Abstract
Predation can be a very strong selective pressure on prey. Many studies have shown the existence of innate anti-predator responses, mostly in the early developmental stages of juvenile vertebrates. Learning to recognize predators is another possible defensive resource, but such a method involves a high death risk. There is evidence that prenatal learning exists in animals but few studies have explicitly tested for embryonic learning. The aim of this study was to test innate and learned predator recognition in cuttlefish embryos. For this, naïve embryos were exposed to chemical and visual cues emanating from predators, non-predators, and ink. Their response was assessed by measuring their ventilation rate (VR). We first show that VR decreased in response to both visual and chemical predatory cues and ink but not to non-predatory cues. Second, we show that when non-predatory cues (visual or chemical) are paired with predatory cues or ink for several days, embryonic VR significantly decreased. Such a response is likely adaptive, especially in a translucent egg, since it results in reduced movement and hence may lower the risk of detection by visual predators. This freezing-like behavior may also reduce the bioelectric field, thus lessening the predation risk by non-visual foragers. Our results report that cuttlefish embryos had an innate capacity to differentiate between harmless and harmful chemical and visual cues. They were also capable of learning to respond to harmless cues when they were paired with danger (predator or ink) based on conditioning. The combination of these behavioral mechanisms is an example of the early adaptability of cephalopods. Such behavioral plasticity may give the newly hatched cuttlefish a selective advantage when dealing with either known or unfamiliar threats. Nevertheless, more experiments are needed to test the efficiency of the embryos’ response faced with known or new predators.
Similar content being viewed by others
Introduction
From the first moments of life, many animals must be able to protect themselves from predators as well as find food. To ensure survival, juveniles must be able to recognize predators at a very early stage in order to avoid them. Predator recognition is based on a strong innate component. In mammals, birds, amphibians, reptiles, fishes or snails, animals that are preyed on must use chemical, visual and/or auditory cues to identify their predators (Amo, López, & Martín, 2005; Balderas-Valdivia, Ramírez-Bautista, & Carpenter, 2005; Barreto, Luchiari, & Marcondes, 2003; Brown, Kreiter, Maple, & Sinnott, 1992; Dalesman, Rundle, Coleman, & Cotton, 2006; Dalesman, Rundle, & Cotton, 2007; Fendt, 2006; Griffiths, Schley, Sharp, Dennis, & Roman, 1998; Hartman & Abrahams, 2000; Hawkins, Magurran, & Armstrong, 2004; Hirsch & Bolles, 1980; Saunders, Ong, & Cuthbert, 2013). Certain prey species, however, need to learn to recognise and thus avoid their predators. Acquired predator recognition has been shown in a diverse range of taxa: birds (Curio, Ernst, & Vieth, 1978); mammals (Kindermann, Siemers, & Fendt, 2009); fishes (Chivers & Smith, 1998; Kelley & Magurran, 2003; Mathis & Smith, 1993); amphibians (Chivers & Smith, 1998; Epp & Gabor, 2008; Ferrari, Manek, & Chivers, 2010; Mathis, Ferrari, Windel, Messier, & Chivers, 2008; Mirza, Ferrari, Kiesecker, & Chivers, 2006; Wisenden, 2003; Woody & Mathis, 1998) and invertebrates (snail: Aizaki & Yusa, 2010; mosquitoe: Ferrari, Messier, & Chivers, 2008; common whelk: Rochette, Arsenault, Justome, & Himmelman, 1998; damselflies: Wisenden, Chivers, & Smith, 1997; planarians: Wisenden & Millard, 2001). One mode of learning is through the pairing of cues linking a predator with an alarm signal (classical conditioning). Indeed, in a marine environment the usual way for prey to detect and identify predators is by recognizing olfactory and visual information (Brown & Smith, 1998; Hartman & Abrahams, 2000; Kats & Dill, 1998; Miklósi, Pongrácz, & Csányi, 1997; Utne-Palm, 2001).
Predator recognition can be learned even in the early stages of development. Within the protective egg-case embryos are able to perceive environmental stimuli that identify risk factors likely to be present in their post-hatching environment. This embryonic learning ability has been extensively studied in amphibians (Ferrari & Chivers, 2009a, 2009b, 2010; Ferrari, Crane, & Chivers, 2016; Ferrari et al., 2010; Golub, 2013; Mathis et al., 2008; Saglio & Mandrillon, 2006). The first study explicitly showing this ability to recognize predators was conducted by Mathis et al. (2008). It demonstrated that when salamander eggs (Ambystoma annulatum) were exposed to chemical predatory cues, larvae showed anti-predatory behaviors such as shelter-seeking and reduced locomotor activity (Mathis et al., 2008). Subsequently, further studies have shown that predator recognition can also be learned and generalized to other similar predators (Ferrari & Chivers, 2009b). By observing post-hatching responses, Ferrari and colleagues have shown that amphibian embryos can learn to recognize chemical cues before hatching by using associative identification cues concerning predators or their diet and/or alarm signals such as the smell of injured congeners (Ferrari & Chivers, 2009a, 2009b, 2010; Ferrari et al., 2010; Garcia, Urbina, Bredeweg, & Ferrari, 2017).
Cuttlefish are oviparous cephalopod molluscs. Embryos develop in soft elastic egg cases and juveniles do not receive direct parental care since adult males die soon after mating and females after egg-laying (Boyle, 1987; Lee, Lin, Chiao, & Lu, 2016). Romagny and collaborators (2012) showed that the different sensory systems in Sepia officinalis are functional before hatching: they observed mantle contractions after tactile, olfactory, and light stimulations. Furthermore, other studies have highlighted indirect prenatal learning (Darmaillacq, Lesimple, & Dickel, 2008; Darmaillacq, Mezrai, O’Brien, & Dickel, 2017; Guibé, Poirel, Houdé, & Dickel, 2012). Indeed, cuttlefish embryos that have been exposed to small crabs before hatching prefer crabs to their innately preferred shrimp prey (Darmaillacq et al., 2008). Likewise, cuttlefish that innately prefer black crabs will preferentially select white crabs following embryonic exposure to them (Guibé et al., 2012). Unlike Sepia officinalis in which the egg case is darkened by maternal ink, in the pharaoh cuttlefish (Sepia pharaonis) eggs are totally transparent. This allows direct observation of embryo response to external stimuli, whether chemosensory or visual, and thus tests of embryonic learning abilities without modification of the egg capsule.
The aim of this study is to see whether Sepia pharaonis embryos have an innate recognition capacity for predators and the ability to learn novel association. In order to test their innate visual and chemical recognition capabilities, embryos were exposed to predatory and non-predatory cues. To test their learned visual and chemical recognition capabilities, a classical conditioning procedure was used, involving the pairing of a neutral stimulus (the sight or odor of a non-predatory fish) with an alarm signal: cuttlefish ink, which can be a relevant warning signal (Derby, 2014). The ink is composed of secretions from two glands: (1) the ink-bag gland that produces melanin-tinted black ink, and (2) the mucus-producing gland in the funnel. Cuttlefish ink is composed not only of melanin, but also of catecholamines, DOPA and dopamine (both monoamines derived from tyrosine), amino acids such as taurine, as well as certain metals such as cadmium, copper, and lead (Derby, 2014; Madaras, Gerber, Peddie, & Kokkinn, 2010; Prota et al., 1981). Cephalopod inking would thus be a defense strategy acting as a direct predator deterrent or a lure (interspecific effects); it can also serve as an alarm cue for nearby conspecifics or embryos, indirectly signaling the presence of danger (intraspecific effect) (Derby, 2014; Hanlon & Messenger, 2018). We can propose the hypothesis that embryos use chemical cues for predator recognition, as do many vertebrates (e.g., amphibians) and some invertebrate species (e.g., mosquitos), but also visual cues due to the characteristics of the egg case. Likewise, we hypothesize that embryos can learn about a new danger through chemosensory and visual cues by associative learning. Unlike Romagny et al. (2012), ventilation rate (VR; number of ventilations per minute) was used as a behavioral measure rather than mantle contractions because VR can be used to monitor more subtle responses to low-intensity stimuli (Boal & Ni, 1996). Indeed, in addition to mantle contractions, decreased ventilation and bradycardia can be observed in cuttlefish after sudden visual or chemical stimulation (King & Adamo, 2006). Unlike heart rate, VR is easily and directly observable in cuttlefish under the microscope, either by noting the rhythmic motion of the collar flaps circulating oxygenated water to the gills, or by the movement of the funnel in response to pressure changes resulting from respiratory movements (inhalation and exhalation).
Materials and methods
Biological model used
Experimental model
The model species used in the study is the pharaoh cuttlefish (Sepia pharaonis). The pharaoh cuttlefish is one of the most important aquaculture species of cephalopod and, in the wild, is widely distributed from the east African coast to the west Pacific Ocean (Anderson et al., 2010). Adults (four females and two males) were fished and housed in a semi-natural area in Academia Sinica Marine Research Station or Aquaticlch Biotech Company Ltd. aquaculture (Yilan, Taiwan), in a concrete pool of ca. 7 × 6 × 0.5 m (25 ± 2°C). After mating, females laid hundreds of eggs under plates (30 × 30 cm) available in the tank. Since females were housed together and laid at the same locations, it was not possible to control for sibship. Eggs were then collected and transferred before organogenesis to the Institute for Behavioral Experiments (Institute of Systems Neuroscience and Department of Life Science, National Tsing Hua University, Hsinchu, Taiwan). The transfer to Hsinchu was made by car and the eggs were transported in large containers (30 × 50 × 30 cm) filled with natural seawater, oxygenated by a bubbler pump. At the institute, eggs were maintained in the laboratory. Tanks were supplied with natural sea water coming from a two closed-circulation aquarium system (700 L each), at a temperature of 25 ± 2°C and on a 12:12 h light:dark cycle; about one-third of the water of the tank was replaced each week with fresh natural sea water. The eggs were separated individually from the clusters and incubated in plastic baskets floating in the culture tank (20 eggs maximum per basket of 15 × 20 × 3cm). The volume of each tank was 300 L.
Embryonic development
The developmental schedule differed for each of the eggs since they are laid singly, and the spawning period may last for several days. It took 22–24 days to complete embryonic development at a water temperature between 22°C and 25°C (Lee et al., 2016). On the basis of morphological characteristics, 30 stages were observed during the embryonic development of S. pharaonis: cleavage from stages 1 to 9, blastulation and gastrulation from stages 10 to15, and organogenesis from stages 16 to 30 (Lee et al., 2016). During embryogenesis the sensory systems start to develop and become functional (Romagny, Darmaillacq, Guibé, Bellanger, & Dickel, 2012) and embryos are able to recognize and respond to chemical cues (Mezrai, Chiao, Dickel, & Darmaillacq, 2019). Indeed, embryos respond to predator odor (Narrow-lined Pufferfish: Arothron manilensis) but not to non-predator odor (Clownfish: Amphiprion percula) as from stage 25 (Mezrai et al., 2019).
Experimental apparatus and stimuli used
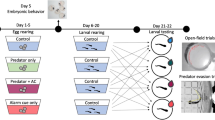
All experiments were conducted in an opaque 36 × 22 × 25cm tank in order to isolate the embryos from any external visual interference (cf. Fig. 1). The top glass is removed so that natural light illuminates the inside of the tank. Each embryo is used only once (one trial per treatment so that embryos are completely naive). Embryonic behavior was recorded with an underwater camera (Olympus Stylus Tough TG-4) and data were analyzed from videos after the experiments. Each cuttlefish embryo (stage 25) was placed on the bottom and in the center of the tank for 5 min (acclimation phase). Then, the chemical or visual stimuli were presented to the embryo (stimulation phase).
Schematic representation of the experimental device used. The cuttlefish egg is placed on the bottom in the center of the tank. The camera is positioned in front of the embryo in order to record its responses. (Left) Chemical stimulation test: The fish is in another tank connected to the experimental tank via a water pump. (Right) Visual stimulation test: The fish is placed in the embryo tank but the embryo is enclosed in a transparent glass container
For the chemical stimulation (see below), the aquarium with the chemical stimulus (predator, non-predator, or diluted ink) was placed next to the embryo’s tank and connected to a water pump. During the stimulation phase, the pump was turned on so that the odor appeared close to the embryo (80 mL/min).
For the visual stimulation (see below), the egg was placed in a transparent glass container (6 × 4 × 4cm) to protect it from predators and avoid chemical exposure, placed in a bigger aquarium. For groups 1 and 2, the fish was directly placed in the embryo’s aquarium. For group 3, 3 mL of black ink was presented close to the embryo’s box.
All fishes and cuttlefish used were maintained in natural seawater (25 ± 2°C; see above) oxygenated by a bubbler pump installed in each aquarium, on a 12:12 h light:dark cycle.
Visual stimuli
Three visual stimulations were presented to the cuttlefish embryos:
-
1)
Predator fishes: The predator used was the Narrow-lined Pufferfish (Arothron manilensis) fed daily on standard food (five frozen-thawed shrimps).
-
2)
Non-predator fishes: The non-predator used was the Clownfish (Amphiprion percula) fed on standard herbivorous aquarium food.
Predators and non-predators were of comparable size (4–6 cm) and displayed similar swimming activity in the experimental tank (size: 20 × 60 × 30 cm).
-
3)
Black ink: Black cuttlefish ink was obtained by approaching 1-week-old cuttlefish (placed in a 300-mL glass container) with a net to induce the ejection of clouds of ink (mucus and ink) until the container became saturated with ink (i.e., the water was totally black and the cuttlefish invisible); the cuttlefish were then returned to their home-tank.
Chemical stimuli
Four different chemical stimulations were presented to the cuttlefish embryos:
-
1, 2) Predatory fishes: the predator used was the Narrow-lined Pufferfish (Arothron manilensis). Two groups of Pufferfish were used: the first group was fed daily on standard food (five frozen-thawed shrimps). Pufferfish in the second group were given one cuttlefish embryo per day.
-
3) Non-predator fishes: the non-predator used was the Clownfish (Amphiprion percula), and this group was fed ad libitum on standard herbivorous aquarium food.
-
4) Ink odor: 3 mL of ink obtained as described above was then added to 150 mL of blank seawater and blended until the cloud of ink was dispersed (diluted) and the solution became clear.
Experiment 1: Innate recognition test
Innate chemical recognition test
Experimental conditions:
-
1)
Blank seawater (“C,” control condition); n=9
-
2)
Clownfish odor (“NP,” non-predator condition); n=8
-
3)
Pufferfish odor when fed with shrimps (“Pshrimp,” predator condition); n=6
-
4)
Pufferfish odor when fed with cuttlefish embryos (“Pembryo,” predator condition); n=6
-
5)
Cuttlefish ink (“I,” ink condition); n=17
Innate visual recognition test
Experimental conditions:
-
1)
Clownfish (“NP,” non-predator condition); n=10
-
2)
Pufferfish (“P,” predator condition); n=10
-
3)
Black cuttlefish ink (“I,” ink condition); n=12
Ventilation rate (VR) was recorded during both the last minute of the acclimation period before the stimulation and the first minute under experimental conditions (stimulation time). Data collection was carried out by manually counting the VR from videos. Preliminary studies showed that embryos responded immediately when exposed to stimulation: during acclimation VR did not change but it did change during the stimulation phase. The observer was blind to the treatments involved.
Experiment 2: Learned recognition test
Conditioning phase
A classical conditioning procedure was used. The Clownfish (NP; non-predator) was used as a conditional stimulus (CS) and the cuttlefish ink (I) was used as an unconditional stimulus (US). Each embryo was exposed to the CS coupled with the US once a day for 30 min over a period of 4 days. The stimuli used in this experiment were obtained according to the same procedure as in the innate recognition tests described above.
Two groups were tested for chemical recognition:
-
1)
The first group, experimental group “NP+I” (n=11), included embryos exposed to a Clownfish odor (Non-Predator) paired with cuttlefish ink odor.
-
2)
The second group, control group “NP” (n=12), included embryos exposed to a Clownfish odor alone (Non-Predator).
Two groups were tested for visual recognition:
-
1)
The first group, experimental group “NP+I” (n=10), included embryos exposed to a Non-Predator (Clownfish) paired with cuttlefish Ink clouds.
-
2)
The second group, control group “NP” (n=12), included embryos exposed to a Non-Predator alone (Clownfish).
Testing phase
On day 5, all embryos were tested with the odor or sight of a Clownfish alone. Data collection was carried out by manually counting the VR 1 min before and after the stimulation phase. The observer was blind to the treatments involved.
Statistical analyses
Data were non-normal. As a consequence, nonparametric statistical tests were used. A permutation paired t-test was used to compare VR during the acclimation and stimulation phase (R©3.6.0). The α level for all analyses was 0.05. As in a previous study (Mezrai et al., 2019), data have been normalized, and for the graphical representation, each histogram bar represents the index calculated as follows:
This index shows whether the VR increases or decreases as a result of stimulation (positive values mean that the VR increases after the stimulation; negative values mean that the VR decreases).
Ethical note
All animals (fishes and cuttlefish) and the entire protocol were approved by the National Tsing Hua University Institutional Animal Care and Use Committee (IACUC Protocol No. 10510). Throughout the protocol, we followed the published guidelines for the care and welfare of cephalopods to avoid stress in test animals (Fiorito et al., 2015).
Results
Innate recognition
The ventilation rate (VR) of each embryo was measured before stimulation (VR acclimation) and after stimulation (VR stimulation). By using the index I, we could then see whether the resulting VR decreased or increased as a result of this stimulation.
Chemical
Embryo VR did not change after exposure to blank seawater (“C” group), to non-predator odor (“NP” group), or to odor of the predator fed on shrimps (“Pshrimp” group) (Fig. 2: C group: T=0.00; p=1; NP: T=0.32; p=0.89; Pshrimp: T=1.17; p=0.516). Embryo VR decreased after exposure to the odor of a predator fed on cuttlefish embryos (“Pembryo” group) and to ink odor (“I” group) (Fig. 2: Pembryo: T=3.313; p=0.046; I: T=3.4165; p=0.004).
Visual
Embryo VR did not change after exposure to a non-predator (“NP” group) (Fig. 2: NP: T=0.524; p=0.69). Embryo VR decreased after exposure to a predator (“P” group) and to ink (“I” group) (Fig. 2: P: T=2.241; p=0.038; I: T=3.881; p=0.002).
Learned recognition
Chemical
After 4 days of repeated exposure to Clownfish odor paired with ink odor, VR significantly decreased when embryos were exposed to Clownfish odor alone on the day 5 (Fig. 3: NP+I: T=2.472; p=0.044). On the contrary, after 4 days of repeated exposure to Clownfish odor alone, VR did not change if embryos were exposed to Clownfish odor alone on day 5 (Fig. 3: NP: T=-0.297; p=0.9).
Visual
After 4 days of repeated exposure to Clownfish paired with ink, VR significantly decreased when embryos were exposed to Clownfish alone on day 5 (Fig. 3: NP+I: T=3.5701; p=0.02). Conversely, after 4 days of repeated exposure to Clownfish odor alone, VR did not change if embryos were exposed to Clownfish odor alone on day 5 (Fig. 3: T=-0.793; p=0.502).
Discussion
In the first part of this study, we investigated whether embryos are able to innately recognize a predator via visual or olfactory cues. We show that the ventilation rate (VR) of S. pharaonis embryos significantly decreased when they were exposed concomitantly to potential predators and ink. Changes in physiological parameters such as heart rate or VR often indicate perception (Colombelli-Négrel, Hauber, & Kleindorfer, 2014; Oulton, Haviland, & Brown, 2013) or attention (Porges & Raskin, 1969; Richards & Casey, 1991), notably when the animal is in a dangerous situation. The VR often increases to prepare an individual for flight from a predator (Misslin, 2003). However, predator detection through visual or chemical stimulus may also induce “freezing-like” behavior (Misslin, 2003) along with a decrease of VR. In mammals, freezing is considered to be a fear response related to a harmful stimulus, characterized by immobility and changes in physiological parameters, such as heart and ventilation rates, and may enhance a prey’s survival when facing predation. In cuttlefish, few studies have focused on changes in VR during such stimulation. In these studies, a change of VR indicates visual or chemical perception (Boal & Golden, 1999; Boal & Ni, 1996; King & Adamo, 2006). Juvenile cuttlefish became motionless (behavioral freezing), hyperinflated their mantle, and decreased their VR and heart rate upon presentation of a sudden visual stimulus (rapidly approaching bird cut-out; King & Adamo, 2006). Likewise, in adult cuttlefish, decreased breathing was associated with a freezing-like response that would seem adaptive since it could reduce the risk of being detected because of their own movements. Similarly, the reduction of the bioelectric field could well prevent attacks by sharks (Bedore, Kajiura, & Johnsen, 2015). S. pharaonis eggs are totally transparent and lack parental care; consequently, fewer movements associated with respiratory decrease and, hence, general activity inside the egg may lower the probability of embryos to be detected by predators, thus increasing its chances of survival. Further experiments could address the efficiency of the decrease of embryonic VR toward visual predators (Pufferfish) to test the hypothesis of the visual detection and non-visual predators (e.g., sharks; Bedore, Kajiura, & Johnsen, 2015) to test the hypothesis of the decrease in bioelectrical activity. VR has also been shown to be a sensitive indicator of fish physiological response to stress. In their study, Barreto et al. (2003) measured the VR of the Nile tilapia (Oreochromis niloticus) before and after the presentation of three stimuli: an aquarium with a harmless fish, a predator, or water (control). Nile tilapia’s VR increased significantly in the group visually exposed to a predator when compared with the two other groups, thus indicating its recognition ability (Barreto et al., 2003).
As in the young of vertebrate species, cuttlefish embryos innately respond to chemical cues from predators but not from non-predators. Indeed, our study shows that embryos respond differently to chemical cues from Pufferfish fed with frozen shrimps (less harmful) than to Pufferfish fed with cuttlefish embryos (harmful). The VR significantly decreased only when embryos were exposed to the latter. This result suggests that embryos do not respond to the chemical cues from the predator itself but rather to the degree of danger represented by the predator via its diet (digesting, excreting, and/or defecating). Such specific recognition is in accordance with the results of a study on the Clownfish Amphiprion percula, in which the larvae remained indifferent to chemosensory cues from non-piscivorous fishes fed with their usual diet, but significantly avoided chemical cues from piscivorous and non-piscivorous fishes fed with a diet containing a fish product (Dixson, Pratchett, & Munday, 2012). Cuttlefish embryos seem to recognize predation rather than predators. There could be a variety of chemical cues present in the predators’ tank, but given that there is no other food in the predators’ tank, it is likely that the embryos respond to the feces odor or to their body odor (Wisenden, 2000).
One of the most noteworthy results of the present study is that predator recognition is not based on chemical cues alone, but also on predatory visual information. Embryo VR decreased when embryos were visually exposed to the Pufferfish but not to the Clownfish. On the one hand, this change of VR cannot be attributed to a lack of oxygen, the egg being enclosed in a box. Indeed, we did not observe any change of VR when embryos were exposed to a non-predator and, secondly, a lack of oxygen would have been more likely to cause an increase in VR (Randall & Shelton, 1963). Which visual predatory cues embryos respond to remains unanswered. Nevertheless, some hypotheses can be proposed.
First, since the size of the fish was controlled, recognition may be based on the predator’s behavior. Indeed, behavior in the experimental tank differed between the two species of fish. Pufferfish can be trained to prey on eggs, cuttlefish embryos being the basis of their diet. As a consequence, during exposure the Pufferfish spent most of the time close to the glass box and directed several attacks at the direction of the egg (personal observation); it represents a higher threat than the clownfish. This is in accordance with results of another study in which S. pharaonis juveniles display secondary defensive behavior (deimatic pattern and inking) when the Pufferfish approaches (Lee, Darmaillacq, Dickel, & Chiao, 2020). On the other hand, the Clownfish stayed away from the egg closest to the side of the aquarium. It is evident that the threat-level is higher with the Pufferfish than with the Clownfish.
Second, a morphometric analysis of 20 different facial features of reef fish was carried out in order to assess cues to possible predator recognition, showing that the shape of the fish’s mouth and the distance between the eyes and the mouth could be different between a carnivorous and a herbivorous fish (Karplus & Algom, 1981). This morphological criterion may be sufficient for good visual recognition of a predator.
Our study highlights the fact that embryos innately respond to the sight of an ink cloud as well as to ink odor at a very low concentration, serving as a warning signal. Again, this response is likely to be adaptive because it decreases the probability of being detected by predators likely to attack eggs. In fish and amphibian species, young individuals innately respond to chemical alarm cues (pheromones) released by injured conspecifics. In cephalopods, threatened individuals eject clouds of black ink, which would make cuttlefish ink a relevant warning signal (Derby, 2014). Cephalopod ink would thus serve as a defense against predators as a direct predator deterrent and as an alarm cue for conspecifics (Derby, 2014; Hanlon & Messenger, 2018). In Loligo opalescens squid ink can cause inking and camouflage (Gilly & Lucero, 1992; Lucero, Farrington, & Gilly, 1994). Furthermore, dopamine at biologically relevant concentrations is sufficient to cause ink ejection (Gilly & Lucero, 1992; Lucero et al., 1994).
In the second part of the study, we showed that embryos can learn to distinguish a harmful stimulus when it is paired with ink. Indeed, the VR of cuttlefish embryos decreased significantly on the simple presentation of a Clownfish (through sight or smell) when it had been coupled with ink over the previous 4 days. Unpublished data show that pairing the Clownfish odor with ink over the previous day leads to the same results on S. officinalis embryos. Furthermore, S. officinalis embryos exposed to cuttlefish ink once a day for 4 days do not subsequently respond to a neutral odor (cinnamon; Mezrai, unpublished data). As a consequence, it is unlikely that this group experienced any form of sensitization. In the present study, it is more likely that embryos learn to recognize a new predator by associative learning. Associative learning, defined as the ability to learn either to associate two related events or a form of behavior and its consequences (Bouton, 2007), has been shown in cuttlefish (adults and juveniles) and other cephalopods including octopuses (O’Brien, Mezrai, Darmaillacq, & Dickel, 2017; Wells, 1968; Young, 1961). Cuttlefish (S. officinalis) can learn the visual characteristics of a prey while inside the egg through mere exposure, which would be non-associative learning because spontaneous juvenile food preferences are altered after embryonic exposure to crabs (Darmaillacq et al., 2008, 2017; Guibé et al., 2012). The present study brings direct evidence that cuttlefish embryos can also learn through classical conditioning. This learning capability may be adaptive in that it can allow cuttlefish embryo to gain information relevant to its future environment while still inside the egg case, hence improving the survival chances of the hatchlings. Indeed, embryos’ predators are also hatchlings’ predators. If embryos learn to recognize them before hatching, they may avoid them after hatching. These results are in accordance with studies on tadpoles and invertebrate larvae, in which embryos have been shown to learn to recognize new predators when they are paired with alarm cues (mosquitoes: Ferrari et al., 2008; damselfly: Wisenden et al., 1997). Predation is a constant threat faced by prey individuals, so learning about predation before hatching could confer advantages for the survival of young animals, especially when they develop without direct parental care.
To conclude, the ability to detect, identify, and learn about potential predators can be beneficial for the embryo while still in its egg case. This prenatal learning may have potential direct benefits for embryos (reduced detection by freezing-like behavior) and probably also after hatching (the hatchling might remember and might display appropriate antipredator behaviors). Furthermore, in a changing environment, these prenatal learning abilities are important when faced with new predators (e.g., invasive species) or predator diet changes. Indeed, in fish the flexibility of feeding behavior is an important adaptive trait because most natural environments undergo constant spatial and temporal change (Dill, 1983; Vehanen, 2003; Wright, Eberhard, Hobson, Avery, & Russello, 2010). Development in a transparent egg enables the use of visual as well as chemosensory information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, A-S. Darmaillacq.
References
Aizaki, K., & Yusa, Y. (2010). Learned predator recognition in a freshwater snail, Pomacea canaliculata. Malacologia, 52(1), 21–29.
Amo, L., López, P., & Martín, J. (2005). Chemical Assessment of Predation Risk in the Wall Lizard, Podarcis muralis, is influenced by Time Exposed to Chemical Cues of Ambush Snakes. The Herpetological Journal, 15(1), 21–25.
Anderson, F. E., Engelke, R., Jarrett, K., Valinassab, T., Mohamed, K. S., Asokan, P. K., … Dunning, M. (2010). Phylogeny of the Sepia pharaonis species complex (Cephalopoda: Sepiida) based on analyses of mitochondrial and nuclear DNA sequence data. Journal of Molluscan Studies, 77(1), 65–75.
Balderas-Valdivia, C. J., Ramírez-Bautista, A., & Carpenter, G. C. (2005). Aversive behavior of beaded lizard, heloderma horridum, to sympatric and allopatric predator snakes. The Southwestern Naturalist, 50(1), 24–31. https://doi.org/10.1894/0038-4909(2005)050<0024:ABOBLH>2.0.CO;2
Barreto, R. E., Luchiari, A. C., & Marcondes, A. L. (2003). Ventilatory frequency indicates visual recognition of an allopatric predator in naïve Nile tilapia. Behavioural Processes, 60(3), 235–239. https://doi.org/10.1016/S0376-6357(02)00127-4
Bedore, C. N., Kajiura, S. M., & Johnsen, S. (2015). Freezing behaviour facilitates bioelectric crypsis in cuttlefish faced with predation risk. Proc. R. Soc. B, 282(1820), 20151886. https://doi.org/10.1098/rspb.2015.1886
Boal, J. G., & Golden, D. K. (1999). Distance chemoreception in the common cuttlefish, Sepia officinalis (Mollusca, Cephalopoda). Journal of Experimental Marine Biology and Ecology, 235(2), 307–317. https://doi.org/10.1016/S0022-0981(98)00187-7
Boal, J. G., & Ni, J. N. (1996). Ventilation rate of cuttlefish, Sepia officinalis, in response to visual stimuli. Veliger, 39(4), 342–347.
Bouton, M. E. (2007). Learning and behavior: A contemporary synthesis (Vol. xiii). Sunderland, MA, US: Sinauer Associates.
Boyle, P. R. (1987). Cephalopod life cycles: Volume II: Comparative Reviews. Academic Press.
Brown, G. E., & Smith, R. J. F. (1998). Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery-reared fish to recognize chemical cues of a predator. Canadian Journal of Fisheries and Aquatic Sciences, 55(3), 611–617.
Brown, M. M., Kreiter, N. A., Maple, J. T., & Sinnott, J. M. (1992). Silhouettes elicit alarm calls from captive vervet monkeys (Cercopithecus aethiops). Journal of Comparative Psychology, 106(4), 350.
Chivers, D. P., & Smith, R. J. F. (1998). Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience, 5(3), 338–352.
Colombelli-Négrel, D., Hauber, M. E., & Kleindorfer, S. (2014). Prenatal learning in an Australian songbird: habituation and individual discrimination in superb fairy-wren embryos. Proceedings of the Royal Society B: Biological Sciences, 281(1797), 20141154.
Curio, E., Ernst, U., & Vieth, W. (1978). Cultural transmission of enemy recognition: one function of mobbing. Science, 202(4370), 899–901.
Dalesman, S., Rundle, S. D., Coleman, R. A., & Cotton, P. A. (2006). Cue association and antipredator behaviour in a pulmonate snail, Lymnaea stagnalis. Animal Behaviour, 71(4), 789–797. https://doi.org/10.1016/j.anbehav.2005.05.028
Dalesman, S., Rundle, S. D., & Cotton, P. A. (2007). Predator regime influences innate anti-predator behaviour in the freshwater gastropod Lymnaea stagnalis. Freshwater Biology, 52(11), 2134–2140. https://doi.org/10.1111/j.1365-2427.2007.01843.x
Darmaillacq, A.-S., Lesimple, C., & Dickel, L. (2008). Embryonic visual learning in the cuttlefish, Sepia officinalis. Animal Behaviour, 76(1), 131–134.
Darmaillacq, A.-S., Mezrai, N., O’Brien, C. E., & Dickel, L. (2017). Visual ecology and the development of visually guided behavior in the cuttlefish. Frontiers in Physiology, 8. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5469150/
Derby, C. D. (2014). Cephalopod Ink: Production, Chemistry, Functions and Applications. Marine Drugs, 12(5), 2700–2730. https://doi.org/10.3390/md12052700
Dill, L. M. (1983). Adaptive flexibility in the foraging behavior of fishes. Canadian Journal of Fisheries and Aquatic Sciences, 40(4), 398–408.
Dixson, D. L., Pratchett, M. S., & Munday, P. L. (2012). Reef fishes innately distinguish predators based on olfactory cues associated with recent prey items rather than individual species. Animal Behaviour, 84(1), 45–51.
Epp, K. J., & Gabor, C. R. (2008). Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology, 114(6), 607–615.
Fendt, M. (2006). Exposure to Urine of Canids and Felids, but not of Herbivores, Induces Defensive Behavior in Laboratory Rats. Journal of Chemical Ecology, 32(12), 2617. https://doi.org/10.1007/s10886-006-9186-9
Ferrari, M. C., & Chivers, D. P. (2009a). Latent inhibition of predator recognition by embryonic amphibians. Biology Letters, 5(2), 160–162.
Ferrari, M. C., & Chivers, D. P. (2009b). Sophisticated early life lessons: threat-sensitive generalization of predator recognition by embryonic amphibians. Behavioral Ecology, 20(6), 1295–1298.
Ferrari, M. C., & Chivers, D. P. (2010). The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behavioral Ecology and Sociobiology, 64(4), 549–555.
Ferrari, M. C., Crane, A. L., & Chivers, D. P. (2016). Certainty and the cognitive ecology of generalization of predator recognition. Animal Behaviour, 111, 207–211.
Ferrari, M. C., Manek, A. K., & Chivers, D. P. (2010). Temporal learning of predation risk by embryonic amphibians. Biology Letters, 6(3), 308–310.
Ferrari, M. C., Messier, F., & Chivers, D. P. (2008). Threat-sensitive learning of predators by larval mosquitoes Culex restuans. Behavioral Ecology and Sociobiology, 62(7), 1079–1083.
Fiorito, G., Affuso, A., Basil, J., Cole, A., de Girolamo, P., D’Angelo, L., … Kuba, M. (2015). Guidelines for the Care and Welfare of Cephalopods in Research–A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Laboratory Animals, 49(2_suppl), 1–90.
Garcia, T. S., Urbina, J. C., Bredeweg, E. M., & Ferrari, M. C. O. (2017). Embryonic learning and developmental carry-over effects in an invasive anuran. Oecologia, 184(3), 623–631. https://doi.org/10.1007/s00442-017-3905-5
Gilly, W. F., & Lucero, M. T. (1992). Behavioral responses to chemical stimulation of the olfactory organ in the squid Loligo opalescens. Journal of Experimental Biology, 162(1), 209–229.
Golub, J. L. (2013). Embryonic learning in threespine stickleback (Gasterosteus aculeatus). CLARK UNIVERSITY. Retrieved from http://gradworks.umi.com/35/38/3538197.html
Griffiths, R. A., Schley, L., Sharp, P. E., Dennis, J. L., & Roman, A. (1998). Behavioural responses of Mallorcan midwife toad tadpoles to natural and unnatural snake predators. Animal Behaviour, 55(1), 207–214. https://doi.org/10.1006/anbe.1997.0596
Guibé, M., Poirel, N., Houdé, O., & Dickel, L. (2012). Food imprinting and visual generalization in embryos and newly hatched cuttlefish, Sepia officinalis. Animal Behaviour, 84(1), 213–217.
Hanlon, R. T., & Messenger, J. B. (2018). Cephalopod behaviour. Cambridge University Press.
Hartman, E. J., & Abrahams, M. V. (2000). Sensory compensation and the detection of predators: the interaction between chemical and visual information. Proceedings of the Royal Society of London B: Biological Sciences, 267(1443), 571–575. https://doi.org/10.1098/rspb.2000.1039
Hawkins, L. A., Magurran, A. E., & Armstrong, J. D. (2004). Innate predator recognition in newly-hatched Atlantic salmon. Behaviour, 141(10), 1249–1262.
Hirsch, S. M., & Bolles, R. C. (1980). On the Ability of Prey to Recognize Predators. Zeitschrift Für Tierpsychologie, 54(1), 71–84. https://doi.org/10.1111/j.1439-0310.1980.tb01064.x
Karplus, I., & Algom, D. (1981). Visual Cues for Predator Face Recognition by Reef Fishes. Zeitschrift Für Tierpsychologie, 55(4), 343–364. https://doi.org/10.1111/j.1439-0310.1981.tb01277.x
Kats, L. B., & Dill, L. M. (1998). The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience, 5(3), 361–394.
Kelley, J. L., & Magurran, A. E. (2003). Learned predator recognition and antipredator responses in fishes. Fish and Fisheries, 4(3), 216–226. https://doi.org/10.1046/j.1467-2979.2003.00126.x
Kindermann, T., Siemers, B. M., & Fendt, M. (2009). Innate or learned acoustic recognition of avian predators in rodents? Journal of Experimental Biology, 212(4), 506–513. https://doi.org/10.1242/jeb.024174
King, A. J., & Adamo, S. A. (2006). The ventilatory, cardiac and behavioural responses of resting cuttlefish (Sepia officinalis L.) to sudden visual stimuli. Journal of Experimental Biology, 209(6), 1101–1111. https://doi.org/10.1242/jeb.02116
Lee, M.-F., Lin, C.-Y., Chiao, C.-C., & Lu, C.-C. (2016). Reproductive Behavior and Embryonic Development of the Pharaoh Cuttlefish, Sepia pharaonis (Cephalopoda: Sepiidae). Zoological Studies, 55(41). Retrieved from http://zoolstud.sinica.edu.tw/Journals/55/55-41.pdf
Lee, Y.-C., Darmaillacq, A.-S., Dickel, L., & Chiao, C. C. (2020). Effects of embryonic exposure to predators on the postnatal defensive behaviors of cuttlefish. Journal of Experimental Marine Biology and Ecology, 524, 151288.
Lucero, M. T., Farrington, H., & Gilly, W. F. (1994). Quantification of L-dopa and dopamine in squid ink: implications for chemoreception. The Biological Bulletin, 187(1), 55–63.
Madaras, F., Gerber, J. P., Peddie, F., & Kokkinn, M. J. (2010). The effect of sampling methods on the apparent constituents of ink from the squid Sepioteuthis australis. Journal of Chemical Ecology, 36(11), 1171–1179.
Mathis, A., Ferrari, M. C. O., Windel, N., Messier, F., & Chivers, D. P. (2008). Learning by embryos and the ghost of predation future. Proceedings of the Royal Society B: Biological Sciences, 275(1651), 2603–2607. https://doi.org/10.1098/rspb.2008.0754
Mathis, A., & Smith, R. J. F. (1993). Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike’s diet. Animal Behaviour, 46(4), 645–656. https://doi.org/10.1006/anbe.1993.1241
Mezrai, N., Chiao, C.-C., Dickel, L., & Darmaillacq, A.-S. (2019). A difference in timing for the onset of visual and chemosensory systems during embryonic development in two closely related cuttlefish species. Developmental psychobiology.
Miklósi, Á., Pongrácz, P., & Csányi, V. (1997). The ontogeny of antipredator behavior in paradise fish larvae (Macropodus opercularis) IV. The effect of exposure to siblings. Developmental Psychobiology, 30(4), 283–291.
Mirza, R. S., Ferrari, M. C. O., Kiesecker, J. M., & Chivers, D. P. (2006). Responses of American toad tadpoles to predation cues: behavioural response thresholds, threat-sensitivity and acquired predation recognition. Behaviour, 143(7), 877–889. https://doi.org/10.1163/156853906778017926
Misslin, R. (2003). The defense system of fear: behavior and neurocircuitry. Neurophysiologie Clinique/Clinical Neurophysiology, 33(2), 55–66. https://doi.org/10.1016/S0987-7053(03)00009-1
O’Brien, C. E., Mezrai, N., Darmaillacq, A.-S., & Dickel, L. (2017). Behavioral development in embryonic and early juvenile cuttlefish (Sepia officinalis). Developmental Psychobiology, 59(2), 145–160.
Oulton, L. J., Haviland, V., & Brown, C. (2013). Predator Recognition in Rainbowfish, Melanotaenia duboulayi, Embryos. PLOS ONE, 8(10), e76061. https://doi.org/10.1371/journal.pone.0076061
Porges, S. W., & Raskin, D. C. (1969). Respiratory and heart rate components of attention. Journal of Experimental Psychology, 81(3), 497.
Prota, G., Ortonne, J. P., Voulot, C., Khatchadourian, C., Nardi, G., & Palumbo, A. (1981). Occurrence and properties of tyrosinase in the ejected ink of cephalopods. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 68(3), 415–419.
Randall, D. J., & Shelton, G. (1963). The effects of changes in environmental gas concentrations on the breathing and heart rate of a teleost fish. Comparative Biochemistry and Physiology, 9, 229–239.
Richards, J. E., & Casey, B. J. (1991). Heart rate variability during attention phases in young infants. Psychophysiology, 28(1), 43–53.
Rochette, R., Arsenault, D. J., Justome, B., & Himmelman, J. H. (1998). Chemically-mediated predator-recognition learning in a marine gastropod. Écoscience, 5(3), 353–360. https://doi.org/10.1080/11956860.1998.11682473
Romagny, S., Darmaillacq, A.-S., Guibé, M., Bellanger, C., & Dickel, L. (2012). Feel, smell and see in an egg: emergence of perception and learning in an immature invertebrate, the cuttlefish embryo. The Journal of Experimental Biology, 215(23), 4125–4130. https://doi.org/10.1242/jeb.078295
Saglio, P., & Mandrillon, A.-L. (2006). Embryonic experience to predation risk affects tadpoles of the common frog (Rana temporaria). Archiv Für Hydrobiologie, 166(4), 505–523.
Saunders, S. P., Ong, T. W. Y., & Cuthbert, F. J. (2013). Auditory and visual threat recognition in captive-reared Great Lakes piping plovers (Charadrius melodus). Applied Animal Behaviour Science, 144(3), 153–162. https://doi.org/10.1016/j.applanim.2013.01.009
Utne-Palm, A. C. (2001). Response of naive two-spotted gobies Gobiusculus flavescens to visual and chemical stimuli of their natural predator, cod Gadus morhua. Marine Ecology Progress Series, 218, 267–274.
Vehanen, T. (2003). Adaptive flexibility in the behaviour of juvenile Atlantic salmon: short-term responses to food availability and threat from predation. Journal of Fish Biology, 63(4), 1034–1045. https://doi.org/10.1046/j.1095-8649.2003.00228.x
Wells, M. J. (1968). Sensitization and the Evolution of Associative Learning. In Neurobiology of Invertebrates (pp. 391–411). Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-8618-0_28
Wisenden, B. D. (2000). Olfactory assessment of predation risk in the aquatic environment. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 355(1401), 1205-1208.
Wisenden, B. D. (2003). Chemically mediated strategies to counter predation. In Sensory processing in aquatic environments (pp. 236–251). Springer. Retrieved from http://link.springer.com/chapter/10.1007/978-0-387-22628-6_12
Wisenden, B. D., Chivers, D. P., & Smith, R. J. F. (1997). Learned Recognition of Predation Risk by Enallagma Damselfly Larvae (Odonata, Zygoptera) on the Basis of Chemical Cues. Journal of Chemical Ecology, 23(1), 137–151. https://doi.org/10.1023/B:JOEC.0000006350.66424.3d
Wisenden, B. D., & Millard, M. C. (2001). Aquatic flatworms use chemical cues from injured conspecifics to assess predation risk and to associate risk with novel cues. Animal Behaviour, 62(4), 761–766.
Woody, D. R., & Mathis, A. (1998). Acquired recognition of chemical stimuli from an unfamiliar predator: associative learning by adult newts, Notophthalmus viridescens. Copeia, 1998(4), 1027–1031.
Wright, T. F., Eberhard, J. R., Hobson, E. A., Avery, M. L., & Russello, M. A. (2010). Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethology Ecology & Evolution, 22(4), 393–404. https://doi.org/10.1080/03949370.2010.505580
Young, J. Z. (1961). Learning and Discrimination in the Octopus. Biological Reviews, 36(1), 32–95. https://doi.org/10.1111/j.1469-185X.1961.tb01432.x
Acknowledgements
This work was supported by a grant from Campus France (Orchid project 2017-2018), by the Agence Nationale de la Recherche (ANR PReSTO’Cog 13-BSV7-0002), and by the Région Normandie. The authors gratefully acknowledge MANCHE 2021 "Plateformes d'exploitation des ressources marines" co-funded by the European Union, Normandy County Council, in the framework of the ERDF-ESF operational program 2014-2020 and Jane Martin for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mezrai, N., Arduini, L., Dickel, L. et al. Awareness of danger inside the egg: Evidence of innate and learned predator recognition in cuttlefish embryos. Learn Behav 48, 401–410 (2020). https://doi.org/10.3758/s13420-020-00424-7
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-020-00424-7