Abstract
The agency facet of extraversion (aE) describes individual differences in goal-directed behavior and has been linked to dopamine function in incentive contexts. Because dopamine presumably modulates the processing of negative feedback/failure, aE may relate to failure processing in incentive contexts. To test this hypothesis, N = 86 participants performed a virtual ball-catching task. An incentive context was created by displaying potential rewards and subtle manipulations of task performance, which either was (control group) or was not (incentive context group) made explicit. To probe the involvement of dopamine, participants received either placebo or the selective dopamine D2 receptor antagonist sulpiride (200 mg). Failure processing was assessed through negative-feedback-evoked differences in the frontal midline theta electroencephalogram power (DFMT) and in the feedback-related negativity event-related potential component (FRN). Before incentives were introduced, DFMT (but not the FRN) was related to neuroticism/anxiety. Importantly, once incentives were displayed, aE was associated with DFMT, FRN, task performance, and changes in self-reported positive affect, which further depended on incentive context group and/or substance group: In the incentive context group but not in the control group, agentic extraverts showed relatively blunted DFMT after placebo. Sulpiride significantly enhanced DFMT, whereas it reduced FRN amplitudes and performance in agentic extra- versus introverts. These findings provide strong support for current dopamine models of aE and failure processing, and also highlight the importance of task context. Moreover, the dissociations of FRN and DFMT suggest the existence of two nonredundant electrophysiological indices of feedback processing, both relating to dopamine and aE.
Similar content being viewed by others
Agentic extraversion (aE) or “agency” is a facet of the broader extraversion construct (Eysenck, 1967), which typically also encompasses other components like affiliation and impulsive sensation seeking (Depue & Collins, 1999). Agentic extraverts are characterized by a strong sense of accomplishing goals, assertiveness and social dominance, relatively high levels of activity, well-being, and positive affect (Depue & Collins, 1999). Conversely, agentic introverts have a propensity for anhedonia (Wacker, Chavanon, & Stemmler, 2010), a cardinal feature of depression (Hasler, Drevets, Manji, & Charney, 2004). One of several relevant mechanisms that may predispose agentic extraverts for their characteristic engagement in and maintenance of goal-directed behavior may be an attenuated processing of failure during goal-directed behavior: Reduced sensitivity to internal or external failure signals may facilitate the anticipation of positive consequences in incentive contexts and thereby support persistence and goal striving in aE. From another perspective, however, aE has been traditionally characterized by enhanced reward anticipation (Depue & Collins, 1999), and may therefore relate to enhanced sensitivity for reward omission, which would suggest potentiated rather than blunted processing of failure during goal directed behavior (Lange, Leue, & Beauducel, 2012; Smillie, Cooper, & Pickering, 2011). In theory, aE may thus relate to either decreased or increased processing of failure.
When humans fail, anterior cingulate cortex (ACC) responses can be recorded on the scalp surface electroencephalogram (EEG; Debener et al., 2005; Gehring & Willoughby, 2002), which manifest as transient differences in frontal midline theta power (DFMT; Cavanagh, Zambrano-Vazquez, & Allen, 2012; Gehring & Willoughby, 2004; Luu, Tucker, & Makeig, 2004) or particular waveform components in the event-related potential (i.e., the error-related negativity [ERN] and feedback-related negativity [FRN]; Gehring, Coles, Meyer, & Donchin, 1995; Miltner, Braun, & Coles, 1997). It has been suggested that the FRN and ERN can be understood in terms of transient failure-induced increases in theta (4–8 Hz) power and cross-trial phase consistency (Cavanagh et al., 2012; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003; Luu et al., 2004), although it has also been noted that the FRN and feedback-evoked changes in theta are not redundant, and may thus provide complementary insights into failure processing (Cohen, Elger, & Ranganath, 2007).
Of relevance, neurobiological models link both aE (Depue & Collins, 1999) and frontomedial failure processing (Holroyd & Coles, 2002) to dopamine (DA), which is supported from molecular genetic (e.g., Reuter & Hennig, 2005; Smillie et al., 2011; Wacker, Mueller, Hennig, & Stemmler, 2012), pharmacological (e.g., Chavanon, Wacker, Leue, & Stemmler, 2007; Jocham & Ullsperger, 2009; Santesso et al., 2008; Zirnheld et al., 2004), and combined genetic–pharmacological (Mueller, Makeig, Stemmler, Hennig, & Wacker, 2011) studies.
An influential theory of extraversion has related aE to high tonic mesocorticolimbic DA activity, which may ultimately render agentic extraverts’ reward-related circuitry more responsive for incentive stimuli than are those of agentic introverts (Depue & Collins, 1999). Dopamine models of failure processing have related the ERN and FRN to phasic dips in mesencephalic DA activity that reflect reinforcement learning negative prediction errors (Holroyd & Coles, 2002). Alternatively, tonic rather than phasic DA may modulate failure processing (Lapish, Kroener, Durstewitz, Lavin, & Seamans, 2007), and we have suggested the existence of a curvilinear relationship between tonic prefrontal DA level and failure processing (Mueller et al., 2011). Unifying the aE–DA account and the curvilinear failure processing–DA accounts would suggest not only that high- versus low-aE individuals show different sensitivities for failure, but also that further increases of DA (e.g., through pharmacological manipulation) may either reduce or enhance failure processing, depending on baseline DA and aE.
Despite the common association with DA, however, a direct link between failure processing and aE has not yet been tested. Associations of failure processing with other extraversion-related measures are somewhat inconsistent (Hoffmann, Wascher, & Falkenstein, 2012; Lange et al., 2012; Smillie et al., 2011), with a large number of null findings (Boksem, Tops, Wester, Meijman, & Lorist, 2006; Luu, Collins, & Tucker, 2000; Pailing & Segalowitz, 2004; Sato et al., 2005), possibly because the previously assessed extraversion-related constructs have been less directly related to DA than the agency facet of extraversion (Depue & Lenzenweger, 2005). In addition, whereas the typical assessment of failure processing in standardized laboratory environments implies that putative relationships between extraversion and failure processing should generalize across situations, including not particularly rewarding laboratory contexts, contextualized dispositional approaches (Allport, 1937; Mischel & Shoda, 1998; Stemmler, 1997) suggest that reliable relationships between personality, physiology, affect, and behavior should emerge only in those situations that activate the underlying neurobehavioral systems (Stemmler & Wacker, 2010).
Prior evidence supports the context dependence of associations between personality and failure processing (Pailing & Segalowitz, 2004; Tops & Boksem, 2010) and of the link between aE and DA-related brain processes (Coan, Allen, & McKnight, 2006; Wacker, Mueller, Pizzagalli, Hennig, & Stemmler, 2013). For example, Pailing and Segalowitz (2004) showed that changes in monetary incentives for task performance modulate the ERN as a function of conscientiousness (aE was not assessed in that study). Moreover, we recently demonstrated in young male participants that an indicator of aE was only related to a putative correlate of DA-system activity (frontal EEG asymmetry during rest) when the participants were treated by highly attractive female experimenters (thus, presumably, providing an incentive context for the male participants), but not when the experiment was run by slightly less attractive (Wacker et al., 2013) or male (Wacker, Chavanon, Leue, & Stemmler, 2008) experimenters. These findings indicate that incentive contexts may be a necessary condition to produce reliable correlations between aE and DA activity and—by extension—between aE and DA-related failure processing.
On the basis of the arguments above, we hypothesized that aE is related to individual differences in failure processing (Hypothesis 1), that the delivery of a DA antagonist should modulate the association between aE and failure processing (Hypothesis 2), and that the link between aE and failure processing should be larger in an incentive than in a neutral context (Hypothesis 3). We tested our hypotheses with a randomized double-blind placebo-controlled pharmacological manipulation of DA (with the DA D2 receptor antagonist sulpiride, 200 mg) and an experimental manipulation of incentive context. For the latter manipulation, all participants were shown a basket of potential rewards that could be won in a virtual ball-catching task. This task started out with a difficult phase in which participants were not very successful, and hence did not get closer to their goal of receiving a reward. We therefore termed this the “non-goal-attainment phase.” After that phase, however, the task became easier, thereby improving task performance and bringing participants closer to the reward. We thus termed that the “pre-goal-attainment phase” (Davidson, 1994). Of relevance, a control group was informed about this change in task difficulty, whereas the experimental group was not. With this procedure, we aimed to manipulate the motivational significance of the context without affecting its physical properties. We reasoned that only the experimental group should attribute their improved performance internally (i.e., to their ability), and should therefore report increased feelings of expectancy and wanting (Stemmler, 2009), since they would expect to receive the reward (incentive context group). In contrast, the control group should attribute their performance externally (nonincentive context control group).
Method
Participants
A total of N = 86 females (M age = 22.62 years, SD = 2.53 years, range = 18–31 years) participated in the present study. After recruitment on campus, exclusion (acute or life-time psychiatric disorder, self-reported physical impairment, habitual smoking, or drug or alcohol abuse) and inclusion (body mass index ≥ 17.5, blood pressure > 90/50) criteria were examined. The ethics committee of the German Psychological Society (Ethikkommission der Deutschen Gesellschaft für Psychologie) approved the study protocol. For completion, participants obtained monetary compensation (€55–€65, approximately US$75–$85). The participants were German natives and unmedicated except for hormonal contraception, which was required because pregnancy and lactation are contraindications of sulpiride subscription. The mean weight of the sample was 62.7 kg (SD = 9.7 kg; mean BMI: 22.32).
Procedure
The present study involved the same participants who had also participated in a prior study, which included different tasks than the present report, a challenge of the opioid (i.e., via naltrexone) rather than dopamine system, and was conducted at least 3 months earlier than this study (Schweiger, Stemmler, Burgdorf, & Wacker, 2013). At the beginning of the session, all volunteers gave written informed consent and said that they had not consumed alcohol, nicotine, or caffeine within the last 12 h. All of the participants were tested to confirm that they were not pregnant (10 mIU/ml human chorionic gonadotropin hCG, VEDA.LAB, Alencon cedex, France), had a light standardized breakfast, and received a sulpiride or placebo capsule (double-blind), depending on the randomized substance condition of the prior study (i.e., individuals randomized to the active-substance condition in Study 1 received placebo in the present study, and vice versa). Thereafter, participants completed (1) several questionnaires assessing their personality and intelligence, (2) an n-back task, (3) an emotion induction procedure combined with a social computer game, and (4) an AX continuous-performance task. Participants then started the catch-the-ball task after a 3-min resting period (see below). They were then debriefed and received their monetary compensation, plus the incentive context reward described below. The whole session lasted approximately 5 h.
Catch-the-ball task
A schematic trial of the task is shown in Fig. 1. A trial started with the standard picture (500–1,500 ms) before the top virtual player threw a ball at a random angle toward the participant’s player (bottom of screen). The ball traveled for a certain number of frames in the so-called trajectory presentation period, and then stopped. The participant was instructed to move his player to one of four positions in order to catch the ball. Then the ball continued its trajectory (duration: 925 ms) and was either caught (“catch trial”) or not caught (“miss trial”). Additional feedback with a duration of 1,000 ms was provided after each trial.
Schematic trial indicating the phase-dependent trajectory presentation period and the different postdecision throwing sequences, depending on whether the participant chose Position 1 (top) or 4 (bottom), leading to a catch and a miss, respectively. Note the different trajectory presentation periods for the non-goal-attainment phase (a) and the pre-goal-attainment phase (b)
The task consisted of “training,” “baseline,” “non-goal-attainment,” and “pre-goal-attainment” phases, which varied in their difficulty due to the different trajectory presentation periods (i.e., the number of frames until the ball stopped; see Fig. 1). Whereas few frames of travel time provide little information to estimate the angle of the ball’s trajectory, more frames allow for a better estimate. The training (44 trials) and baseline (88 trials) phases had medium trajectory presentation periods (four frames, 270 ms) or moderate difficulty; the non-goal-attainment phase (88 trials) was relatively difficult due to a short trajectory presentation period (two frames, 110 ms), whereas the pre-goal-attainment phase (88 trials) was relatively easy, due to a long trajectory presentation period (five frames, 350 ms).
A basket of potential rewards (including pralines, gift certificates, etc.) was shown before the non-goal-attainment and pre-goal-attainment phases, and participants were promised one item from the basket for good task performance. Individuals in the incentive context group were not informed about the change in task difficulty from the non-goal-attainment to the pre-goal-attainment phase, whereas participants in the nonincentive context control group were told about this manipulation and the possible emotions that might emerge.Footnote 1
As a behavioral measure of task performance, the percentage of catch trials was calculated for each phase. Because participants were never instructed to perform quickly and could take as much time as they wanted to make a decision, the analysis of reaction times was not informative.
Sulpiride
The substituted benzamide sulpiride is a selective D2-receptor antagonist. A single acute dose of 200 mg of sulpiride presumably blocks presynaptic autoreceptors, thereby elevating DA levels (Kuroki, Meltzer, & Ichikawa, 1999; Mereu, Casu, & Gessa, 1983). Sulpiride is generally well tolerated and has low affinity to histaminergic, cholinergic, serotonergic, adrenergic, or GABA receptors. Sulpiride is slowly absorbed from the gastrointestinal tract. The peak serum levels occur within 1–6 h after oral ingestion, whereas the average elimination half life is between 3 and 10 h (Mauri, Bravin, Bitetto, Rudelli, & Invernizzi, 1996).
Agentic extraversion
aE was measured by first standardizing and then averaging the NEO-PI-R (by Costa and McCrae; translated into German by Ostendorf & Angleitner, 2004), MPQ (Tellegen & Waller, 2008), and BIS/BAS (Carver & White, 1994) scales that loaded onto an agentic extraversion factor in a larger independent data set (i.e., loadings > .3; the MPQ scales were Social Potency and Well Being; the BIS/BAS scales were Drive and Reward Responsiveness; and the NEO Self and Other Ratings were Assertiveness, Activity, and Positive Emotions; Wacker et al., 2012). The internal consistency of the aE score was satisfactory (when entering the standardized scales as items, Cronbach’s alpha was .80). Affiliation and impulsivity scores were determined similarly by first standardizing and then averaging the associated scales (for affiliation: MPQ Social Closeness and NEO Gregariousness, Warmth, and Positive Emotions scales; Cronbach’s alpha = .82; for impulsivity: MPQ Control (inverted) and BAS Fun Seeking scales; Cronbach’s alpha = .68). To allow for comparison with earlier studies, we also computed a Broad Extraversion score by first standardizing and then averaging all of the scales that went into the aE, affiliation, and impulsivity scores.
Self-report of emotion
After the training, baseline, non-goal-attainment, and pre-goal-attainment phases, participants rated their feeling states on several dimensions using one-item measures. According to Stemmler (2009), aE is particularly associated with the expectancy–wanting emotion system, which motivates an individual to achieve success when striving for reward. For the item that assessed expectancy–wanting, participants indicated the degree to which they had feelings of positive anticipation, were full of expectancy, felt quickened, had a zest for action, and felt confident (the original German wording was Vorfreude, erwartungsvoll, beflügelt, Tatendrang, zuversichtlich) on a scale from 0 (not at all) to 8 (very much).
Electroencephalography (EEG)
EEG was recorded at a sampling rate of 512 Hz using a 64-channel Active Two (BioSemi, Amsterdam, Netherlands) active electrode system with driving right leg and common mode sense as the active and passive references, respectively. EEG was re-referenced to average reference, down-sampled to 128 Hz, band-pass (0.5–50 Hz) filtered and manually screened for data epochs with nonocular artifacts. Independent component analysis was then applied to the EEG data in order to remove ocular artifacts.
Data reduction
The continuous EEG was segmented into 2-s epochs ranging from –1,000 to 1,000 ms, relative to the first frame onset of the postdecision throwing sequences (see Fig. 1). Theta power was determined by performing a fast Fourier transform (FFT) at the single-trial level for the whole 2-s epochs, which were convolved with a 10 % Hamming window in order to avoid leakage effects and simultaneously to attenuate the relative contributions of the predecision throwing sequence and the additional explicit feedback at the end of the trial. To achieve individual measures of condition-specific theta, power spectra (0.5-Hz resolution) were averaged across trials (separately for each phase and trial type) and log-transformed. In exploratory analyses we also analyzed 500ms-segments (–500–0, 0–500, and 500–1000 ms relative to the first frame onset) with a reduced (2-Hz) resolution were, in order to get further information about the timing of observed effects. To compute the FRN, EEG segments were averaged across trials, and grand-average waveforms were computed across all individuals. The FRN was measured as the mean amplitude from 250 to 400 ms at channel Cz, where it was maximal (see Fig. 2 in the Results).
Topography of differential frontal midline theta (DFMT, panel a) and differential FRN (panel b) for the whole group. The top scalp map shows the difference of theta power after miss versus catch trials (red indicates more power during miss trials). The bottom scalp map shows the difference in event-related potential voltage after miss trials minus catch trials (red indicates a more-negative amplitude during miss trials)
Statistical analyses
To reduce between-subjects variance unrelated to our hypotheses, all of the statistical analyses were conducted with difference scores. A continuous measure of differential frontal midline theta (DFMT) was computed by subtracting the log-transformed theta after catch trials from the log-transformed theta after miss trials. If theta were to increase after miss versus catch trials, this differential score would be positive, and more positive values indicate relatively more theta for miss than for catch trials. A continuous measure of differential FRN was computed by subtracting the FRN for miss trials from the FRN for catch trials. Assuming more negative FRN during miss than during catch trials, the differential scores should be positive, and more positive values indicate an increased (negative) FRN for miss versus catch trials. A differential measure of performance was computed by subtracting performance in the baseline phase from performance in the later phases. Positive values would indicate relatively increased performance, whereas negative values would indicate reduced performance. Similarly, a differential measure of expectancy–wanting was computed by subtracting expectancy wanting in the baseline phase from expectancy–wanting in the later phases. Positive values would indicate an increase of expectancy–wanting, whereas negative values would indicate a decrease of expectancy–wanting.
In a first step, these four dependent variables (differential performance, expectancy–wanting, DFMT, and FRN) were analyzed with separate Phase (non-goal-attainment vs. pre-goal-attainment) × Context Group (incentive context group vs. nonincentive context group) × Substance Group (placebo vs. sulpiride) analyses of variance (ANOVAs). These ANOVAs were conducted in order to test for general (i.e., personality independent) effects and to confirm that incentive context had been successfully manipulated in the incentive versus nonincentive context groups. In addition, one-sample t tests were conducted on DFMT and the differential FRN to confirm that these measures reliably captured brain activity sensitive for miss versus catch trials.
In a second step, the three primary hypotheses were tested. To test Hypothesis 1 (relationship between aE and failure processing), the correlations of the dependent variables with aE in the placebo group were tested for significance. To test Hypothesis 2 (influence of DA on aE–failure processing relationships), the correlations of the dependent variables were also computed for the sulpiride group and compared to placebo using the Fisher (1950) transformation. To test Hypothesis 3 (influence of incentive context on aE–failure processing relationships), correlations of the dependent variables and aE were computed separately for the incentive context and nonincentive context within the substance groups and compared using the Fisher transformation.
Results
General effects
Performance
The Phase (non-goal-attainment vs. pre-goal-attainment) × Context Group × Substance Group ANOVA on baseline-corrected performance revealed a main effect of phase [F(1, 82) = 1566.12, p < .001, η p 2 = .95]. As intended, participants performed more poorly in the non-goal-attainment phase (34.7 % correct responses) than in the baseline phase (75.5 %), and showed a strong increase in performance in the pre-goal-attainment phase (79.5 % correct responses). No other main effects or interactions reached significance (ps > .08).
Expectancy–wanting
A Phase × Context Group × Substance Group ANOVA on baseline-corrected expectancy–wanting confirmed an increase of expectancy–wanting from non-goal-attainment to pre-goal-attainment [main effect of phase: F(1, 82) = 19.99, p < .001, η p 2 = .20] and higher levels of expectancy–wanting in the incentive context group [main effect of context group: F(1, 82) = 6.94, p = .010, η p 2 = .08], which were further qualified by a significant Phase × Context interaction [F(1, 82) = 5.21, p = .025, η p 2 = .06]. As intended, we found a significant expectancy–wanting increase from baseline to pre-goal-attainment in the incentive context group [t(43) = 4.07, p < .0002], but not in the nonincentive context group [t(41) = –1.1, p > .2]. This indicates that we successfully manipulated the motivational significance of the task context, such that only individuals in the incentive group reported increased pre-goal-attainment-related positive affect in that phase. No other significant main effects or interactions emerged (ps > .2).
DFMT
As is shown in Fig. 2, DFMT was maximal at channel FCz. A Phase × Context Group × Substance Group ANOVA on DFMT at channel FCz revealed a main effect of phase [F(1, 82) = 4.72, p = .033, η p 2 = .05], indicating that the DFMT score was more pronounced in the pre- than in the non-goal-attainment phase. The DFMT score was significantly larger than zero for both phases [non-goal-attainment phase, t(85) = 5.79, p < .00001; pre-goal-attainment phase, t(85) = 6.21, p < .00001], indicating that DFMT reliably captured increased theta for miss versus catch trials. Furthermore, the DFMT score was not confounded with the number of correct responses that an individual made [r(84) = –.07, p > .5].
FRN
The differential FRN score (i.e., positive minus negative feedback) was maximal at channel Cz. A Phase × Context Group × Substance Group ANOVA on the differential FRN at Cz revealed a main effect of phase [F(1, 82) = 124.47, p < .0001, η p 2 = .60], indicating that the differential FRN effect was significantly smaller than zero in the non-goal-attainment phase, in which positive feedback was unexpected [t(85) = 5.16, p < .0001], but significantly larger than zero in the pre-goal-attainment phase, in which negative feedback was unexpected [t(85) = 9.76, p < .0001], thereby supporting the expectedness account of the FRN (Donkers, Nieuwenhuis, & van Boxtel, 2005; Ferdinand, Mecklinger, Kray, & Gehring, 2012). We found no other main effects or interactions (ps > .2). Of relevance, the differential FRN and DFMT were not correlated across participants in either phase (ps > .5).
Failure processing and aE
DFMT
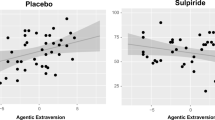
Consistent with Hypothesis 1 (“aE is associated with failure processing”), we observed a negative correlation between aE and DFMT in the placebo group during the pre-goal-attainment phase [r(41) = –.37, p = .019]. In line with Hypothesis 2 (“dopamine modulates the relationship between aE and failure processing”), this association was absent in the sulpiride group [r(45) = .08, p > .5], with the two correlations differing significantly (Z = 2.18, p < .03). Finally, when the placebo group was further subdivided into context groups, the correlation was only significant in the incentive context subgroup [r(20) = –.57, p = .009; Fig. 3]Footnote 2 but not in the nonincentive context subgroup [r(21) = .03, p > .8], and again, the two correlations differed significantly (Z = 2.00, p < .05), thereby supporting Hypothesis 3 (“a larger absolute aE–failure processing correlation in an incentive vs. a nonincentive context”). When DFMT was computed separately for the segments –500 to 0 ms, 0 to 500 ms, and 500 to 1,000 ms relative to feedback onset, only theta power in the segment from 0 to 500 ms was significantly correlated with aE [r(20) = –.49, p = .029], indicating that the relationship between aE and DFMT is primarily driven by DFMT during the actual postdecision throwing sequence (i.e., 0 to 500 ms), when early failure processing should occur.
Scatterplots for agentic extraversion and differential frontal midline theta (a), feedback-related negativity (b), expectancy–wanting (c), and performance (d) for the pre-goal-attainment phase. Trend lines are shown for placebo (black) versus sulpiride (gray) and for incentive (solid) versus nonincentive (dashed) context group
It should be noted that in the incentive context placebo group, DFMT also correlated with the broad extraversion score [r(20) = –.48, p = .031], and marginally with the impulsivity facet [r(20) = –.41, p = .069]. However, the small association between impulsivity and DFMT disappeared when the other extraversion facets (agency and affiliation) were partialed out (p > .3). In contrast, the association between aE and DFMT remained significant, when affiliation and impulsivity were partialed out [r(16) = –.52, p = .028], indicating that the link between DFMT and extraversion was predominantly driven by agentic introversion/extraversion rather than impulsivity. Moreover, the correlation of aE and DFMT remained significant when partialing out behavioral performance [r(16) = –.71, p = .001]. We found no significant associations between aE and DFMT during the non-goal-attainment phase, thereby further supporting Hypothesis 3.
One interpretation of the significantly different aE–DFMT correlations for the placebo versus sulpiride groups is that sulpiride affects the association between aE and DFMT. Another perspective on the same interaction is that individual differences in aE modulate the effect of sulpiride on DFMT. To illustrate this view, we dichotomized aE by median split and performed an Agency (agentic introversion [aI] vs. aE) × Substance ANOVA. As would be expected from the continuous aE measure, this ANOVA revealed an interaction of Agency and Substance [F(1, 82) = 6.58, p = .012, η p 2 = .07; Fig. 4]. Follow-up t tests indicated that sulpiride induced a reduction of DFMT in agentic introverts [t(41) = 2.17, p = .036], whereas it had a (nonsignificant) opposite effect on agentic extraverts [t(41) = –1.42, p = .163]. Thus, the present data also allow the interpretation that individual differences in aE modulate how a dopaminergic substance influences neural failure responses.
Barplots depicting mean (with SEMs) differential (i.e., negative minus positive feedback) frontal midline theta power (left panel) and differential (i.e., positive minus negative feedback) FRN amplitude (right panel), depending on dichotomized agentic introversion/extraversion (i.e., agentic introversion, aI; agentic extraversion, aE) and substance (i.e., placebo, white; sulpiride, gray)
Feedback-related negativity
In the pre-goal-attainment phase, the difference wave FRN showed a marginally significant positive association with aE in the placebo group [r(41) = .30, p = .061] and a nonsignificant negative association in the sulpiride group [r(45) = –.18, p = .243]; these two correlations differed significantly from each other (Z = 2.20, p = .014). aE–FRN correlations were not significantly different between incentive context groups (ps > .2), and no associations were apparent between aE and FRN in the non-goal-attainment phase (ps > .3).
A dichotomized Agency × Substance ANOVA on the FRN in the pre-goal-attainment phase revealed a significant interaction of Agency and Substance [F(1, 82) = 5.73, p = .019, η p 2 = .07; Fig. 4], supporting the interpretation that individual differences in aE modulate the effect of sulpiride on failure processing.
Expectancy–wanting
We found no significant association between expectancy–wanting increase and aE in the placebo group [r(41) = –.04, p > .8], although aE did predict a reduced increase of expectancy–wanting in the sulpiride group [r(45) = –.33, p = .030]. Of relevance, when the sulpiride group was further subdivided into the incentive context versus nonincentive context groups, the correlation between expectancy–wanting increase and aE was significant only in the incentive context group [r(24) = –.59, p = .003], but not in the nonincentive context group [r(21) = –.05, p > .8; comparison of correlation coefficients: Z = 1.95, p = .05]. No associations emerged between aE and baseline-corrected expectancy–wanting in the non-goal-attainment phase.
Performance
As with self-reported expectancy–wanting, aE and baseline-corrected performance in the pre-goal-attainment phase were uncorrelated in the placebo group [r(41) = –.14, p > .3], but were correlated in the sulpiride group [r(45) = –.51, p = .0004]. Although this indicated that substance affected the correlation between aE and performance, such that agentic extraverts performed relatively worse than introverts after sulpiride but not placebo intake, the direct comparison of the correlation coefficients failed to reach significance (Z = 1.88, p = .059, two-sided). The correlation of aE and performance was unaffected by context within the substance groups, and was absent across groups during the non-goal-attainment phase.
Failure processing and anxiety
As is shown in Fig. 5, we further found a positive correlation between the NEO Neuroticism Anxiety scale and DFMT [r(85) = .29, p = .008], but not FRN (p > .5), in the baseline phase, thereby providing partial support for the previously reported link between neuroticism/anxiety and high failure processing in standard laboratory contexts (Hajcak, McDonald, & Simons, 2003; Luu et al., 2000; Mueller, Stemmler, Hennig, & Wacker, 2013). Contrasting the DFMT–aE relationships, the DFMT–anxiety association was unaffected by both sulpiride (p > .7) and incentive context (p > .6), and was absent in the task phases after the reward had been introduced (p > .3).
Scatterplot for differential frontal midline theta (DFMT) and anxiety for the baseline phase. The trend lines show the linear relationships across all groups (r = .3, p < .008). This relationship is significantly affected neither by placebo (black) versus sulpiride (gray) nor by incentive (solid) versus nonincentive (dashed) context (ps > .3)
Discussion
The present study indicates that aE is associated with individual differences in failure processing, and that this association is related to dopamine and incentive context. Specifically, aE was correlated with reduced failure-evoked DFMT during a pre-goal-attainment phase under placebo (confirming Hypothesis 1: “aE is linked to failure processing”), this association was attenuated following intake of a dopamine-blocker (confirming Hypothesis 2: “the link between aE and failure processing is modulated by dopamine”) and was significantly stronger in the incentive versus nonincentive context group (confirming Hypothesis 3: “the link between aE and failure processing is larger in an incentive vs. nonincentive context”). Moreover, a dopamine blocker changed the association between aE and FRN, thereby providing further support for Hypothesis 2. Together, these findings indicate that dopaminergic mechanisms may connect aE to failure processing, particularly in incentive motivational contexts, and they converge with prominent dopamine theories of both, aE (Depue & Collins, 1999) and failure processing (Holroyd & Coles, 2002).
aE, failure processing, and the role of incentive context
Because incentive motivational contexts presumably activate individual differences in specific brain processes, behavior, and affect as a function of aE, particular care was taken to create such a context by (a) displaying a basket with potential rewards and (b) inducing an improvement in performance that should lead to subjective goal acquisition if success is attributed internally (incentive context group). Consistent with this aim, the personality-independent analyses revealed an increase in pre-goal-attainment positive affect (i.e., expectancy–wanting) in the pre-goal-attainment phase in the incentive context, but not in the nonincentive context control group.
In the incentive motivational context that was so established, agentic extraverts under placebo showed reduced neural failure responses as measured with DFMT. Assuming that frontomedial failure signals indicate a negatively valenced affective response (Gehring & Willoughby, 2002; Pailing & Segalowitz, 2004), the negative DFMT–aE correlation may indicate that failing in a potentially rewarding situation is more unpleasant for agentic introverted than extraverted individuals. Attenuated sensitivity for failure in incentive contexts may predispose agentic extraverts to give up less easily during goal-directed behavior and to maintain reward-seeking behavior.
Importantly, the correlation between aE and DFMT was specific to the incentive context group and phase. It may be relevant to discriminate between failing in an incentive context (in which failing may lead to reward omission; i.e., C+) and failing in a nonincentive context (in which a potential consequence of failing is negative social evaluation or other forms of punishment; i.e., C–). Although the former may be particularly linked to dopaminergic reward pathways and aE, the latter may be more closely linked to the neural processing of aversive events and anxiety. This interpretation converges with the present double dissociation, in which aE is linked to DFMT in an incentive but not in a nonincentive context, whereas anxiety relates to DFMT in the baseline phase but not when a performance-related reward is salient (for similar associations between anxiety and failure processes in nonincentive contexts, see Boksem et al., 2006; Hajcak et al., 2003; Luu et al., 2000; note, however, that these studies reported associations of anxiety with the FRN/ERN rather than DFMT; (Mueller et al., 2013). Moreover, the dopaminergic manipulation via sulpiride affected only the association between aE and DFMT, but not between anxiety and DFMT. Together, the DFMT findings support the contextualized dispositional account (Stemmler, 1997) and converge with prior reports of context-specific associations of failure processing and personality (Olvet & Hajcak, 2009; Tops & Koole, 2012).
In contrast to DFMT, the FRN–aE relationship was not affected by context group (although it did show some contextual modulation, since the FRN was only related to aE in the pre-goal-attainment phase, but not in the non-goal-attainment phase). Moreover, the FRN showed a positive rather than a negative correlation with aE, which converges with prior reports on broad extraversion and FRN (Lange et al., 2012; Smillie et al., 2011) and indicates that early failure detection processes, potentially associated with reinforcement learning (Holroyd & Coles, 2002) and/or N200-like conflict detection (Baker & Holroyd, 2011), are enhanced rather than attenuated in aE.
Dopamine modulates failure processing, task performance, and expectancy–wanting in aE
Consistent with dopaminergic models of aE and failure processing, the aE–DFMT and the aE–FRN correlations were significantly modulated in individuals who received a DA blocker as compared to placebo. We recently proposed a U-shaped relationship between prefrontal DA level and ERN/FRN such that low and high tonic prefrontal DA levels allow for stronger phasic failure signals than medium DA levels (Mueller et al., 2011), possibly by enhancing neural network instability through relative hyperactivation of D2 versus D1 receptors (Durstewitz & Seamans, 2008). From this perspective, the relationship between FRN and aE may relate to individual differences in tonic prefrontal D2 versus D1 receptor activation states. Consistent with this interpretation, aE has been associated with the VAL allele of the COMT polymorphism (Wacker & Gatt, 2010; Wacker et al., 2012), which presumably relates to low prefrontal DA and D2 versus D1 receptor states (Durstewitz & Seamans, 2008), and enhanced ERN (Osinsky, Hewig, Alexander, & Hennig, 2012) and FRN (Marco-Pallares et al., 2009) amplitudes. After sulpiride intake, these levels may shift such that VAL-allele carriers (and agentic extraverts) show medium prefrontal-DA levels, D1 versus D2 receptor states, and reduced ERN/FRN amplitudes (Mueller et al., 2011). Furthermore, it could be speculated that DFMT captures an affective response to negative feedback that is regulated by phasic subcortical DA release, which is presumably inversely related to tonic prefrontal DA levels (Bilder, Volavka, Lachman, & Grace, 2004).
Regardless of the exact mechanisms, the different correlations after placebo versus sulpiride intake (see also Fig. 3) imply that the level of aE determines whether sulpiride enhances or reduces failure signaling. Of relevance, sulpiride not only enhanced DFMT and reduced FRN in agentic extraverts versus introverts, but further led to relatively worse task performance in agentic extraverts than in introverts, thereby mirroring prior reports that sulpiride reduced the relative performance of agentic extraverts versus introverts in a working memory task (Chavanon et al., 2007).
After sulpiride, aE was further characterized by a relatively reduced increase of expectancy–wanting in the incentive context. Thus, after sulpiride, those individuals who are typically characterized by increased expectancy–wanting and high reward motivation reported a relatively low increase in positive affect in the pre-goal-attainment phase. Conversely, agentic introverts, characterized by a propensity for anhedonia (Wacker et al., 2010), showed relatively strong increases of expectancy–wanting after sulpiride. This finding converges not only with the efficacy of sulpiride for the treatment of anhedonic symptoms (Serra et al., 1990), but it further shows that sulpiride is specifically beneficial for individuals with high aI. Overall, sulpiride seems to make agentic introverts become more like agentic extraverts with regard to performance, positive affect, and frontomedial error processing (and vice versa).
Measurement of failure processing and aE
In the present study, failure processing was measured as negative-feedback-related increases in frontal midline theta power (Cavanagh et al., 2012; Cohen et al., 2007; De Pascalis, Varriale, & Rotonda, 2012; Gehring & Willoughby, 2004) and as negative-feedback-related increases in (negative) ERP amplitude (Gehring & Willoughby, 2002; Miltner et al., 1997). Both indices showed the expected sensitivity for failure, and both indices were stronger in the pre- than in the non-goal-attainment phase, in which failure is less likely, and hence particularly salient. Despite these similarities, the two indices had slightly different fronto- and centromedial topographies, were uncorrelated across individuals (Cohen et al., 2007), and showed different associations with aE and incentive context. Since event-related potential amplitudes depend not only on single-trial activity (which would be reflected in spectral power), but also on the cross-trial phase consistency, the FRN amplitude does not necessarily correspond to theta power, even if theta is the primary frequency that contributes to the FRN (Cavanagh et al., 2012; Gehring & Willoughby, 2004). The present dissociations of DFMT and FRN are consistent with prior work, and they suggest that future studies on personality and feedback processing may utilize these two measures, which may provide complementary insights into feedback processing (Cohen et al., 2007; Holroyd, HajiHosseini, & Baker, 2012).
Limitations and conclusion
First, because we used a task in which information on whether or not a reaction was successful gradually increased as the ball came closer to the participant, no temporally consistent time point was associated with the feedback stimulus. As a consequence, event-related potentials may have been smeared in the time domain. However, the assessed FRN showed the expected topography and significantly differed between catch and miss trials. Similarly, the robust theta increase after miss versus catch trials and the frontomedial topography of this effect provide important support for the present operationalization of frontal midline theta power as a highly sensitive measure of failure processing. We therefore suggest that these measures can also be considered for other paradigms in which task-related information gradually increases (e.g., cyberball task; Eisenberger, Lieberman, & Williams, 2003). Second, the investigated sample was exclusively female. Although the general tendency of sulpiride to reverse aE–physiology and aE–behavior relationships converges with prior studies with male samples, future work may test whether the present findings on failure processing also generalize to men. Finally, with cell sizes between 21 and 24, the power to detect correlations between personality and indices of failure processing within the substance and context groups was relatively low, and the confidence intervals around the observed correlation coefficients were relatively broad. Thus, the observed DA- and context-dependent aE–failure processing relationships may exceed the actual relationship in the population. To more accurately estimate the magnitude of aE–failure processing associations, future studies with larger sample sizes will be needed.
Notwithstanding these limitations, the present study has demonstrated that aE is associated with FRN and failure-evoked frontal midline theta, particularly in an incentive-motivational context. Because these relationships are modulated by the selective D2 receptor blocker sulpiride, the present findings speak to the relevance of DA in linking aE to failure processing and support current models, which assume prominent roles of DA in both failure processing (Holroyd & Coles, 2002) and agentic introversion/extraversion (Depue & Collins, 1999).
Notes
Whether individuals were placed in the incentive or the nonincentive context group was determined through randomization in Study 1 (three months before the present study). The individuals randomized to a warmth-liking condition in Study 1 were now placed in the incentive context group, whereas individuals randomized to an expectancy–wanting condition in Study 1 were now placed in the nonincentive context group. Participants were evenly distributed across the experimental groups (placebo in incentive context, n = 20; placebo in nonincentive context, n = 21; sulpiride in incentive context, n = 24; sulpiride in nonincentive context, n = 21).
Note that these correlations are based on the DFMT difference (catch trials vs. miss trials) score. When correlations in the nonincentive placebo group between aE and theta power were computed separately for catch and miss trials, aE correlated with theta after miss trials (r(21) = –.46, p < .05), but not after catch trials [r(20) = –.28, p > .2].
References
Allport, G. (1937). Personality: A psychological interpretation. New York, NY: Holt.
Baker, T. E., & Holroyd, C. B. (2011). Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological Psychology, 87, 25–34. doi:10.1016/j.biopsycho.2011.01.010
Bilder, R. M., Volavka, J., Lachman, H. M., & Grace, A. A. (2004). The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology, 29, 1943–1961. doi:10.1038/sj.npp. 1300542
Boksem, M. A., Tops, M., Wester, A. E., Meijman, T. F., & Lorist, M. M. (2006). Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research, 1101, 92–101. doi:10.1016/j.brainres.2006.05.004
Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67, 319–333. doi:10.1037/0022-3514.67.2.319
Cavanagh, J. F., Zambrano-Vazquez, L., & Allen, J. J. (2012). Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology, 49, 220–238. doi:10.1111/j.1469-8986.2011.01293.x
Chavanon, M.-L., Wacker, J., Leue, A., & Stemmler, G. (2007). Evidence for a dopaminergic link between working memory and agentic extraversion: An analysis of load-related changes in EEG alpha 1 activity. Biological Psychology, 74, 46–59. doi:10.1016/j.biopsycho.2006.07.001
Coan, J. A., Allen, J. J., & McKnight, P. E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72, 198–207. doi:10.1016/j.biopsycho.2005.10.003
Cohen, M. X., Elger, C. E., & Ranganath, C. (2007). Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage, 35, 968–978. doi:10.1016/j.neuroimage.2006.11.056
Davidson, R. J. (1994). Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology, 6, 741–758.
De Pascalis, V., Varriale, V., & Rotonda, M. (2012). EEG oscillatory activity associated to monetary gain and loss signals in a learning task: Effects of attentional impulsivity and learning ability. International Journal of Psychophysiology, 85, 68–78. doi:10.1016/j.ijpsycho.2011.06.005
Debener, S., Ullsperger, M., Siegel, M., Fiehler, K., von Cramon, D. Y., & Engel, A. K. (2005). Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience, 25, 11730–11737. doi:10.1523/JNEUROSCI.3286-05.2005
Depue, R. A., & Collins, P. F. (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–517. doi:10.1017/S0140525X99002046. disc. 518–569.
Depue, R. A., & Lenzenweger, M. F. (2005). A neurobehavioral dimensional model of personality disturbance. In M. L. A. J. Clarkin (Ed.), Theories of personality disorders (2nd ed.). New York, NY: Guilford Press.
Donkers, F. C., Nieuwenhuis, S., & van Boxtel, G. J. (2005). Mediofrontal negativities in the absence of responding. Cognitive Brain Research, 25, 777–787. doi:10.1016/j.cogbrainres.2005.09.007
Durstewitz, D., & Seamans, J. K. (2008). The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biological Psychiatry, 64, 739–749. doi:10.1016/j.biopsych.2008.05.015
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science, 302, 290–292. doi:10.1126/science.1089134
Eysenck, H. J. (1967). Biological basis of personality. Springfield, IL: Charles C. Thomas.
Ferdinand, N. K., Mecklinger, A., Kray, J., & Gehring, W. J. (2012). The processing of unexpected positive response outcomes in the mediofrontal cortex. Journal of Neuroscience, 32, 12087–12092. doi:10.1523/JNEUROSCI.1410-12.2012
Fisher, R. A. (1950). Statistical methods for research workers (11th ed.). Edinburgh, UK: Oliver & Boyd.
Gehring, W. J., Coles, M. G., Meyer, D. E., & Donchin, E. (1995). A brain potential manifestation of error-related processing. Electroencephalography and Clinical Neurophysiology, 44(Suppl), 261–272.
Gehring, W. J., & Willoughby, A. R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295, 2279–2282. doi:10.1126/science.1066893
Gehring, W. J., & Willoughby, A. R. (2004). Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In M. U. M. Falkenstein (Ed.), Errors, conflicts, and the brain: Current opinions on performance monitoring (pp. 14–20). Leipzig, Germany: Max Planck Institute of Cognitive Neuroscience.
Hajcak, G., McDonald, N., & Simons, R. F. (2003). Anxiety and error-related brain activity. Biological Psychology, 64, 77–90.
Hasler, G., Drevets, W. C., Manji, H. K., & Charney, D. S. (2004). Discovering endophenotypes for major depression. Neuropsychopharmacology, 29, 1765–1781. doi:10.1038/sj.npp.1300506
Hoffmann, S., Wascher, E., & Falkenstein, M. (2012). Personality and error monitoring: An update. Frontiers in Human Neuroscience, 6, 171. doi:10.3389/fnhum.2012.00171
Holroyd, C. B., & Coles, M. G. H. (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109, 679–709. doi:10.1037/0033-295X.109.4.679
Holroyd, C. B., HajiHosseini, A., & Baker, T. E. (2012). ERPs and EEG oscillations, best friends forever: Comment on Cohen et al. Trends in Cognitive Sciences, 16, 192. doi:10.1016/j.tics.2012.02.008. author reply 193.
Jocham, G., & Ullsperger, M. (2009). Neuropharmacology of performance monitoring. Neuroscience & Biobehavioral Reviews, 33, 48–60. doi:10.1016/j.neubiorev.2008.08.011
Kuroki, T., Meltzer, H. Y., & Ichikawa, J. (1999). Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics, 288, 774–781.
Lange, S., Leue, A., & Beauducel, A. (2012). Behavioral approach and reward processing: Results on feedback-related negativity and P3 component. Biological Psychology, 89, 416–425. doi:10.1016/j.biopsycho.2011.12.004
Lapish, C. C., Kroener, S., Durstewitz, D., Lavin, A., & Seamans, J. K. (2007). The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology, 191, 609–625. doi:10.1007/s00213-006-0527-8
Luu, P., Collins, P., & Tucker, D. M. (2000). Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General, 129, 43–60. doi:10.1037/0096-3445.129.1.43
Luu, P., Tucker, D. M., Derryberry, D., Reed, M., & Poulsen, C. (2003). Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science, 14, 47–53.
Luu, P., Tucker, D. M., & Makeig, S. (2004). Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology, 115, 1821–1835. doi:10.1016/j.clinph.2004.03.031
Marco-Pallares, J., Cucurell, D., Cunillera, T., Kramer, U. M., Camara, E., Nager, W., & Rodriguez-Fornells, A. (2009). Genetic variability in the dopamine system (dopamine receptor D4, catechol-O-methyltransferase) modulates neurophysiological responses to gains and losses. Biological Psychiatry, 66, 154–161. doi:10.1016/j.biopsych.2009.01.006
Mauri, M. C., Bravin, S., Bitetto, A., Rudelli, R., & Invernizzi, G. (1996). A risk-benefit assessment of sulpiride in the treatment of schizophrenia. Drug Safety, 14, 288–298.
Mereu, G., Casu, M., & Gessa, G. L. (1983). (-)-Sulpiride activates the firing rate and tyrosine hydroxylase activity of dopaminergic neurons in unanesthetized rats. Brain Research, 264, 105–110.
Miltner, W. H. R., Braun, C. H., & Coles, M. G. H. (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience, 9, 788–798.
Mischel, W., & Shoda, Y. (1998). Reconciling processing dynamics and personality dispositions. Annual Review of Psychology, 49, 229–258.
Mueller, E. M., Makeig, S., Stemmler, G., Hennig, J., & Wacker, J. (2011). Dopamine effects on human error processing depend on catechol-o-methyltransferase VAL158MET genotype. Journal of Neuroscience, 31, 15818–15825. doi:10.1523/JNEUROSCI.2103-11.2011
Mueller, E. M., Stemmler, G., Hennig, J., & Wacker, J. (2013). 5-HTTLPR and anxiety modulate brain–heart covariation. Psychophysiology, 50, 441–453. doi:10.1111/psyp.12016
Olvet, D. M., & Hajcak, G. (2009). The effect of trial-to-trial feedback on the error-related negativity and its relationship with anxiety. Cognitive, Affective, & Behavioral Neuroscience, 9, 427–433. doi:10.3758/CABN.9.4.427
Osinsky, R., Hewig, J., Alexander, N., & Hennig, J. (2012). COMT Val158Met genotype and the common basis of error and conflict monitoring. Brain Research, 1452, 108–118. doi:10.1016/j.brainres.2012.02.054
Ostendorf, F., & Angleitner, A. (2004). NEO-Persönlichkeitsinventar nach Costa und McCrae, revidierte Form (NEO-PI-R). Göttingen, Germany: Hogrefe.
Pailing, P. E., & Segalowitz, S. J. (2004). The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology, 41, 84–95. doi:10.1111/1469-8986.00124
Reuter, M., & Hennig, J. (2005). Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. NeuroReport, 16, 1135–1138.
Santesso, D. L., Evins, A. E., Frank, M. J., Schetter, E. C., Bogdan, R., & Pizzagalli, D. A. (2008). Single dose of a dopamine agonist impairs reinforcement learning in humans: Evidence from event-related potentials and computational modeling of striatal-cortical function. Human Brain Mapping, 7, 1963–1976. doi:10.1002/hbm.20642
Sato, A., Yasuda, A., Ohira, H., Miyawaki, K., Nishikawa, M., Kumano, H., & Kuboki, T. (2005). Effects of value and reward magnitude on feedback negativity and P300. NeuroReport, 16, 407–411.
Schweiger, D., Stemmler, G., Burgdorf, C., & Wacker, J. (2013). Opioid receptor blockade and warmth-liking: Effects on interpersonal trust and frontal asymmetry. Social Cognitive and Affective Neuroscience. Advance online publication. doi:10.1093/scan/nst152
Serra, G., Forgione, A., D’Aquila, P. S., Collu, M., Fratta, W., & Gessa, G. L. (1990). Possible mechanism of antidepressant effect of L-sulpiride. Clinical Neuropharmacology, 13(Suppl. 1), S76–S83.
Smillie, L. D., Cooper, A. J., & Pickering, A. D. (2011). Individual differences in reward-prediction-error: Extraversion and feedback-related negativity. Social Cognitive and Affective Neuroscience, 6, 646–652. doi:10.1093/scan/nsq078
Stemmler, G. (1997). Selective activation of traits: Boundary conditions for the activation of anger. Personality and Individual Differences, 22, 213–233.
Stemmler, G. (2009). Der Emotionsprozess. In G. Stemmler (Ed.), Enzyklopädie der Psychologie, Serie Motivation und Emotion: Psychologie der Emotion (Vol. 3, pp. 1–19). Göttingen, Germany: Hogrefe.
Stemmler, G., & Wacker, J. (2010). Personality, emotion, and individual differences in physiological responses. Biological Psychology, 84, 541–551. doi:10.1016/j.biopsycho.2009.09.012
Tellegen, A., & Waller, N. G. (2008). Exploring personality through test construction: Development of the multidimensional personality questionnaire. In G. J. Boyle, G. Matthews, & D. H. Saklofske (Eds.), The Sage handbook of personality and assessment (Vol. 2, pp. 161–292). London, UK: Sage.
Tops, M., & Boksem, M. A. (2010). Absorbed in the task: Personality measures predict engagement during task performance as tracked by error negativity and asymmetrical frontal activity. Cognitive, Affective, & Behavioral Neuroscience, 10, 441–453. doi:10.3758/CABN.10.4.441
Tops, M., & Koole, S. L. (2012). An updated update to personality and error monitoring. Frontiers in Human Neuroscience, 6, 283. doi:10.3389/fnhum.2012.00283
Wacker, J., Chavanon, M.-L., Leue, A., & Stemmler, G. (2008). Is running away right? The behavioral activation-behavioral inhibition model of anterior asymmetry. Emotion, 8, 232–249. doi:10.1037/1528-3542.8.2.232
Wacker, J., Chavanon, M.-L., & Stemmler, G. (2010). Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality, 44, 167–179. doi:10.1016/j.jrp.2009.12.004
Wacker, J., & Gatt, J. M. (2010). Resting posterior versus frontal delta/theta EEG activity is associated with extraversion and the COMT VAL(158)MET polymorphism. Neuroscience Letters, 478, 88–92. doi:10.1016/j.neulet.2010.04.071
Wacker, J., Mueller, E. M., Hennig, J., & Stemmler, G. (2012). How to consistently link extraversion and intelligence to the catechol-O-methyltransferase (COMT) gene: On defining and measuring psychological phenotypes in neurogenetic research. Journal of Personality and Social Psychology, 102, 427–444. doi:10.1037/a0026544
Wacker, J., Mueller, E. M., Pizzagalli, D. A., Hennig, J., & Stemmler, G. (2013). Dopamine-d2-receptor blockade reverses the association between trait approach motivation and frontal asymmetry in an approach-motivation context. Psychological Science, 24, 489–497. doi:10.1177/0956797612458935
Zirnheld, P. J., Carroll, C. A., Kieffaber, P. D., O’Donnell, B. F., Shekhar, A., & Hetrick, W. P. (2004). Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience, 16, 1098–1112. doi:10.1162/0898929041502779
Author note
This research was supported by the Deutsche Forschungsgemeinschaft (Grant Nos. DFG Ste405/15-1 and DFG WA 2593/5-1)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mueller, E.M., Burgdorf, C., Chavanon, ML. et al. Dopamine modulates frontomedial failure processing of agentic introverts versus extraverts in incentive contexts. Cogn Affect Behav Neurosci 14, 756–768 (2014). https://doi.org/10.3758/s13415-013-0228-9
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0228-9