Abstract
Emotion perception deficits could be due to disrupted connectivity of key nodes in the salience and emotion network (SEN), including the amygdala, subgenual anterior cingulate cortex (sgACC), and insula. We examined SEN resting-state (rs-)fMRI connectivity in rMDD in relation to Facial Emotion Perception Test (FEPT) performance. Fifty-two medication-free people ages 18 to 23 years participated. Twenty-seven had major depressive disorder (MDD) in remission (rMDD, 10 males), as MDD is associated with emotion perception deficits and alterations in rsfMRI. Twenty-five healthy controls (10 males) also participated. Participants completed the FEPT during fMRI, in addition to an 8-minute eyes-open resting-state scan. Seed regions of interest were defined in the amygdala, anterior insula and sgACC. Multiple regression analyses co-varied diagnostic group, sex and movement parameters. Emotion perception accuracy was positively associated with connectivity between amygdala seeds and regions primarily in the SEN and cognitive control network (CCN), and also the default mode network (DMN). Accuracy was also positively associated with connectivity between the sgACC seeds and other SEN regions, and the DMN, particularly for the right sgACC. Connectivity negatively associated with emotion perception was mostly with regions outside of these three networks, other than the left insula and part of the DMN. This study is the first to our knowledge to demonstrate relationships between facial emotion processing and resting-state connectivity with SEN nodes and between SEN nodes and regions located within other neural networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Accurate facial emotion perception is essential for adequate social functioning, as it allows individuals to understand and respond to the feelings and nonverbal communications of others (Russell, Bachorowski, & Fernandez-Dols, 2003). Multiple brain regions are engaged during facial emotion perception, including the medial prefrontal cortex (Phan, Wager, Taylor, & Liberzon, 2002) fusiform gyri, right lateral occipital gyrus, right superior temporal sulcus, inferior frontal gyri, amygdala, and entorhinal cortex (Kesler et al., 2001), visual cortex, and cerebellum (Fusar-Poli et al., 2009). Many facial emotion processing areas are within the salience and emotion network (SEN), a network of brain regions that integrates sensory information with visceral, autonomic, and hedonic signals in order to facilitate attention leading to behavioral decisions (Seeley et al., 2007). The SEN is primarily comprised of the anterior cingulate cortex ACC, orbital frontal and anterior insular regions, and the amygdalae. These regions perform related but separate functions and tend to differentially activate in response to faces displaying specific emotions. For example, viewing happy, sad, and fearful faces tends to activate the amygdala, whereas angry or disgusted faces do not (Fusar-Poli et al., 2009). The amygdala has also been shown to be involved with emotion discrimination (Gur et al., 2002), such that it responds more to fearful faces than happy, sad, or neutral faces (Breiter et al., 1996; Fusar-Poli et al., 2009; Phan et al., 2002) and more to happy faces than to neutral ones (Breiter et al., 1996). The insula is more sensitive to disgust than to anger but activates when individuals are exposed to either type of facial emotion (Gur et al., 2002). The ACC also plays an important role in emotion processing, with the dorsal-caudal ACC reported to be involved in the evaluation and appraisal of emotion, and the rostral-ventral ACC reportedly involved in emotion regulation and control (Etkin, Egner, & Kalisch, 2011). These important studies have utilized fMRI to examine emotion processing; however, few studies have examined functional connectivity, in particular resting-state connectivity of networks such as the SEN, in relation to facial emotion perception.
SEN activity reduces with engagement of the default mode network (DMN), the set of brain regions that are involved in more self-referential and internally focused activities, among a myriad of “non-task” processes (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001). The SEN is important for switching between the DMN and the cognitive control network (CCN; Manoliu et al., 2014; Sridharan, Levitin, & Menon, 2008), a network including dorsolateral frontal and parietal cortices (Seeley et al., 2007) that is responsible for inhibitory control, mental flexibility and switching, and other executive functions. Models of major depressive disorder (MDD) describe imbalances among these primary networks, and their connectivity as a potential mechanism increasing risk for mental disorders (Langenecker, Jacobs & Passarotti, 2014; Menon, 2011). Mayberg et al. (1999) suggested that MDD is characterized by a reduction of top-down cortical control over limbic regions, or reduced CCN–SEN connectivity. For example, amygdala hyperactivity has been reported during facial emotion processing in MDD (Fournier, Keener, Almeida, Kronhaus, & Phillip, 2013). Studies have also shown that the subgenual ACC, possibly part of the SEN, has reduced gray matter (Drevets & Savitz, 2008) and greater connectivity to the DMN (Greicius et al., 2007) in MDD. Furthermore, hyperconnectivity within the DMN and from the DMN to the caudate and dorsomedial prefrontal cortex may underlie rumination in MDD (Jacobs et al., 2014; Wang, Hermens, Hickie, & Lagopoulos, 2012; Whitfield-Gabrieli & Ford, 2012; Zhu et al., 2012). Poor emotion perception (Kohler, Hoffman, Eastman, Healey, & Moberg, 2011; Phillips, Drevets, Rauch, & Lane, 2003) and emotion regulation (Joormann & D’Avanzato, 2010) in individuals with MDD are consistent with connectivity findings demonstrating deficiencies in emotion-related and self-referential networks (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015; Kaiser & Pizzagalli, 2015). Emotion processing deficits can persist despite recovery from illness; for example, individuals with remitted MDD (rMDD) show hyperactivity in the dorsomedial prefrontal cortex/ACC and lateral orbitofrontal cortex when using emotion regulation strategies (Kanske, Heissler, Schonfelder, & Wessa, 2012), suggesting that there may be residual markers of disease in brain function.
We aimed to examine the relationship between resting-state connectivity and facial emotion perception in both healthy controls and individuals with rMDD, with the intention of measuring a varied range of task performance to better understand individual differences. Our hypothesis was that resting-state connectivity of SEN seeds would be meaningfully related to performance. Although performance-related functional connectivity can provide useful insights into task-responsive networks, our goal was to identify the association between individual differences in performance and intrinsic “trait-like” networks. Therefore, we chose to examine resting-state rather than within-task connectivity because of concerns that within-task connectivity would be corrupted to an extent by actual performance. We chose to examine young, medication-free individuals with rMDD to minimize the potential confounds of medication, developmental variance, acute disease effects, chronic illness burden, and neural scar effects. Recent studies have sought to examine the relationships between synchronized neural network metrics at rest and task performance in rMDD (e.g. Rao et al., 2016). Given previous findings that the SEN is implicated in facial emotion perception, we expected that better facial emotion perception performance would be associated with increased functional coupling between these SEN regions at rest. We were interested in examining the association between emotion perception performance and connectivity including diagnosis as a covariate, rather than between-group differences, because we have already previously demonstrated diagnostic differences in resting-state connectivity between individuals with rMDD and HCs (Jacobs et al., 2016; Jacobs et al., 2014; Stange et al., 2017). Furthermore, within an intermediate phenotype view of emotion processing that would be consistent with Research Domain Criteria (RDoC), dimensional analyses like the proposed one offer unique individual differences information that is distinct from disease related information (Cuthbert & Insel, 2010). We chose three bilateral seeds within the SEN—the amygdala, anterior insula, and subgenual ACC—and hypothesized that more accurate facial emotion perception would be associated with increased resting-state connectivity between these three seeds and other regions in the SEN, regardless of diagnostic history or sex. Conversely, we expected that poorer emotion perception would not be associated with increased within-SEN connectivity but would be associated with increased connectivity between SEN regions and DMN and CCN regions.
Method
Participants
Total participants enrolled in the study included 52 individuals, ages 18 to 23 years. The rMDD group comprised 27 (10 male) individuals with a history of depression (1–3 prior episodesFootnote 1) who were in full remission (rMDD), as defined by Diagnostic and Statistical Manual of Mental Disorders (4th ed., TR; American Psychiatric Association, 2000) criteria.
The mean number of years well was 2.85 (SD = 1.70). The healthy control (HC) group comprised 25 participants (10 male) with no previous psychiatric diagnosis themselves or in an immediate family member. Participants were recruited from the Chicago community. Diagnosis was assessed via a clinical interview with the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) and confirmed with a parent/guardian interview using a modified Family Interview for Genetic Studies (Maxwell, 1992) or treatment records for a past MDD diagnosis. Three rMDD and three HC participants were left-handed. Exclusion criteria were substance abuse or dependence within the past year, psychoactive medication (other than a psychostimulant) use within 30 days, a psychostimulant use within the past 2 days, regular smoking, major chronobiological disruption or phase shift within the preceding month, suicide attempt within the past 6 months or hospitalization for suicidal intent within the past 3 months, neurological condition, personal or family history of psychosis, or contraindications for MRI. Current depression severity was assessed using the Hamilton Depression Rating Scale (HDRS), using a cutoff score of 7 (Zimmerman et al., 2013) for both groups.
There were no significant differences between groups in demographic variables, including the proportion of males and females (HC: 10 males/15 females; rMDD:10 males/17 females); age (HC: M = 21.04, SD = 1.86; rMDD: M = 21.48, SD = 1.48) or years of education (HC: M = 14.56, SD = 1.47; rMDD M = 14.52, SD = 1.37). Estimated verbal IQ score was assessed using the Shipley Institute of Living Scale: Vocabulary Test (Shipley, 1940). There was no significant difference in estimated verbal IQ score between the HC (M = 102.28, SD = 9.32) and rMDD (M = 103.35, SD = 9.50) groups. The HC group had significantly lower HDRS scores (M = 0.20, SD = 0.50) than the rMDD group (M = 2.59, SD = 5.77), p < .05.
Measures
Facial Emotion Perception Test
The Facial Emotion Perception Test (FEPT; Langenecker et al., 2005, 2007; Rapport et al., 2002;) rapidly presents participants with faces expressing anger, happiness, sadness, fear, or a neutral state. Each trial begins with a fixation cross (500 ms), followed by a face (or an animal in the control condition) presented for 300 ms, then a visual grayscale mask for 100 ms to prevent visual afterburn phenomena. The visual mask was a square-shaped pixilation of an animal stimulus that was matched to the experimental stimuli for brightness and contrast. Participants identified which emotion they perceived by responding in the subsequent 3,100 ms using a five-button response “claw” (Psychology Software Tools) to indicate perception of an angry, happy, sad, fearful or neutral face. The face stimuli were color pictures from the NimStim (MacBrain) set (Tottenham et al., 2009). A prescanner practice version used face stimuli from the Ekman and Friesen (1976) set. The control condition required participants to identify cats, dogs, primates, fish, and birds to control for activity related to visual processing, praxis, response selection, and execution. Blocks of faces or animal stimuli were interspersed with rest blocks across five runs of 4 minutes, 20 seconds. Each run had one or two animal blocks spread amongst four or five faces blocks. Accuracy was recorded. The emotions were counterbalanced so that the response for each emotion and animal had a different finger for different participants (task version). Participants were randomly assigned to a task version. Groups did not differ in the proportions of participants that completed the different task versions, χ2(4) = 0.95, p= .92.
Procedures
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation. Informed consent was obtained according to the guidelines of the Institutional Review Board of The University of Illinois, Chicago and consistent with the Declaration of Helsinki, 1975, as revised in 2008. Participants were compensated for their involvement.
Subjects completed a practice trial of the FEPT prior to fMRI scanning. FEPT and resting-state fMRI were completed during the second half of a 90-minute fMRI session. Resting-state fMRI data were always collected after the FEPT, during an eyes open, 8-minute session. Participants completed other tasks earlier in the scanning session; however, the results of these tasks will be reported separately.
MRI acquisition
Whole-brain images were acquired with a gradient-echo axial echo-planar imaging sequence performed with a 3T GE Discovery scanner. The image matrix was 64 × 64 over a 22-cm FOV with 3-mm slice thickness (0 gap) for a 3.44-mm × 3.44-mm in-plane resolution. TR = 2,000 ms, TE = minFull (22.2 ms), 90° flip, 44 slices (ascending, interleaved). The four initial scans were discarded, resulting in 126 volumes for each of five runs, each lasting 4 minutes, 20 seconds. The anatomical scan was a T1SPGR echo, with a 22-cm FOV, 256 × 256 matrix size, 1-mm slice thickness, for a 0.86-mm × 0.86-mm in-plane resolution, including 182 slices during a scan time of around 7 minutes.
MRI processing
The fMRI data were preprocessed according to our established pipeline of scripts that utilize both SPM and FSL functions, as follows. Images were slice-time corrected in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), then realigned to the 10th volume in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Brain extraction of anatomical images was performed with FSL’s Brain Extraction Tool (Smith, 2002) then coregistered to functional images and spatially normalized to Montreal Neurological Institute (MNI) space in SPM8, with a final reconstructed spatial resolution of 2 × 2 × 2 mm3. Smoothing was completed in SPM8 with a full-width at half-maximum filter of 5-mm isotropic. Physiologic correction was performed by regressing out the top five principal components from white matter and cerebral spinal fluid sequentially (Behzadi, Restom, Liau, & Liu, 2007). Motion parameters of deviations in translation and rotations in x, y, and z planes were regressed out (along with first temporal derivative and squares of parameters) within first-level models (Jo et al., 2013). The global signal was not regressed due to colinearity violations with gray-matter signal, problematic misestimates of and introductions of anticorrelations (Fox, Zhang, Snyder, & Raichle, 2009) and effect on distance-micro-movement relationships (Jo et al., 2013). Lastly, time series were band-pass filtered over the range 0.01–0.10 Hz.
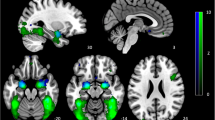
Each seed region of interest was defined and verified in MNI space on the average anatomy of scans collected within this study, with a 2.9-mm radius (19 voxels). Seeds from within the salience and emotion network (SEN) were consistent with those used by our group previously (Bhaumik et al., 2016; Jacobs et al., 2014) and were derived based on previous literature examining resting-state connectivity of the amygdala (McCabe & Mishor, 2011; Pannekoek et al., 2013), sgACC (Kelly et al., 2009; Margulies et al., 2007), and anterior insula (Horn et al., 2010; Sridharan et al., 2008). The following coordinates were used: amygdala +/−23, −5, −19 mm; sgACC +/−4, 21, −8; anterior insula +/−36, 13, 5 mm. Time-course data were averaged from each seed region for each participant. Correlation coefficients were calculated between mean time course for seed regions and all other voxels of the brain, resulting in three-dimensional correlation coefficient images (r images), transformed to z scores using a Fisher transformation and compared in SPM8.
Statistical analysis
Although the SEN is here conceived as a unified network, because hub regions of this network have been implicated in distinct but complementary functions (see Introduction), we elected to perform voxel-wise analyses for each seed separately to test the relationship between resting-state SEN connectivity and emotion processing performance. Six multiple linear regressions were calculated within SPM8, one for each of the three SEN seeds bilaterally. Each included mean accuracy in response to faces (across the entire task) as the predictor of connectivity to the seed region of interest. Diagnostic group and sex were included as covariates. We included diagnostic group as a covariate only because we have already previously investigated diagnostic differences in resting-state connectivity between individuals with rMDD and HCs (Jacobs et al., 2016), and in the present study we wanted to use an individual differences approach in line with RDoC to examine the association between FEPT performance and rs-fMRI connectivity, independent of previous history of depression. For overall accuracy for facial expressions, a univariate ANOVA found no significant main effect of diagnostic group, F(1, 48) = 1.99, p = .17; sex, F(1, 48) = 1.80, p = .19; and no interaction of Diagnostic Group × Sex, F(1, 48) = 0.18, p = .68. Sex differences in resting-state connectivity were also of interest and are reported in the Supplementary Material. The standard deviation (SD) values of the estimated movement parameters from MCFLIRT of translation in x, y, and z planes during the rsfMRI were included as covariates of no interest. There were no diagnostic group differences in the standard deviation values of translation in x, t(50) = −0.32, p = .75; y, t(50) = 0.79, p = .43; or z planes t(50) = 0.70, p = .48. The 3dClustSim program was used to generate a Monte Carlo threshold for significance that combines the height and extent of activation, here p < .005, k > 55 (which is equivalent to 440 mm3).Footnote 2 As mentioned above, we chose to examine resting-state rather than within-task connectivity because of concerns that within-task connectivity would be corrupted to an extent by actual performance, resulting in a circular line of argument for interpreting any effects. However, for maximal interpretability and transparency, we also calculated within-task connectivity. Because functional connectivity during task performance is going to be affected by factors specific to the task, such as the specific visual stimuli, decision-making, and motor responses, we regressed out task events and movement values from the connectivity model, as we were not interested in demonstrating that functional connectivity during a task is related to performing that task. A comparison between these results and those of the resting-state connectivity analysis is included in the Supplementary Materials.
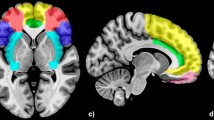
An understanding of integration and segregation within the brain is crucial for determining principles of brain function and development (Uddin, Supekar, & Menon, 2010), and facilitates parsimony of interpretation. For this reason, Tables 1 and 2 report the percentage of each of the significant clusters derived from the whole-brain analysis that are part of the salience and emotion network (SEN), cognitive control network (CCN), and default mode network (DMN), consistent with the predominant triple network model proposed by Menon (2011) as implicated in psychopathology. We created three network masks based on Yeo et al. (2011), who identified and replicated a seven-network cortical parcellation by measuring the intrinsic functional connectivity of 1,000 people. The 7 Networks MNI 152 Freesurfer conformed 1-mm liberal parcellations were used to create masks using the MarsBaR toolbox for SPM8. We used the DMN mask as created by Yeo et al., and created a SEN mask by combining the ventral attention (which Yeo et al., 2011, p. 1138, describe as the salience network) and limbic parcellations in order to capture both attention-related and bottom-up emotion-related components of salience processing. We created a CCN mask by combining the dorsal attention and fronto-parietal parcellations, in line with the work of Menon (2011). Finally, we calculated partial correlations of the extracted signal from each significant cluster and accuracy, adjusting for diagnostic group, sex, and movement. These correlations are reported in the final column of Tables 1 and 2.
Results
Regions of connectivity with salience and emotion network positively associated with facial emotion perception accuracy
Connectivity within regions in salience and emotion network
Better emotion perception accuracy was positively associated with within-SEN connectivity, particularly for the amygdala, with the right amygdala demonstrating the largest cluster of connectivity, more than double the size of that of the largest left amygdala cluster (see Fig. 1, Table 1). Scatter plots illustrating these relationships are presented in the Supplementary Material.
Approximately 20% to 30% of the regions significantly connected with the left and right amygdala, respectively, were located within the SEN (see Table 1). The largest cluster that was connected to the right amygdala that was positively associated with facial emotion perception peaked in the left inferior frontal gyrus (IFG), and over a third of this cluster fell within the SEN, with another third forming part of the DMN (see Table 1). Connectivity between both the left and right amygdala and the left supramarginal gyrus was positively associated with emotion perception accuracy. Emotion perception was also positively associated with connectivity between the left amygdala and the left superior temporal gyrus/insula. It was also positively associated with connectivity between the right amygdala and the right insula as well as a portion of the right posterior cingulate that forms part of the SEN. Emotion perception accuracy was also positively associated with connectivity between the left sgACC and the left superior temporal gyrus, and the right sgACC and the left medial orbital cortex (Brodmann’s area; BA12). These sgACC connections to SEN regions represented around 15% to 20% of the total connections for the right and left sgACC, respectively (see Table 1).
Connectivity between salience and emotion network and regions in the cognitive control network
The amygdalae were the only seeds for which emotion perception was positively associated with connectivity to the CCN. These connections were between both amygdala and the right middle frontal gyrus (BA9) and left supramarginal gyrus, as well as between the right amygdala and the left inferior frontal gyrus (BA44). Amygdalar connections to CCN regions formed around 20% of all regions that were positively associated with facial emotion perception accuracy (see Table 1).
Connectivity between salience and emotion network and regions in default mode network
Emotion perception accuracy was also positively associated with connectivity from SEN seeds to the DMN. This included connectivity between the left amygdala and the left superior temporal gyrus and between the right amygdala and the bilateral inferior frontal gyri (BA47). Emotion perception accuracy was also positively associated with connectivity between both the left and right sgACC and the left middle temporal gyrus, and between the right sgACC and the right middle temporal and anterior cingulate gyri. These connections between the right sgACC and DMN regions formed two thirds of the connections of the right sgACC that were positively associated with facial emotion perception accuracy.
Connectivity between salience and emotion network and regions outside of the SEN, CCN, and DMN
None of the connections to the left or right insula that were positively associated with emotion perception accuracy fell within any of the networks of interest. Rather, these connections were to subcortical and cerebellar regions. The left amygdala and the left sgACC also showed significant connectivity to the cerebellum that was positively associated with facial emotion perception. Other significant connections that were positively associated with facial emotion perception were between the left amygdala and right cuneus, right amygdala and left inferior and middle temporal gyri, the left sgACC and the left parahippocampal gyrus, and the right sgACC and the right posterior cingulate.
Connectivity negatively associated with facial emotion perception
Emotion perception was also negatively associated with connectivity of SEN seeds (after adjusting for group and sex), although to a lesser extent than the positive associations reported above. The seeds involved were only the left amygdala, left insula, left sgACC, and right sgACC seeds (see Fig. 2, Table 2). Notably, emotion perception was only negatively associated with connectivity to right hemisphere or midline regions. The left insula connection to the ACC that was negatively associated with emotion perception was located in the DMN. This was the only connection that was to any region of the three major networks.
Discussion
To our knowledge, this study is the first to evaluate associations between resting-state connectivity in the SEN and facial emotion processing. In a sample of individuals who were healthy or who had rMDD, we found that facial emotion perception was positively associated with within-SEN connectivity, mostly of amygdala to other SEN regions. We also found that facial emotion perception accuracy was positively associated with cross-network connectivity, mostly of amygdala to CCN regions and of sgACC to DMN regions. In contrast, emotion perception was only negatively associated with connectivity between the left insula seed and a part of the ACC (BA24) of the DMN. All other regions of connectivity that were negatively associated with accuracy were outside of the SEN, CCN, and DMN. Amygdala connectivity was the most relevant to better FEPT performance, whereas a surprising lack of connectivity was found between the anterior insula and performance within the SEN. These findings may have implications for advancing our understanding of the neural networks that underlie processing of emotional faces, which are important given that connectivity of these neural networks are altered in individuals with active mood disorders (e.g. Anand et al., 2005; Carballedo et al., 2011; Dannlowski et al., 2009; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). This disrupted network activity has been theorized to account for findings of emotion perception impairments in individuals with depressive disorders (e.g., meta-analysis by Kohler et al., 2011). Although our remitted sample did not demonstrate differences in behavioral performance from the HC group, our individual differences analysis of connectivity patterns was based on the theory that, early in the course of depression, the neural strata of risk may be evident before the behavioral manifestation of facial emotion perception impairment.
Connectivity within regions in salience and emotion network
Overall, results suggested that accuracy during perception of emotional faces was positively associated with connectivity within the SEN. This pattern of results is consistent with the notion that greater integrity, or possibly efficiency, of the SEN may subserve the ability to process emotionally salient stimuli (Seeley et al., 2007), an important ability for social cognition and effective interpersonal interactions. Given that the amygdala is important for the direction of attention to potentially threatening stimuli (Morris et al., 1998; Surguladze et al., 2003), the association between accurate classification of faces and SEN connectivity in the current study is consistent with the presence of a more cohesive SEN subserving these abilities. In addition, lesion studies have suggested that the IFG, which has portions in both the SEN and CCN (Yeo et al., 2011), is associated with emotional empathy (Shamay-Tsoory, Aharon-Peretz, & Perry, 2009), and a meta-analysis has also implicated this region in facial emotion perception (Fusar-Poli et al., 2009). Thus, perhaps stronger SEN coherence, including connectivity between the amygdala and IFG, facilitates the ability to recognize others’ facial expressions of emotions.
Connectivity between salience and emotion network and regions in the cognitive control network
We found a positive association between connectivity between the amygdala and regions in the cognitive control network and facial emotion perception performance, after adjusting for diagnostic group, sex, and movement. This is consistent with literature suggesting that connectivity between the SEN and CCN may facilitate superior adaptive behavior in the context of emotionally salient information and possible need for emotion regulation. Previous studies in active MDD (aMDD), albeit not relating to performance, have reported reduced connectivity between cortical regulatory areas and limbic regions such as the amygdala (Anand et al., 2005; Carballedo et al., 2011; Dannlowski et al., 2009; Siegle et al., 2007). We found a positive association between facial emotion perception performance and connectivity of both the left and right amygdalae to the right middle frontal gyrus (BA9). The middle frontal gyrus is involved in cognitive control (Kubler, Dixon, & Garavan, 2006; Langenecker, Nielson, & Rao, 2004; Sarazin et al., 1998) including planning (Crozier et al., 1999). It has also been implicated in working memory (Babiloni et al., 2005; Wager & Smith, 2003), including for the identification of emotional vocal expressions (Rama et al., 2001).
Connectivity between the right amygdala and the CCN component of the left IFG (BA44; see Table 1) was positively associated with emotion perception performance. This region is closely located to that of BA47, which Briceño et al. (2013) reported be inversely associated with facial emotion perception in females with aMDD. They found that a greater ratio of left-to-right hemisphere IFG activity predicted poorer emotion perception performance. This area of the IFG may be linguistically mediating connectivity between the SEN and CCN. Previous findings also suggest that the ventral IFG may play an adaptive role in MDD, in that activity in the left ventrolateral prefrontal cortex /lateral orbitofrontal cortex is negatively associated with depression severity (Drevets, Gadde, & Krishnan, 2004). Thus, our results suggest that retention of this relationship between the cognitive control and salience and emotion networks is important in sustaining good performance and wellness. Our results also extend the literature to examining connectivity between the SEN and CCN at rest, as it relates to emotion processing, and suggest that superior cognitive control of emotion (or cognitive control abilities in general) may improve the ability to accurately discriminate between different types of emotional faces. Furthermore, this could suggest that one reason that prior studies of MDD have found an attenuated ability to accurately process emotional faces is that individuals with MDD have attenuated connectivity between the SEN and CCN, impairing their performance on such tasks. Support for this hypothesis comes from an fMRI study that found that decreased DLPFC activity during a cognitive task was significantly associated with increased amygdala activity during an emotional task, and that this relationship was reduced in patients with MDD (Siegle et al., 2007). This hypothesis is also consistent with a meta-analysis that reported altered resting-state connectivity in MDD, including hypoconnectivity between the SEN and midline CCN regions (Kaiser et al., 2015). Since this is the first study, to our knowledge, to relate resting-state connectivity to facial emotion perception task performance covarying for rMDD, further research is necessary to replicate this relationship between facial emotion perception accuracy and resting-state connectivity alterations.
Connectivity between salience and emotion network and regions in default mode network
Facial emotion perception was positively associated with connectivity of the right sgACC to areas in the DMN, including the middle temporal gyrus and rostral anterior cingulate. The DMN is argued to represent functions that are integral to the self (Raichle et al., 2001), and Gusnard et al. (2001) argue that part of this involves some assessment of salience for the individual.
Connectivity negatively associated with emotion perception
In contrast with the findings discussed above, we found a negative association between facial emotion perception accuracy and connectivity between the SEN and regions primarily outside of the three networks. This suggests that connectivity between the SEN and areas outside of the network may represent poorer integrity of the SEN, resulting in poorer performance on tasks that require the recruitment of these neurocognitive resources when processing emotional faces. We also found a negative association between facial emotion perception and connectivity between the left anterior insula and the right rostro-ventral anterior cingulate, which is located within the DMN. This finding is consistent with the strong functional coupling between the insula and the anterior cingulate cortex (Menon & Uddin, 2010). It is interesting that separate regions within SEN–DMN connectivity were both positively and negatively associated with emotion perception accuracy. A dissociation was observed whereby connectivity between the amygdala and sgACC seeds was positively but not negatively related to facial emotion perception performance, whereas connectivity between the (left) insula seed and rostro-ventral ACC was negatively but not positively related to facial emotion perception. This finding may be explained by the disparate functions of the insula, which is not only involved in salience detection, but also in switching between other major networks and in cognitive control (Menon & Uddin, 2010). Thus, the insula is important for identifying salient stimuli then disengaging the DMN and activating the CCN, and our finding is consistent with the idea that, in those individuals who have less disengagement of the DMN by the insula, emotion perception accuracy is reduced.
Task-based functional connectivity
The primary aim of this study was to investigate resting-state connectivity and performance. We also examined connectivity during the task performance, regressing out task events (Fair et al., 2007), movement parameters (in same manner as above), and the derived physiological parameters (as above). There were some regions of convergence between resting-state and task connectivity (see Supplementary Material Fig. S5 and S6), and these were primarily in the left midposterior insula (amygdala seeds) and the thalamus (left insula seed). Bressler and Kelso (2001) argue that involvement of cortical areas in coordinated networks is an indicator of their facilitated cooperation, and coordinated networks allow the system to rapidly accomplish functions that require combined and coordinated actions of numerous distributed areas. They assert that the system has relative coordination, can perform multiple functions, and is flexible, allowing it to adaptively transition between cognitive processes, leading to a behavioral goal. As an example, Jiang, He, Zang, and Weng (2004) showed change of the motor network from rest to movement is dynamic and can be modulated in different neural activities. Jiang et al. speculated that the functional network is in existence during rest and maintains a dynamic equilibrium. They note that the resting-state connectivity network seemed much looser than its movement counterpart, and that tighter network coupling implies accomplishment of function (i.e., planning, initiation, execution, and termination). Thus, there were fewer areas in the resting-state network that are responsible for task execution. The lack of convergence between the resting-state connectivity and the task connectivity networks in the present study can therefore be explained by the fact that the SEN performs variable functions, including signaling the salience of internal and external events, some, but not all of which may be associated with facial emotion perception. In contrast, connectivity during a facial emotion perception task, even when task events are removed, is specific to performance of an emotion perception task. Finally, although we have previously demonstrated that within-network SEN connectivity did not differ by group (Stange et al., 2017, Supplement) and there were no significant group differences in performance in the present study, to ensure that there were no differential associations between performance and connectivity by group, we conducted moderation analyses on the extracted beta values from the significant clusters in Tables 1 and 2. These results are reported in the Supplementary Material. Across seeds, there were 39 total clusters (i.e., reported clusters in Tables 1 and 2 of the main manuscript), and we observed seven significant moderation models of the 39. As detailed in the Supplementary Material, of these seven, four were positively associated with performance and three were negatively associated with performance. Further analysis revealed that three clusters showed a significant correlation between the seed and FEPT accuracy in the HC group only ( p < .01), whereas only one cluster showed a significant correlation in the rMDD group only (p < .05), suggesting that rMDD diagnosis was not a major moderator of the relationship between connectivity and performance.
Limitations
Although there is strong evidence that the seeds for our connectivity analysis are located within the SEN, the seed-based connectivity approach could be considered a limitation in comparison to an analysis of complete networks. We did, however, report the proportions of significant connectivity with each seed that were contained within three major networks of theoretical interest (Menon, 2011). In doing so, we chose to reduce the number of intrinsic connectivity networks reported by Yeo et al. (2011), at the exclusion of networks of lesser theoretical interest, including visual and motor networks, to facilitate parsimony in interpretation. Replication of our results by future research in an independent sample is required to provide support for this and other methodological choices made in this study, such as the significance threshold. In addition, there is continuing work toward elucidating the correct strategies for height by cluster extent correction. A final limitation of this study is that resting-state fMRI data were collected after the FEPT, which could result in carryover effects.
Conclusions
This study is the first to our knowledge to demonstrate relationships between facial emotion processing and resting-state connectivity within nodes located in the SEN and between SEN nodes and regions in the CCN and DMN. Among healthy individuals with and without history of MDD, facial emotion perception accuracy was positively associated with connectivity within the SEN as well as cross-network connectivity (particularly to CCN). These results represent an important step in understanding the relationship between resting-state connectivity of brain regions that underlie emotion processing and accuracy of emotion processing. Future work should compare network connectivity among individuals with rMDD and active MDD, at rest and during emotion processing, to better elucidate state versus trait effects. These future studies might also subdivide rMDD into those with and without performance weaknesses in this task to better articulate any risk for illness by subtype.
Notes
Mean = 1.67 episodes. However, one person with five episodes and one with six episodes were included with seasonal affective components to the illness.
An alternative, post hoc approach to calculating smoothness and cluster correction (e.g., 3dClustSim with ACF estimating the long tail of autocorrelation that deviates from the Gaussian) was also calculated for the interested reader. Those results that survive this more conservative threshold are k > 261/259 for L/R amygdala, k > 637/261 for L/R insula, and k > 481/588 for L/R subgenual cingulate. Those that survive cluster level correction in SPM8 are also so noted.
References
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author.
Anand, A., Li, Y., Wang, Y., Wu, J. W., Gao, S. J., Bukhari, L., … Lowe, M. J. (2005). Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biological Psychiatry, 57(10), 1079–1088. https://doi.org/10.1016/j.biopsych.2005.02.021
Babiloni, C., Ferretti, A., Del Gratta, C., Carducci, F., Vecchio, F., Romani, G. L., & Rossini, P. M. (2005). Human cortical responses during one-bit delayed-response tasks: An fMRI study. Brain Research Bulletin, 65(5), 383–390. https://doi.org/10.1016/j.brainresbull.2005.01.013
Behzadi, Y., Restom, K., Liau, J., & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042
Bhaumik, R., Jenkins, L. M., Gowins, J. R., Jacobs, R. H., Barba, A., Bhaumik, D. K., & Langenecker, S. A. (2016). Multivariate pattern analysis strategies in detection of remitted major depressive disorder using resting state functional connectivity. NeuroImage: Clinical. https://doi.org/10.1016/j.nicl.2016.02.018
Breiter, H. C., Etcoff, N. L., Whalen, P. J., Kennedy, W. A., Rauch, S. L., Buckner, R. L., … Rosen, B. R. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17(5), 875–887.
Bressler, S. L. & Kelso, J. A. S. (2001). Cortical coordination dynamics and cognition. Trends in Cognitive Sciences, 5(1), 26–36.
Briceño, E. M., Weisenbach, S. L., Rapport, L. J., Hazlett, K. E., Bieliauskas, L. A., Haase, B. D., … Langenecker, S. A. (2013). Shifted inferior frontal laterality in women with major depressive disorder is related to emotion processing deficits. Psychological Medicine, 43(7), 1433–1445.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. In A. Kingstone & M. B. Miller (Eds.), Year in cognitive neuroscience 2008 (Vol. 1124, pp. 1–38). Malden: Wiley-Blackwell.
Carballedo, A., Scheuerecker, J., Meisenzahl, E., Schoepf, V., Bokde, A., Moller, H. J., … Frodl, T. (2011). Functional connectivity of emotional processing in depression. Journal of Affective Disorders, 134(1/3), 272–279. https://doi.org/10.1016/j.jad.2011.06.021
Crozier, S., Sirigu, A., Lehericy, S., van de Moortele, P. F., Pillon, B., Grafman, J., … LeBihan, D. (1999). Distinct prefrontal activations in processing sequence at the sentence and script level: An fMRI study. Neuropsychologia, 37(13), 1469–1476. https://doi.org/10.1016/s0028-3932(99)00054-8
Cuthbert, B., & Insel, T. (2010). The data of diagnosis: New approaches to psychiatric classification. Psychiatry-Interpersonal and Biological Processes, 73(4), 311–314. https://doi.org/10.1521/psyc.2010.73.4.311
Dannlowski, U., Ohrmann, P., Konrad, C., Domschke, K., Bauer, J., Kugel, H., … Suslow, T. (2009). Reduced amygdala-prefrontal coupling in major depression: Association with MAOA genotype and illness severity. International Journal of Neuropsychopharmacology, 12(1), 11–22. https://doi.org/10.1017/s1461145708008973
Drevets, W. C., Gadde, K., & Krishnan, K. R. R. (2004). Neuroimaging studies of depression. In D. S. Charney, E. J. Nestler, & B. S. Bunney (Eds.), The neurobiological foundation of mental illness. New York: Oxford University Press.
Drevets, W. C., & Savitz, J. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13(8), 663–681.
Ekman, P., & Friesen, W. (1976). Pictures of facial affect. Palo Alto: Consulting Psychologists Press.
Etkin, A., Egner, T., & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. https://doi.org/10.1016/j.tics.2010.11.004
Fair, D. A., Schlaggar, B. L., Cohen, A. L., Miezin, F. M., Dosenbach, N. U. F., Wenger, K. K., … Petersen, S. E. (2007). A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage, 35(1), 396–405.
Fournier, J. C., Keener, M. T., Almeida, J., Kronhaus, D. M., & Phillip, M. L. (2013). Amygdala and whole-brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disorders, 15(7), 741–752. https://doi.org/10.1111/bdi.12106
Fox, M. D., Zhang, D. Y., Snyder, A. Z., & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. https://doi.org/10.1152/jn.90777.2008
Fusar-Poli, P., Placentino, A., Carletti, F., Landi, P., Allen, P., Surguladze, S., … Politi, P. (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34(6), 418–432.
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., … Schatzberg, A. F. (2007). Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62(5), 429–437. https://doi.org/10.1016/j.biopsych.2006.09.020
Gur, R. C., Schroeder, L., Turner, T., McGrath, C., Chan, R. M., Turetsky, B. I., … Gur, R. E. (2002). Brain activation during facial emotion processing. NeuroImage, 16(3), 651–662. https://doi.org/10.1006/nimg.2002.1097
Gusnard, D. A., Akbudak, E., Shulman, G. L., & Raichle, M. E. (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(7), 4259–4264.
Horn, D. I., Yu, C., Steiner, J., Buchmann, J., Kaufmann, J., Osoba, A., … Walter, M. (2010). Glutamatergic and resting-state functional connectivity correlates of severity in major depression—The role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience, 4. https://doi.org/10.3389/fnsys.2010.00033
Jacobs, R. H., Barba, A., Gowins, J. R., Klumpp, H., Jenkins, L. M., Mickey, B. J., … Langenecker, S. A. (2016). Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychological Medicine, 46(5), 1055–1067. https://doi.org/10.1017/s0033291715002615
Jacobs, R. H., Jenkins, L. M., Gabriel, L. B., Barba, A., Ryan, K. A., Weisenbach, S. L., … Welsh, R. C. (2014). Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS ONE, 9(8), 11. https://doi.org/10.1371/journal.pone.0104366
Jenkinson, M. Bannister, P., Brady, J. M., Smith, S. M. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841.
Jiang, T., He, Y., Zang, Y., & Weng, X. (2004). Modulation of functional connectivity during the resting state and the motor task. Human Brain Mapping, 22, 63–71.
Jo, H. J., Gotts, S. J., Reynolds, R. C., Bandettini, P. A., Martin, A., Cox, R. W., & Saad, Z. S. (2013). Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state fMRI. Journal of Applied Mathematics, 9. https://doi.org/10.1155/2013/935154
Joormann, J., & D’Avanzato, C. (2010). Emotion regulation in depression: Examining the role of cognitive processes. Cognition & Emotion, 24(6), 913–939. https://doi.org/10.1080/02699931003784939
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., & Pizzagalli, D. A. (2015). Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–611. https://doi.org/10.1001/jamapsychiatry.2015.0071
Kaiser, R. H., & Pizzagalli, D. A. (2015). Dysfunctional connectivity in the depressed adolescent brain. Biological Psychiatry, 78(9), 594–595. https://doi.org/10.1016/j.biopsych.2015.08.016
Kanske, P., Heissler, J., Schonfelder, S., & Wessa, M. (2012). Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage, 61(3), 686–693. https://doi.org/10.1016/j.neuroimage.2012.03.089
Kelly, A. M. C., Di Martino, A., Uddin, L. Q., Shehzad, Z., Gee, D. G., Reiss, P. T., … Milham, M. P. (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex (New York, N.Y. : 1991), 19(3), 640–657. https://doi.org/10.1093/cercor/bhn117
Kesler, M. L., Andersen, A. H., Smith, C. D., Avison, M. J., Davis, C. E., Kryscio, R. J., & Blonder, L. X. (2001). Neural substrates of facial emotion processing using fMRI. Cognitive Brain Research, 11(2), 213–226. https://doi.org/10.1016/s0926-6410(00)00073-2
Kohler, C. G., Hoffman, L. J., Eastman, L. B., Healey, K., & Moberg, P. J. (2011). Facial emotion perception in depression and bipolar disorder: A quantitative review. Psychiatry Research, 188(3), 303–309. https://doi.org/10.1016/j.psychres.2011.04.019
Kubler, A., Dixon, V., & Garavan, H. (2006). Automaticity and reestablishment of executive control: An fMRI study. Journal of Cognitive Neuroscience, 18(8), 1331–1342. https://doi.org/10.1162/jocn.2006.18.8.1331
Langenecker, S. A., Bieliauskas, L. A., Rapport, L. J., Zubieta, J. K., Wilde, E. A., Berent, S. (2005). Face emotion perception and ex- ecutive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology, 27, 320–333.
Langenecker, S. A., Caveny, A. F., et al. (2007). The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Research, 152, 143–154.
Langenecker, S. A., Jacobs, R. H., & Passarotti, A. M. (2014). Current neural and behavioral dimensional constructs across mood disorders. Current Behavioral Neuroscience Reports, 1(3), 144–153.
Langenecker, S. A., Nielson, K. A., & Rao, S. M. (2004). fMRI of healthy older adults during Stroop interference. NeuroImage, 21(1), 192–200. https://doi.org/10.1016/j.neuroimage.2003.08.027
Manoliu, A., Meng, C., Brandl, F., Doll, A., Tahmasian, M., Scherr, M., … Sorg, C. (2014). Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in Human Neuroscience, 7, 17. https://doi.org/10.3389/fnhum.2013.00930
Margulies, D. S., Kelly, A. M. C., Uddin, L. Q., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37(2), 579–588. https://doi.org/10.1016/j.neuroimage.2007.05.019
Maxwell, E. (1992). Family Interview for Genetic Studies (FIGS): A manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program: National Institute of Mental Health.Retrieved from https://www.nimhgenetics.org/interviews/figs/FIGS%201.0%20Manual%20-%20Aug%201992.pdf
Mayberg, H. S., Liotti, M., Brannan, S. K., McGinnis, S., Mahurin, R. K., Jerabek, P. A.,…Fox, P. T. (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry, 156(5), 675–682.
McCabe, C., & Mishor, Z. (2011). Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage, 57(4), 1317–1323. https://doi.org/10.1016/j.neuroimage.2011.05.051
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. https://doi.org/10.1016/j.tics.2011.08.003
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5/6), 655–667. https://doi.org/10.1007/s00429-010-0262-0
Morris, J. S., Friston, K. J., Buchel, C., Frith, C. D., Young, A. W., Calder, A. J., & Dolan, R. J. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121, 47–57. https://doi.org/10.1093/brain/121.1.47
Nurnberger, J. I., Blehar, M. C., Kaufmann, C. A., Yorkcooler, C., Simpson, S. G., Harkavyfriedman, J., … Ritz, A. L. (1994). Diagnostic Interview for Genetic Studies: Rationale, unique features, and training. Archives of General Psychiatry, 51(11), 849–859.
Pannekoek, J. N., Veer, I. M., van Tol, M. J., van der Werff, S. J. A., Demenescu, L. R., Aleman, A., … van der Wee, N. J. A. (2013). Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. Journal of Affective Disorders, 145(1), 29–35. https://doi.org/10.1016/j.jad.2012.07.006
Phan, K. L., Wager, T., Taylor, S. F., & Liberzon, I. (2002). Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16(2), 331–348. https://doi.org/10.1006/nimg.2002.1087
Phillips, M. L., Drevets, W. C., Rauch, S. L., & Lane, R. (2003). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54(5), 515–528. https://doi.org/10.1016/s0006-3223(03)00171-9
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676
Rama, P., Martinkauppi, S., Linnankoski, I., Koivisto, J., Aronen, H. J., & Carlson, S. (2001). Working memory of identification of emotional vocal expressions: An fMRI study. NeuroImage, 13(6, Pt. 1), 1090-1101. https://doi.org/10.1006/nimg.2001.0777
Rao, J. A., Jenkins, L. M., Hymen, E., Feigon, M., Weisenbach, S. L., Zubieta, J. K., & Langenecker, S. A. (2016). Differential resting state connectivity patterns and impaired semantically cued list learning test performance in early course remitted major depressive disorder. Journal of the International Neuropsychological Society, 22(2), 225–239. https://doi.org/10.1017/s1355617716000011
Rapport, L. J., Friedman, S. R., Tzelepis, A., Van Voorhis, A. (2002). Experienced emotion and affect recognition in adult attention- deficit hyperactivity disorder. Neuropsychology, 16(1), 102–110.
Russell, J. A., Bachorowski, J. A., & Fernandez-Dols, J. M. (2003). Facial and vocal expressions of emotion. Annual Review of Psychology, 54, 329–349. https://doi.org/10.1146/annurev.psych.54.101601.145102
Sarazin, M., Pillon, B., Giannakopoulos, P., Rancurel, G., Samson, Y., & Dubois, B. (1998). Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions. Neurology, 51(1), 142–148.
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. https://doi.org/10.1523/jneurosci.5587-06.2007
Shamay-Tsoory, S. G., Aharon-Peretz, J., & Perry, D. (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132, 617–627. https://doi.org/10.1093/brain/awn279
Shipley, W. C. (1940). A self-administering scale for measuring intellectual impairment and deterioration. The Journal of Psychology: Interdisciplinary and Applied, 9, 371–377.
Siegle, G. J., Thompson, W., Carter, C. S., Steinhauer, S. R., & Thase, M. E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry, 61(2), 198–209. https://doi.org/10.1016/j.biopsych.2006.05.048
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155.
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574. https://doi.org/10.1073/pnas.0800005105
Stange, J. P., Bessette, K. L., Jenkins, L. M., Peters, A. T., Feldhaus, C., Crane, N. A., … Langenecker, S. A. (2017). Attenuated intrinsic connectivity within cognitive control network among individuals with remitted depression: Temporal stability and association with negative cognitive styles. Human Brain Mapping, 38(6), 2939–2954.
Surguladze, S. A., Brammer, M. J., Young, A. W., Andrew, C., Travis, M. J., Williams, S. C. R., & Phillips, M. L. (2003). A preferential increase in the extrastriate response to signals of danger. NeuroImage, 19(4), 1317–1328. https://doi.org/10.1016/s1053-8119(03)00085-5
Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., … Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249.
Uddin, L. Q., Supekar, K., & Menon, V. (2010). Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Frontiers in Systems Neuroscience, 4, 21. https://doi.org/10.3389/fnsys.2010.00021
Wager, T. D., & Smith, E. E. (2003). Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(4), 255–274. https://doi.org/10.3758/cabn.3.4.255
Wang, L., Hermens, D. F., Hickie, I. B., & Lagopoulos, J. (2012). A systematic review of resting-state functional-MRI studies in major depression. Journal of Affective Disorders, 142(1/3), 6–12. https://doi.org/10.1016/j.jad.2012.04.013
Whitfield-Gabrieli, S., & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. In S. NolenHoeksema (Ed.), Annual review of clinical psychology (Vol. 8, pp. 49–76). Palo Alto: Annual Reviews.
Yeo, B. T. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. https://doi.org/10.1152/jn.00338.2011
Zhu, X. L., Wang, X., Xiao, J., Liao, J., Zhong, M. T., Wang, W., & Yao, S. Q. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry, 71(7), 611–617. https://doi.org/10.1016/j.biopsych.2011.10.035
Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I., Dalrymple, K. (2013). Severity classification on the Hamilton Depression Rating Scale. Journal of Affective Disorders, 150(2), 384–388.
Acknowledgements
This work was supported in part by the BRAINS RO1 (to SAL; MH 091811), UIC CTSS (1UL1RR029879), UM MCRU (UL1RR029879), internal support from the Depression and Neuropsychology Sections of the Department of Psychiatry, University of Michigan Medical Center, and internal support from the University of Illinois Department of Psychiatry, Cognitive Neuroscience Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 33314 kb)
Rights and permissions
About this article
Cite this article
Jenkins, L.M., Stange, J.P., Barba, A. et al. Integrated cross-network connectivity of amygdala, insula, and subgenual cingulate associated with facial emotion perception in healthy controls and remitted major depressive disorder. Cogn Affect Behav Neurosci 17, 1242–1254 (2017). https://doi.org/10.3758/s13415-017-0547-3
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-017-0547-3