between IEG activity and the degree to which a female copied her demonstrator (the social learning score).
Methods
Subjects and housing
The subjects were 48 adult zebra finches (35 females, 13 males; > 90 days of age) bred at the University of St Andrews. All birds were housed in colony room wire cages of same-sexed individuals (either 100 × 50 × 50 cm or 140 × 71 × 122 cm). Colony rooms were kept on a 14:10 light:dark cycle (lights on at 07:00) with dusk and dawn lighting for ~10 min at the start and end of each day and temperature at ~20 °C and humidity at ~50%. Each cage had several perch sizes (1–2 cm diameter) and types (plastic, wooden, rope) and the floors were covered with pressed wood pellets. Birds were given free access to mixed seeds, water and cuttle bone, oyster shell and vitamin block. Three times a week water was supplemented with calcium and vitamin D3, and food was supplemented with fresh greens (e.g., spinach). Four observer birds were tested in each trial during the light period (between the hours 07:00 and 21:00; four observers per bird demonstrator). The observer birds were always female and the demonstrator birds were always males.
The work was conducted with the approval of the University of St Andrews Animal Welfare and Ethics Committee.
Apparatus
The behavioural experiment was carried out in a test room containing one demonstrator cage and four observer wire cages. The apparatus was the same as that used by Guillette and Healy (Guillette & Healy, 2017; see Fig. 1 from Guillette & Healy, 2017). White opaque material curtains were hung between each of the cages to allow vocal, but not visual, contact among the four cages. The observer cage (100 × 50 × 50 cm, Kings Cages) contained two water dispensers, six perches, a cuttlefish bone and vitamin block. Two grey-coloured food bowls were located on the opposite side of the cage from where the viewing monitor and experimental feeders would be located. During the test phase (described below) coloured feeders – one purple, one pink (wrapped in coloured opaque paper) – were attached to each cage. A video screen (ViewsSonic Thin Film Transistor, model # VS15804) was located 15 cm from the long side of the observer cage and concealed behind a white opaque curtain. The demonstrator cage was identical to the observer cages, with the exception that the baited coloured feeder was the only food source available to the demonstrator for the duration of the experiment.
Scale schematic of the top-down view of an observer cage. The dashed line represents a white opaque barrier between the observer cage and the video screen. The video screen (monitor) was in place at all times with the exception of the observation phase. The food bowls were removed from the observer cage 2 h prior to the start of the observation phase. The demonstrator cage (not pictured) was identical to the observer cages barring one exception: the food bowls were never present, thus the baited colour feeder (only one colour in each trial) was the only food source available to the demonstrator. This figure originally appeared in Guillette and Healy (2017) and is open access under CC BY license.
Each cage contained three cameras (SpyCameraCCTV, Bristol, UK) connected to a laptop computer. A GoPro Hero3 (GoPro, Incorporated, California, USA) recorded and live-streamed the behaviour of the demonstrator. The mini-HDMI connection from the GoPro was connected to a 1 × 4 HDMI Splitter (HDelity by Cablesson), which was connected to each of the four video screens via a separate HDMI cable, thus the birds could vocally but not visually interact. Using this live-streaming set-up zebra finch females will copy the feeder colour choices of male demonstrators (Guillette & Healy, 2017) as they do when demonstrators are live (Guillette & Healy, 2014).
Behavioural testing
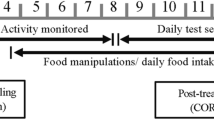
We followed the protocol as described in Guillette and Healy (2017). Each trial lasted approximately 24 h. Birds were randomly assigned to treatment groups. The observer bird viewed the behaviour of the demonstrator bird via a video screen. All birds were in the same room and could vocally but not visually interact because of the white opaque curtains that hung between each cage. The video that the observer viewed was streamed live from the GoPro (30fps 1080p).
On Day 1, one bird was placed in each of the observer cages and in a demonstrator cage. At this time, all opaque curtains were in place, including between the observer and the video screen so birds could not see one another or the video screen. The only food available to the demonstrator bird was provided in one of the two feeders (pink or purple), and thus the demonstrator bird learned which feeder contained the food during the observation phase (described below).
On Day 2 food was removed from each cage 2 h post-light onset. In each trial there were two phases, the observation phase and then the test phase. The observation phase started after the 2 h of food deprivation. In the observation phase the second coloured feeder was added to the demonstrator’s cage so that the demonstrator had two feeders – one pink, one purple – and only one feeder contained food.
The 30-min observation period began when the opaque curtain between the observer cage and the video screen was removed so that the observer could view the behaviour of the demonstrator via the video screen. The demonstrator and observer behaviour and vocalizations were recorded via in-cage video cameras scored at a later date.
After the 30-min observation phase we returned the opaque barrier so the observer could no longer see the demonstrator. Each observer was also given one pink and one purple feeder, both containing food. With the exception of the observer phase, these feeder colours were novel to the observers. The spatial location of the feeders on the observer cages mirrored that of the demonstrator cage such that location and colour cues were redundant. The test phase then began and lasted 60 min, during which the behaviour of the observers was video recorded. Please see Online Supplementary Material (OSM) Fig. S1 for a schematic timeline of the experiment showing the intervals during which behavioural measures were scored.
Brain tissue collection
Immediately after the test phase ended each observer bird was removed from their cage and terminally anaesthetized (0.3 ml intraperitoneal injection of pentobarbital). The brain was quickly removed and placed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 0.1M pH = 7.4) and stored at 4 °C. Six days later brains were submerged in 15% sucrose in 4% PFA for 24 h at 4 °C and were then transferred to 30% sucrose in PBS for 24 h at 4 °C. Each brain was then frozen on powered dry ice, wrapped in foil, and stored in individual labelled plastic bags at −80 °C. Brains were transported to the Roslin Institute, University of Edinburgh on dry ice and the stored at −80 °C until processing. The forebrain was coronally sectioned at 50 μm on a freezing microtome. The free-floating sections were collected in 0.1M PBS and stored at −20 °C in a cryoprotectant and were processed for Fos immunocytochemistry within 4 weeks.
Fos immunohistochemistry
All brains were processed in the same immunohistochemical run, and each brain was processed in one Corning netwell basket. Sections were washed four times for 15 min in 0.2% Triton X-100 in 0.1 M PBS (PBS-T) on a shaking platform. Sections were then rinsed three times in 0.1 M PBS for 5 min. Sections were then incubated in 0.3% hydrogen peroxide for 20 min, then washed in PBS-T three times for 10 min, after which they were incubated in 10% Normal Goat serum (NGS) in PBS-T for 60 min and incubated in Fos antibody (1:5000; Santa Cruz Biotechnology Inc.) rabbit antibody (c-Fos [K-25, sc-253]) diluted in 10% normal goat serum in 0.1 M PBS-T for 120 min at room temperature and then at 4 °C for approximately 20 h. Following primary antibody incubation the tissue was washed three times in PBS-T for 15 min. A Vectastain elite rabbit kit (Vector Laboratories, PK6101) was used to localize and amplify the antibody-antigen complexes and sections were incubated for 60 min (anti-rabbit secondary antibody and NGS in PBS-T). Sections were washed three times in 0.1 M PBS-T for 10 min and then incubated for 60 min in Avidin/Biotinylated horseradish-peroxidase complex (ABC; Vector Laboratories) in PBS-T. The tissue was then washed three times in 0.1 M PBS-T for 10 min, rinsed in 0.1 M PBS for 5 min, and briefly rinsed in 0.1 M sodium acetate buffer. Following these washes the antibody-avidin-biotin complexes were visualized in 0.4% diaminobenzidene solution (Sigma Fast DAB) for 90 s and then rinsed four times with 0.1M PBS to stop the reaction. The tissue was then float-mounted onto gelatinised glass microscope slides, allowed to air dry for 24 h before being counterstained with a 0.5% methyl-green solution (ethyl-violet, Sigma) in 0.1M sodium acetate buffer pH = 4.2 for 5 min, rinsed in distilled water, and dehydrated in 95% and then 100% ethanol. Finally, the slides were cleared in xylene and cover-slipped with glass coverslips using DePex (VWR).
Behavioural analysis
Observation phase
We quantified the behaviour of the observers and the demonstrators 90–60 min prior to sacrifice. The time delay between the behaviour and sacrifice provided time for the accumulation of Fos protein in the brain. For observers we scored the number of scratches (with feet), hops, drinks, preens, vocalizations and pecks while for demonstrators we scored the number of pecks to each feeder and the number of vocalizations.
Test phase
From the video recordings of the test phase for each trial we used Solomon Coder (beta version 16.06.26) to count the number of pecks delivered to each feeder by the observer. To quantify feeder colour preference, we calculated the proportion of pecks by the observer bird to the demonstrator’s feeder colour. The test phase occurred from 60 min pre-sacrifice. From these data we determined the copying behaviour of the observers ranging from 0 (exclusively eating from the non-demonstrated colour feeder) to 0.5 (eating equally from both the demonstrated and non-demonstrated colour feeder) to 1 (eating exclusively from the demonstrated colour feeder).
Quantification of Fos immunoreactivity (Fos-ir)
Fos immunoreactivity (Fos-ir) was quantified in brain regions that form part of the neural social behaviour network (Dere et al., 2015; Goodson, 2005; Goodson & Wang, 2006; O’Connell & Hofmann, 2011): AH, BSTmd, BSTmv, BSTI, LScv, LScvl, LSr, MS, POM and VMH; areas associated with learning and memory: dHP, mHP (O’Connell & Hofmann, 2011); and the anterior motor pathway: AMV (Edwards et al., 2020; Feenders et al., 2008; Hall et al., 2014; Heimovics & Riters, 2007).
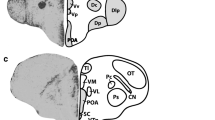
Each brain region of interest was located using full section architecture and regional anatomy using brain atlases of the zebra finch (Nixdorf-Bergweiler & Bischof, 2007) and canary (Serinus canaria; Stokes et al., 1974). For each area adjacent coronal sections were inspected to locate the midpoint region (at 2.5 × magnification) and images of these regions (at 40 × magnification) were taken in both hemispheres, using Nikon E600 Brightfield Microscope camera and Zen 2 software, across five coronal sections for dHP and mHP and three coronal sections for the remaining areas of interest (Fig. 2). The total number of Fos-ir cells were manually counted in the field-of-view (161 × 120 μm) using Zen 2 software after applying the ‘set exposure’ and ‘auto-white balance’ functions. The number of suitable sections differed across individuals, therefore the number of Fos-ir nuclei in each brain region was summed and averaged to yield a single value for each brain region in each bird (e.g., Hall et al; Edwards et al., Heimovics & Riters, 2007). To avoid unconscious bias during Fos-ir quantification, birds were randomly assigned an identification number so that the experimenter was blind to the social learning score of the bird.
Brain regions quantified for Fos-ir in the zebra finch brain. The location along the sagittal plane of two transverse sections (1, 2) showing the location of the regions quantified bilaterally for Fos-ir. The full brain drawing is pictured with the beak on the left. Representative photomicrographs of sampling squares taken in tissue stained to label neurons producing Fos-ir in LScvl. Scale bar represents 50 μm
Statistical analyses
In two trials (n = 6 observers) the demonstrator did not eat and in one trial the demonstrator pecked at the demonstrated feeder only six times (average = 150, standard deviation = 55; n = 3 observers), so these observers were removed from the experiment. Two observers did not eat during the test phase and three more observers were removed due to technical difficulties (1 = video failed; 2 = demonstrator feeder moved during the observation phase). One brain was damaged during extraction. The final number for all behavioural and neural measures was 20 females.
An independent-samples t-test was conducted to test for differences in the number of pecks by the demonstrator to the feeder that contained seed when the demonstrated container was pink versus purple. To test for systematic copying by the observers a one-sample Wilcoxon signed rank test on the proportion of pecks to the demonstrated colour feeder was conducted with the chance level set to 0.5 (which means no preference). Fos-ir was significantly positively correlated r(20) = 0.56, p = 0.01 in the two sub-regions sampled in the hippocampus and thus these sub-regions were combined into one measure for the hippocampus (Hp, hereafter). A one-way repeated-measures (within-subjects) test was conducted to test for differences in Fos-ir neurons across the 12 brain areas and sub-divisions, the Huynh-Feldt correction for the violation of the sphericity assumption and all reported values were Bonferroni-corrected to allow for multiple comparisons. Spearman correlations were performed on the average Fos-ir in each of the brain areas and the proportion of pecks to the demonstrated colour (social learning score) by the observer birds in the test phase. T-tests were conducted to test for differences between birds that copied (social learning score > 0.5, n = 10) and birds that did not copy (social learning score < 0.5, n = 10) the feeder colour of the demonstrator in the average Fos-ir in each brain area. In cases of unequal variances between groups (n = 2, LSr, BSTmv) an adjusted t-distribution was used. Stepwise linear regressions were used to test whether the observers’ behaviour during the observation phase (number of scratches, hops, drinks, preen, vocalizations, pecks) predicted Fos-ir in each of the brain areas. Spearman correlations were performed to test whether the behaviour of the demonstrator during the observation phase (i.e., the behaviour that the observers could view/hear: pecks and vocalizations) was related Fos-ir in each of the brain areas of the observers. All analyses were conducted in IBM SPSS Statistics 26 or 28.
Results
Demonstrator performance
The demonstrators pecked only at the feeder that contained seeds and did not peck more at one colour-baited feeder than at the other (number of pecks to the purple feeder: 168 ± 48, range 133–237; pink: 137 ± 59, range 90–220; t7=-0.86, p = 0.42).
Observer performance
The number of pecks to feeders by the observers ranged from 91 to 558. Overall, the female observers did not prefer the feeder colour of that used by their demonstrator (Wilcoxon signed-rank test, Wstandardized = -0.51, N = 20, p = 0.61; Fig. 3). As on further investigation we found that observers tended to prefer pink over purple feeders (Wstandardized = 1.91, N = 20, p = 0.056), we conducted two additional one-sample Wilcoxon signed rank tests: one for birds that watched a demonstrator feeding from a pink feeder (Wstandardized = 1.10, N = 12, p = 0.27), and the second for the females that watched a male feeding from a purple feeder (Wstandardized = -1.68, N = 8, p = 0.09). Neither of these comparisons significantly differed from no preference (0.5).
Relationship between Fos-ir and observer and demonstrator behaviour in the observation phase
Several observer behaviours predicted Fos-ir significantly across four of the brain areas measured, see Table 1 for all β and P values: observers that drank more had lower Fos-ir in the AH, while increased vocalizations, pecks and preens were related to lower Fos-ir in the VMH. There was one positive significant relationship: observers that preened more had higher Fos-ir in the LScv. Demonstrator behaviour, the number of pecks, was negatively correlated with Fos-ir in two brain areas: more pecks by the demonstrator was correlated with lower Fos-ir in the LScv and LScvl; see Table 2 for Pearson r and p values.
Differences in Fos-ir among the different brain regions
There were significant differences in Fos-ir neurons across the brain areas we measured F(1,5.37) = 10.56, p < 0.0001, η2partial = 0.36). See Fig. 4 for the mean Fos-ir number by brain region and OSM Table S1 for the significance levels that are Bonferroni-corrected to allow for multiple comparisons.
Relationship between social learning score and neural score
There were no significant correlations between the social learning score and Fos-ir in any brain region measured with the exception of the BSTmv: birds with lower social learning scores had more Fos-ir in the BSTmv (p = 0.05; Fig. 5; Table 2 for Spearman R and P values, all ns = 20). When observers were separated into those females that copied (n = 10) and those females that did not copy (n = 10), the demonstrator’s feeder colour choice, Fos-ir in the BSTI (p = 0.06) and BSTmv (p = 0.07, Fig. 6; Table 2 for T and P values, all dfs = 18), tended to be higher in those birds that did not copy the demonstrator.
Discussion
Some females copied the novel colour feeder demonstrated by males, while others did not. There was also variation in the extent to which females used the social information: the proportion of choices to the feeder that matched the demonstrated colour was not all-or-none, and most of the females opted to eat from both novel-coloured feeders. We found some variation in the Fos-ir among some brain regions (and sub-divisions): female observers that copied the novel feeder colour used by male demonstrators had fewer Fos-ir positive neurons in the BSTmv and also tended to have fewer Fos-ir positive neurons in the lateral subdivision of the BST. Females that drank more had fewer Fos positive neurons in the anterior hypothalamus, and increased pecking, vocalizing and preening was associated with lower Fos activity in the ventromedial hypothalamus. Moreover, the more pecks a demonstrator male made to a feeder while the females observed him, the lower Fos-ir in her lateral septum.
We had two unexpected results: (1) we did not find group-wide copying of male demonstrator foraging choices by female observers; (2) we found little immediate early gene activity related to copying. The lack of group-wide copying may have been due to a preference for pink over purple feeders: females tended not to copy when their demonstrator fed from a purple feeder colour rather than if he fed from a pink feeder. In a previous experiment by the same researchers, female zebra finches had an overarching colour preference for white rather than black feeders, and this preference obscured evidence of social learning (Guillette et al., 2014). Unexplained pre-existing colour preferences for both food (e.g., Rosa et al., 2012) and nest material (e.g., Muth et al., 2013) can make interpreting the results of social learning experiments difficult, especially when there are no pre-tests for colour preferences, as in the current experiment. On the other hand, testing for pre-existing colour preferences prior to a social intervention (e.g., a conspecific foraging from one of two pre-tested feeders), can also obscure social information acquisition and/or use because as an individual collects asocial information (via the pre-test) they may be less likely to socially learn because their uncertainty about the environment is reduced (Guillette et al., 2014; Laland, 2004; Rosa et al., 2012).
Our second unexpected result was that we found little evidence for Fos-ir activity in brain regions associated with social behaviour that were related to the observed variation in social learning. Indeed, female zebra finches that copied the novel feeder colour used by male demonstrators had fewer Fos-ir positive neurons in the BSTmv, a sub-region of the extended medial amygdala that is involved with social and appetitive arousal. This result is consistent with that of Goodson et al. (2005), who found that Fos-ir in the BSTm (they did not divide this region into sub-regions) was lower as the sociality of finch species increased (sociality = average group size). In that experiment Goodson et al. (2005) exposed male and female finches to a social stimulus (same-sex conspecifics), and found the lowest Fos-ir in colonial species (zebra finches and spice finches Lonchura punctulata), moderate Fos-ir in the social, but not colonial, Angolan blue waxbill Uraeginthus angolensis, and the highest Fos-ir in the territorial violet-eared waxbill Uraeginthus granatina. Why this relationship is negative is unclear, but Goodson et al. (2005) proposed that a higher response may indicate social stress. If this is the case, then it could be that the females in our experiment that were more stressed were thereby less likely to copy a male’s feeder choice. Future work might, then, involve examining whether female acute stress response (corticosterone) during social information acquisition is associated with social learning (copying). Nonetheless, the BSTm does appear to regulate behavioural responses to conspecifics, and may therefore play a role in whether or not individuals are more, or less, likely to acquire or use social information. Other work, again using finch species that vary in sociality, showed that neuropeptide receptor densities in the BSTm and other regions in the septal complex increased as the species-typical group size increased (Goodson & Wang, 2006). Moreover, vasotocin immunoreactive neurons in the BSTm increased in IEG expression in gregarious species that were exposed to conspecifics but decreased in expression in territorial species that were exposed to conspecifics (Goodson & Wang, 2006).
We also share with Goodson et al. (2005) changes in Fos-ir in the lateral septum: in both cases as the demonstrators did more demonstrating, less activity was found in the lateral septum of the observers. Goodson et al. (2005) found that territorial birds (less social) had a significantly higher IEG response compared to more social species. Work in song sparrows (Melospiza melodia) indicates that multiple subdivisions in the LS show IEG responses to stressors, and our lateral septum data may, as suggested above, be a result of social stress (Goodson & Evans, 2004).
Conclusion
To our knowledge this is the first study describing an attempt to link an individual’s social learning on a foraging task to the IEG activity in their social behaviour network for the period of time during which they learned information from a demonstrator. We hypothesized that the neural activity in the SBN during information acquisition (learning) may influence the degree to which an individual learns from a demonstrator and therefore predicted among-individual co-variation in social learning score and neural activity. Overall, we found weak evidence of co-variation between neural activity in subdivisions of the LS and BST and what individuals watched during learning and their behavioural output (i.e., evidence of social learning), respectively. Moreover, the direction of the co-variation maps onto earlier work on social information processing conducted among finch species (e.g., Goodson et al., 2006). Although there is agreement between our data and the Goodson et al. work, we did not find strong relationships in IEG activity with the degree to which females learned from observing a male’s choice of feeder colour. One explanation is that our social learning score (i.e., whether or not the female copied the foraging choice of her demonstrator) was taken from a period of time after we took a snapshot of IEG activity. If a female acquired social information from her demonstrator but did not use it, our social learning score would not have correlated with her IEG activity. We suggest at least two possible future avenues for work: (1) Examining IEG activity in the social behaviour network in the interval when the observer puts learned information to use; and (2) determining more specifically the functions of the various regions in the social behaviour network. This would usefully be coupled with a clearer articulation of the aspects of social learning that differ from learning in an asocial context (e.g., Heyes, 2012; Heyes & Pearce, 2015). Given the considerable body of work on social learning, it now seems timely that effort would be especially directed to addressing these issues.
Data availability
The data for all experiments are available upon request from the corresponding author; none of the experiment was preregistered.
Abbreviations
- AH:
-
anterior hypothalamus
- AMV*:
-
anterior ventral mesopallium
- BSTmd:
-
bed nucleus of the stria terminalis, dorsalmedial
- BSTmv:
-
bed nucleus of the stria terminalis, ventromedial
- BSTl:
-
bed nucleus of the stria terminalis, lateral
- dHP:
-
dorsal hippocampus
- LScv:
-
lateral septum, ventral caudal
- LScvl:
-
lateral septum, lateral ventral caudal
- LSr:
-
lateral septum, rostral
- mHP:
-
media hippocampus
- MS:
-
medial septum
- POM:
-
medial preoptic area
- VMH:
-
ventromedial hypothalamus
References
Aplin, L. M., Farine, D. R., Cockburn, A., Thornton, A., & Sheldon, B. C. (2015). Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature, 518, 538–341. https://doi.org/10.1038/nature13998
Auersperg, A. M. I., von Bayern, A. M. I., Weber, S., Szabadvari, A., Bugnyar, T., & Kacelnik, A. (2014). Social transmission of tool use and tool manufacture in Goffin cockatoos (Cacatua goffini). Proceedings of the Royal Society B, 281, 20140972.
Belzner, S., Voigt, C., Catchpole, C. K., & Leitner, S. (2009). Song learning in domesticated canaries in a restricted acoustic environment. Proceedings of the Royal Society B, 276, 2881–2886. https://doi.org/10.1098/rspb.2009.0669
Carter, A. J., Marshall, H. H., Heinsohn, R., & Cowlishaw, G. (2014). Personality predicts the propensity for social learning in a wild primate. PeerJ, 2014(1), 1–22. https://doi.org/10.7717/peerj.283
Dere, E., Winkler, D., Ritter, C., Ronnenberg, A., Poggi, G., Patzig, J., Gernert, M., Müller, C., Nave, K. A., Ehrenreich, H., & Werner, H. B. (2015). Gpm6b deficiency impairs sensorimotor gating and modulates the behavioral response to a 5-HT2A/C receptor agonist. Behavioural Brain Research, 277(SI), 254–263. https://doi.org/10.1016/j.bbr.2014.04.021
Duffy, G. A., Pike, T. W., & Laland, K. N. (2009). Size-dependent directed social learning in nine-spined sticklebacks. Animal Behaviour, 78(2), 371–375. https://doi.org/10.1016/j.anbehav.2009.05.015
Edwards, S. C., Hall, Z. J., Ihalainen, E., Bishop, V. R., Nicklas, E. T., Healy, S. D., & Meddle, S. L. (2020). Neural Circuits Underlying Nest Building in Male Zebra Finches. Integrative and Comparative Biology, 60(4), 943–954. https://doi.org/10.1093/icb/icaa108
Feenders, G., Liedvogel, M., Rivas, M., Zapka, M., Horita, H., Hara, E., Wada, K., Mouritsen, H., & Jarvis, E. D. (2008). Molecular mapping of movement-associated areas in the avian brain: A motor theory for vocal learning origin. PLoS ONE, 3(3). https://doi.org/10.1371/journal.pone.0001768
Galef, B. G. (2009). Strategies for Social Learning. Testing Predictions from Formal Theory. Advances in the Study of Behavior, 39, 117–151. https://doi.org/10.1016/S0065-3454(09)39004-X
Goodson, J. L. (2005). The vertebrate social behavior network: evolutionary themes and variations. Hormones and Behavior, 48(1), 11–22. https://doi.org/10.1016/j.yhbeh.2005.02.003
Goodson, J. L. (2008). Nonapeptides and the evolutionary patterning of sociality. Progress in Brain Research, 170, 3–15. https://doi.org/10.1016/S0079-6123(08)00401-9
Goodson, J. L., & Evans, A. K. (2004). Neural responses to territorial challenge and nonsocial stress in male song sparrows: Segregation, integration, and modulation by a vasopressin V 1 antagonist. Hormones and Behavior, 46(4), 371–381. https://doi.org/10.1016/j.yhbeh.2004.02.008
Goodson, J. L., Evans, A. K., Lindberg, L., & Allen, C. D. (2005). Neuro-evolutionary patterning of sociality. Proceedings of the Royal Society B: Biological Sciences, 272(1560), 227–235. https://doi.org/10.1098/rspb.2004.2892
Goodson, J. L., Evans, A. K., & Wang, Y. (2006). Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Hormones and Behavior, 50(2), 223–236. https://doi.org/10.1016/j.yhbeh.2006.03.005
Goodson, J. L., & Wang, Y. (2006). Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 17013–17017. https://doi.org/10.1073/pnas.0606278103
Griffin, A. S. (2004). Social learning about predators: A review and prospectus. Learning & Behavior, 32(1), 131–140.
Guillette, L. M., & Healy, S. D. (2014). Mechanisms of copying behaviour in zebra finches. Behavioural Processes, 108, 177–182. https://doi.org/10.1016/j.beproc.2014.10.011
Guillette, L. M., & Healy, S. D. (2017). The roles of vocal and visual interactions in social learning zebra finches: A video playback experiment. Behavioural Processes, 139, 43–49. https://doi.org/10.1016/j.beproc.2016.12.009
Guillette, L. M., Morgan, K. V, Hall, Z. J., Bailey, I. E., & Healy, S. D. (2014). Food preference and copying behaviour in zebra finches, Taeniopygia guttata. Behavioural Processes, 109, 145–150. https://doi.org/10.1016/j.beproc.2014.04.013
Guillette, L. M., Scott, A. C. Y., & Healy, S. D. (2016). Social learning in nest-building birds: a role for familiarity. Proceedings of the Royal Society B: Biological Sciences, 283, 20152685.
Hall, Z. J., Bertin, M., Bailey, I. E., Meddle, S. L., & Healy, S. D. (2014). Neural correlates of nesting behavior in zebra finches (Taeniopygia guttata). Behavioural Brain Research, 264, 23–33. https://doi.org/10.1016/j.bbr.2014.01.043
Heimovics, S. A., & Riters, L. V. (2007). ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris). Behavioural Brain Research, 176(2), 333–343. https://doi.org/10.1016/j.bbr.2006.10.023
Heyes, C. (1994). Social learning in animals: categories and mechanisms. Biological Reviews of the Cambridge Philosophical Society, 69, 207–231. https://doi.org/10.1111/j.1469-185X.1994.tb01506.x
Heyes, C. (2012). What’s social about social learning? Journal of Comparative Psychology, 126(2), 193–202. https://doi.org/10.1037/a0025180
Heyes, C., & Pearce, J. M. (2015). Not-so-social learning strategies. Proceedings of the Royal Society B, 282, 20141709. https://doi.org/10.1098/rspb.2014.1709
Johnson, Z. V., & Young, L. J. (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neuroscience and Biobehavioral Reviews, 76, 87–98. https://doi.org/10.1016/j.neubiorev.2017.01.034
Johnson, Z. V, & Young, L. J. (2015). Neurobiological mecahnisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences, 3, 38–44. https://doi.org/10.1016/j.cobeha.2015.01.009.Neurobiological
Kis, A., Huber, L., & Wilkinson, A. (2015). Social learning by imitation in a reptile (Pogona vitticeps ). Animal Cognition, 18, 325–331. https://doi.org/10.1007/s10071-014-0803-7
Laland, K. N. (2004). Social learning strategies. Learning & Behavior, 32(1), 4–14.
Leadbeater, E., & Chittka, L. (2007). Social Learning in Insects — From Miniature Brains to Consensus Building Review. Current Biology, 703–713. https://doi.org/10.1016/j.cub.2007.06.012
Mello, C. V., Kaser, T., Buckner, A. A., Wirthlin, M., & Lovell, P. V. (2019). Molecular architecture of the zebra finch arcopallium. Journal of Comparative Neurology, 527(15), 2512–2556. https://doi.org/10.1002/cne.24688
Mesoudi, A., Chang, L., Dall, S. R. X., & Thornton, A. (2016). The Evolution of Individual and Cultural Variation in Social Learning. Trends in Ecology and Evolution, 31(3), 215–225. https://doi.org/10.1016/j.tree.2015.12.012
Muth, F., Steele, M., & Healy, S. D. (2013). Colour preferences in nest-building zebra finches. Behavioural Processes, 99, 106–111. https://doi.org/10.1016/j.beproc.2013.07.002
Nixdorf-Bergweiler, B., & Bischof, H.-J. (2007). A Sterotaxic Atlas of the Brain of the Zebra Finch, Taeniopygia guttata, with Special Emphasis on the Telencephalic and Song System Nuclei in Transverse and Saggital Sections. National Center for Biotechnology Information (US). http://pub.uni-bielefeld.de/publication/1870999
O’Connell, L. A., & Hofmann, H. A. (2011). The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. Journal of Comparative Neurology, 519(18), 3599–3639. https://doi.org/10.1002/cne.22735
Olsson, A., Knapska, E., & Lindström, B. (2020). The neural and computational systems of social learning. Nature Reviews Neuroscience https://doi.org/10.1038/s41583-020-0276-4
Pike, T. W., & Laland, K. N. (2010). Conformist learning in nine-spined sticklebacks’ foraging decisions. Biology Letters, 6(4), 466–468. https://doi.org/10.1098/rsbl.2009.1014
Rendell, L., Fogarty, L., Hoppitt, W. J. E., Morgan, T. J. H., Webster, M. M., & Laland, K. N. (2011). Cognitive culture: Theoretical and empirical insights into social learning strategies. Trends in Cognitive Sciences, 15(2), 68–76. https://doi.org/10.1016/j.tics.2010.12.002
Rosa, P., Nguyen, V., & Dubois, F. (2012). Individual differences in sampling behaviour predict social information use in zebra finches. Behavioral Ecology and Sociobiology, 66(9), 1259–1265. https://doi.org/10.1007/s00265-012-1379-3
Slagsvold, T., & Wiebe, K. L. (2011). Social learning in birds and its role in shaping a foraging niche. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 366(1567), 969–977. https://doi.org/10.1098/rstb.2010.0343
Stokes, T. M., Leonard, C. M., & Nottebohm, F. (1974). The Telencephalon, Diencephalon, and Mesencephalon of the Canary, Serinus canaria, in Sterotaxic Coordinates. Journal of Comparative Neurology, 156, 337–374.
Watson, S. K., Vale, G. L., Hopper, L. M., Dean, L. G., Kendal, R. L., Price, E. E., Wood, L. A., Davis, S. J., Schapiro, S. J., Lambeth, S. P., & Whiten, A. (2018). Chimpanzees demonstrate individual differences in social information use. Animal Cognition, 21(5), 639–650. https://doi.org/10.1007/s10071-018-1198-7
Yusufishaq, S., & Rosenkranz, J. A. (2013). Post-weaning Social Isolation Impairs Observational Fear Conditioning. Behavioural Brain Research, 242, 142–149. https://doi.org/10.1016/j.bbr.2012.12.050.Post-weaning
Acknowledgements
This work was supported by Biotechnology and Biological Sciences Research Council Anniversary Future Leaders Fellowship; NSERC Discovery Launch Accelerator DGECR-2019-00173; NSERC Discovery Grant RGPIN-2019-04733 and a Start-Up Grant from the Faculty of Science at the University of Alberta to Lauren Guillette, and Roslin Institute Strategic grant funding from the Biotechnology and Biological Sciences Research Council, UK (BB/P013759/1) to Simone Meddle. We thank Sophie Edwards, Eira Ihalainen and the animal care staff at the University of St Andrews for assistance with the birds. We also thank two reviewers for their thoughtful comments on an earlier version of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
*Note that Mello et al., 2019, propose that AMV should refer to the medial ventral arcopallium
Supplementary information
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
Guillette, L.M., Vámos, T.I.F., Healy, S.D. et al. Involvement of the neural social behaviour network during social information acquisition in zebra finches (Taeniopygia guttata). Learn Behav 50, 189–200 (2022). https://doi.org/10.3758/s13420-022-00511-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-022-00511-x