Abstract
Recent research using the resting electroencephalogram (EEG) showed that posterior versus anterior theta activity (around 4–8 Hz) is consistently associated with agency, reflecting the dopaminergic core of extraversion (i.e., incentive motivation, positive emotion). Neuroimaging studies using various methodologies and experimental paradigms have converged on the anterior cingulate cortex (ACC) as a neurophysiological correlate of extraversion. The aim of the present study is integrate these lines of research by testing the hypothesis that posterior versus anterior EEG theta is at least partly based on ACC theta activity. Resting EEG data were analyzed in N = 78 healthy, male participants extremely high or low in agentic extraversion (aE). Using the low-resolution electromagnetic tomography algorithm, we localized the sources of aE-dependent intracerebral theta activity within rostral subdivisions of the ACC. The posterior versus anterior index and theta current density within the rostral ACC were significantly correlated (r = -.52), and both displayed high retest stability across 5 hr and were associated with traits from the aE spectrum. These neurophysiological correlates of aE and their possible functional significance are discussed.
Similar content being viewed by others
Individuals differ in the degree to which they respond to positive incentives, and these differences are based on a neurobiological system. This idea, first raised by Jeffrey A. Gray (1970), represents the foundation of Depue's influential theory of agentic extraversion (aE; Depue & Collins, 1999). aE encompasses lower order traits, such as drive, assertiveness, enthusiasm, dominance, and ambition, but excludes both the affiliative component of extraversion (i.e., enjoying close interpersonal relations, being warm and affectionate; Depue & Morrone-Strupinsky, 2005) and traits from the impulsivity sensation-seeking spectrum, the latter of which are typically included in measures based on Gray’s behavioral activation system (BAS) theory (e.g., the BAS scale of Carver & White, 1994).Footnote 1
Depue and Collins (1999) view aE as resulting from individual differences in a broad motivational system that they termed the behavioral facilitation system (BFS) and that is involved in the processing of reward or incentive salience and in generating approach behavior. The BFS modulates or “facilitates” behavior motivated by positive incentives through increasing their motivational salience. Since activation of the BFS is accompanied by affective states of positive activation (e.g., feelings of desire, wanting, enthusiasm, and energy), individuals high in aE, whose BFS is thought to be more reactive, are not only generally more enduring, motivated, and vigorous in pursuing positive incentive goals, but also more apt to feel these positive affective states. Neurobiologically, the BFS, much like J. A. Gray’s (1994) BAS, is centered around the mesocorticolimbic dopamine system originating from the dopaminergic cells of the ventral tegmental area projecting most strongly to limbic and cortical areas such as the nucleus accumbens, septum, amygdala, hippocampus, medial orbital prefrontal cortex, and cingulate cortex (Wise, 2004).
Depue, Luciana, Arbisi, Collins, and Leon (1994) provided initial evidence for the dopamine theory of aE by demonstrating strong and specific correlations between aE, as measured by Tellegen’s Multidimensional Personality Questionnaire (MPQ, Positive Emotionality scale; Tellegen & Waller, 2008), and several characteristics of the dopamine agonist-induced inhibition of prolactin secretion. Furthermore, several recent studies reported associations between extraversion spectrum traits and genetic polymorphisms associated with individual differences in functional dopamine activity (e.g., Reuter & Hennig, 2005; Smillie, Cooper, Proitsi, Powell, & Pickering, 2009; Wacker & Gatt, 2010). However, a complete account of how individual differences in functional dopamine activity translate into aE will also need to incorporate the level of individual differences in neural activity.
Agentic extraversion and posterior versus anterior EEG theta activity
Recordings of electrical brain activity, although low in spatial resolution, bear the considerable advantage that sample sizes large enough to investigate associations to personality traits with sufficient statistical power can be collected at minimal cost.Footnote 2 Furthermore, in contrast to fMRI measures of blood flow, electroencephalogram (EEG) activity can be assessed in a quiet, nonthreatening environment under rest, a condition suggested to be ideally suited to capture tonic, trait-like differences in functional brain activity (but see Stemmler & Wacker, 2010).
Inspired by promising early results (Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997), studies using EEG measures to identify brain activity correlates of aE (and trait BAS) have strongly focused on frontal asymmetry in the alpha band. However, our recent meta-analysis of all the available data demonstrated that the link between aE/trait-BAS and left versus right resting frontal cortical activity (i.e., right vs. left resting frontal inhibitory alpha activity) is considerably weaker and less consistent than was initially hoped (Wacker, Chavanon, & Stemmler, 2010). Instead, accumulating evidence suggests that the posterior–anterior distribution of resting EEG activity (Hewig, Hagemann, Seifert, Naumann, & Bartussek, 2004, 2006; Knyazev, 2009), particularly in the delta and theta frequency range (Koehler et al., 2010; Wacker et al., 2010; Wacker & Gatt, 2010), is consistently associated with traits from the extraversion spectrum.
It has long been known that two different manifestations of EEG theta rhythm can be distinguished (Schacter, 1977). First, there is a frontal midline variant, which has been linked to alert states characterized by focused attention, self-regulation, emotional processing, and mental effort (Aftanas & Golocheikine, 2001; Gevins, Smith, McEvoy, & Yu, 1997; Ishii et al., 1999; Jensen & Tesche, 2002; Luu, Tucker, & Makeig, 2004; Onton, Delorme, & Makeig, 2005). Second, a more posterior and more widely distributed variant exists that is associated with decreased prestimulus alertness and less focused, more freely floating attention (e.g., during hypnagogic states). In the last decade, frontal midline theta has attracted major attention in neuroscience. It has been shown that the generator of this oscillatory component is located in or near the ACC (Asada, Fukuda, Tsunoda, Yamaguchi, & Tonoike, 1999; Ishii et al., 1999). Regarding posterior or parietal theta, research is rather sparse, and its sources are not well defined. Because the mental processes associated with the two types of theta rhythm are, at least in part, mutually exclusive, the activity in brain sources of frontal midline and parietal midline theta should also be reciprocally related. Thus, we proposed that the difference between frontal and parietal midline theta activity could capture a meaningful aspect of EEG theta activity in a single measure (Wacker, Chavanon, & Stemmler, 2006).
In an initial test of this hypothesis, we indeed found not only that a simple index of resting posterior minus anterior EEG theta activity specifically correlated with trait aE, but also that this association was completely reversed after administration of the selective dopamine D2/D3 receptor antagonist sulpiride, supporting an association of this novel index with the presumed neurochemical basis of aE (Wacker et al., 2006). Meanwhile, we consistently observed a correlation between aE and posterior minus anterior EEG theta activity in six independent studies (total N > 1,500), collected in three different laboratories (Koehler et al., 2010; Wacker et al., 2010; Wacker & Gatt, 2010). Others reported similar effects for trait BAS in the adjacent alpha band (Hewig et al., 2004, 2006). In addition, we predicted and found associations between posterior minus anterior EEG theta activity and genetic polymorphisms implicated in the modulation of prefrontal dopamine levels (catechol-o-methyltransferase Val158Met; Wacker & Gatt, 2010) and dopamine D2 receptor functioning (Koehler et al., 2010).
To our knowledge, so far no other measure of brain activity has demonstrated an equally consistent link to aE in a comparably large sample. The psychological construct validity of posterior minus anterior EEG theta activity as a correlate of aE is, thus, already well established, indicating that this easily obtainable measure may have considerable utility in the study of aE. However, the neural sources of resting EEG theta activity contributing to individual differences in posterior versus anterior EEG theta activity have yet to be determined—an important next step in developing an integrative account of how individual differences in neural systems are linked to aE.
Agentic extraversion and anterior cingulate cortex activity
Several neuroimaging studies have reported associations between extraversion and activation within dopaminergically innervated, reward-sensitive regions such as the ventral striatum (i.e., caudate, putamen, nucleus accumbens), the amygdala, and the anterior cingulate cortex (ACC; Johnson et al., 1999; O'Gorman et al., 2006). Various findings highlight the importance of the reward system's sensitivity (and its positive emotional impact) for individual differences in extraversion under emotional/motivational stimulus processing (e.g., Barros-Loscertales et al., 2010; Beaver et al., 2006; Cohen, Young, Baek, Kessler, & Ranganath, 2005). Especially the ACC, as one of the major targets of reward-related midbrain dopamine projections (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001), appears to be a central structure of the reward circuit related to extraversion and related traits in a variety of experimental settings, ranging from resting condition (e.g., Ebmeier et al., 1994; Hermes, 2007; Johnson et al., 1999; Sugiura et al., 2000) to emotional/motivational (e.g., Canli, Amin, Haas, Omura, & Constable, 2004; Canli et al., 2001; Eisenberger, Lieberman, & Satpute, 2005; Fruehholz, Prinz, & Herrmann, 2010; Haas, Omura, Amin, Constable, & Canli, 2006; Hermes, 2007; Keightley et al., 2003; Rapp et al., 2008) and cognitive (e.g., Gray & Braver, 2002; Gray et al., 2005; Kumari, ffytche, Williams, & Gray, 2004) tasks. Recent research on the neurobiology of major depression has also focused on the ACC. More specifically, the major depression symptom of anhedonia, characterized by reward-insensitive behavior and blunted positive emotionality — and hence, apparently, the extremely low end of aE (Depue, 1995) — has been associated with abnormally low levels of activity in the ventral-rostral ACC (rACC) regions (Pizzagalli, Peccoralo, Davidson, & Cohen, 2006) and with blunted nucleus accumbens responses to reward signals (Wacker, Dillon, & Pizzagalli, 2009). Furthermore, pharmacological challenge studies have demonstrated that the ACC responds to dopaminergic drugs (e.g., Völlm et al., 2004), and ACC activity also qualifies as a predictor for psychopharmacological treatment responses (for selective norepinephrine and serotonin reuptake inhibitors, see Korb, Hunter, Cook, & Leuchter, 2009).
Consequently, despite using relatively small samples and quite variable methodological approaches generally not involving personality scales specifically designed to measure aE, the neuroimaging work shows at least some convergence on the ACC as a neurophysiological correlate of this trait. In addition, the ACC has been identified as a generator of frontal midline theta (e.g., Asada et al., 1999), receives dense dopaminergic inputs, and is considered part of the brain’s reward circuitry (Allman et al., 2001). We therefore hypothesize (1) that posterior minus anterior EEG theta is at least partly based on ACC theta activity and (2) that aE correlates with the latter. Using low-resolution electromagnetic tomography (LORETA; Pascual-Marqui, Michel, & Lehmann, 1994) to compute estimates of intracerebral activity in the ACC and other brain regions, the present study provides an initial test of these hypotheses, aiming to provide a basis for integrating the EEG and neuroimaging literature on brain correlates of aE and developing a model of the functional significance of resting posterior versus anterior EEG theta activity.
Method
Participants
To select participants either extremely high or low in aE, we recruited a pool of N = 422 male university or high school student volunteers to fill in a German Positive Emotionality short scale (the Marburg Agentic Extraversion [MAE] scale; for details, see Wacker et al., 2006). This scale contains three positively correlated 10-item primary scales corresponding to the MPQ scales (Tellegen & Waller, 2008) most relevant to positive emotionality (Well-Being, Achievement, and Social Potency; Cronbach's alpha for primary scales, α ≥ .82; for the total score, α = .88). To obtain greater homogeneity within aE groups, we used extreme group selection: Participants scoring above the median in each of the three primary scales constituted the high-aE group, whereas individuals with scores below or equal to the median in all three primary scales constituted the low-aE group. With regard to the total score, this selection procedure formed two groups: one above the 67th percentile and the other below the 33 rd percentile of the aE distribution. All the participants were required to be right-handed, free from medication and illegal drugs for 3 months, without past or current Axis I disorders, physically healthy, and willing to participate in a placebo-controlled pharmacological study. N = 88 volunteers participated in return for monetary compensation (€80, approximately US$120) for about 10 hr of involvement in the whole study (see below). All the participants gave written informed consent to the study protocol, which had been approved by the ethics committee of the German Psychological Society.
Because of technical problems (n = 2) and excessive artifacts (n = 7; more than two corrupted EEG channels or fewer than 20 artifact-free epochs; see below), data from 9 participants were excluded from further analysis. One participant was excluded from analysis because he had smoked just before the experimental session, leaving N = 78 participants for analysis (high aE, n = 40; low aE, n = 38). The average age was M = 23.2 years (range 19–31, SD = 2.69).
Procedure
The experiment was conducted in two separate sessions. In a first session, the experimenter checked lifetime absence of DSM Axis I psychiatric disorders, using a standardized clinical interview, and the participants filled out several personality questionnaires. At the end of Session 1, the experimenter reminded participants to abstain from caffeine, alcohol, and nicotine before Session 2 (on average, 1.5 days later; range 1–9 days). At the beginning of Session 2, electrodes were attached, and participants were told to relax with their eyes open for a 10-min rest period with five embedded 1-min EEG recordings at minutes 2, 4, 6, 8, and 10. Recording breaks were inserted to allow for resetting the DC level of the EEG amplifier and, if necessary, for reminding participants to keep their eyes open. At the end of the rest period, participants performed an emotion self-report (see Wacker et al., 2006). After the rest period, the participants received one of four substances (placebo; 50, 200, or 400 mg of dopamine antagonist Sulpiride) and completed another 6 hr of study protocol, in which working memory tasks and rest periods alternated (pharmaco-EEG data will be presented elsewhere). Some of the selection criteria mentioned above (abstinence from medication and drugs, physical and psychiatric health, and male gender) were due to this pharmacological manipulation. Because data on the stability of posterior versus anterior theta activity are lacking, we calculated test–retest correlations for the first recording analyzed here and another resting EEG recording conducted 5 hr later. In order to obtain substance-free data, we used the placebo subsample (n = 21) for this calculation.
Personality questionnaires
Besides our aE short scale used for selection of extreme groups, we also administered the MPQ Negative Emotionality scale (MPQ–NE; Tellegen & Waller, 2008), as well as the MPQ Social Closeness scale (MPQ–SC). MPQ–NE is characterized by perceptions of the world as threatening, problematic, and distressing; it is similar to neuroticism as defined by many personality measures. Its primary scales are Stress Reactivity, Alienation, and Aggression. The internal consistencies for the subscales are high, ranging from .80 to .89 (e.g., Tellegen & Waller, 2008). In line with Depue and colleagues (Morrone, Depue, Scherer, & White, 2000; Morrone-Strupinsky & Depue, 2004; Morrone Strupinsky & Lane, 2007), we used the MPQ–SC scale to measure the affiliative extraversion facet (Depue & Collins, 1999). This scale has a 30-day retest correlation of about .90 and an internal consisteny of about .85 (Tellegen & Waller, 2008).
In addition, a German version of the revised Eysenck Personality Questionnaire (EPQ–R; Ruch, 1999) was included to measure Eysenck’s neuroticism, extraversion, and psychoticism. For all EPQ scales, satisfactory internal consistencies (.68–.85) and test–retest reliabilities (.78–.89) have been observed in normal adults (Ruch, 1999). Note that Eysenck’s Extraversion scale assesses both agency and affiliative aspects (Tellegen & Waller, 2008).
Data aquisition, recording, and analysis
To record eye blinks and vertical eye movements, electrodes were placed midline above and below the right eye. Electrodes on the outer canthi of both eyes were attached to record horizontal eye movements. EEG was recorded from 29 Ag/AgCl sintered ring electrodes located in a head cap (Easy Caps, Germany), in accordance with the International 10–20 system (Jasper, 1958; impedances <5 kOhm for EEG, <1 kOhm for the ground electrode AFz, <10 kOhm for the electrooculogram [EOG]). All sites were online referenced to Cz. EEG and EOG signals were amplified with a 32-channel SynAmps 5083 amplifier (EEG, gain = 500; EOG, gain = 100). Data were digitally filtered (band-pass, 1–50 Hz for EEG; low-pass, 1 kHz for EOG; 50-Hz notch filter) and stored (sampling rate: 2 kHz). Then signals were down-sampled to 250 Hz and converted to physical units. Subsequent preprocessing was carried out using EEGLAB (Delorme & Makeig, 2004; http://sccn.ucsd.edu/eeglab). Low-pass filters were located at 30 Hz, and high-pass filters at 1 Hz. After visual rejection of data portions containing nonstereotyped artifacts (large muscle artifacts, swallowing, cable movement, etc.), concatenated EEG data were submitted to extended infomax-independent component analysis (EEGLAB runica.m). Independent components reflecting eye blinks, lateral eye movements, line noise, and electrocardial activity were identified visually and discarded by back-projecting all but these components to the data space. Corrupted channels defined as artifact contaminated for more than one quarter of the time were excluded from ICA and were estimated using spherical spline interpolation after ICA (except for Pz, Fz, and mastoids). Please note that a maximum of two channels were interpolated. All data portions with more than two corrupted channels, as well as corrupted signals from Fz, Pz, or mastoid electrodes, were rejected prior to ICA. Participants with less than 70 s of ICA-usable data were excluded form further analyses. Following ICA and interpolation, all data epochs of 2.048 s were once again semiautomatically screened for artifacts, as recommended in the EEGLAB manual. Any trials that still contained visible artifacts were removed. Participants with fewer than 20 epochs were excluded.
On average, estimates of resting current density and the posterior–anterior theta index were based on 58.9 artifact-free epochs [i.e., about 40% of the recorded 5 min.; SD = 23.1 epochs; range 25–143 epochs; no significant differences between high and low aE; Wilcoxon–Mann–Whitney z(76) = -0.91, p = .36].
Posterior versus anterior theta index
For posterior versus anterior theta index, all artifact-free epochs were referenced to an average montage and submitted to a fast Fourier transform (50% Hamming-windowed, padded symmetrically with zeros up to 1,024 data points).Footnote 3 The resulting estimates of power density (μV2/Hz; resolution 0.25 Hz) were clustered into delta (1.5–3.5 Hz), theta (4.0–7.5 Hz), broad alpha (8.0–12.5 Hz), and lower (13.0–18.5 Hz), middle (19.0–21.5 Hz), and upper (22.0–29.5 Hz) beta frequency bands and were log10 transformed in order to normalize distributions (see, e.g., Davidson, Jackson, & Larson, 2000). The posterior versus anterior EEG index was computed separately for each band as power at Pz minus power at Fz. Because Knyazev (2009) reported extraversion-related differences in an anterior–posterior gradient for 1 to 30 Hz, we analyzed the aE effects in the Pz - Fz difference separately for 0.5-Hz bins within the range of 1–29.5 Hz as well.
LORETA source modeling
Following prior reports (Pizzagalli et al., 2006; Wacker et al., 2009), LORETA software was applied to compute resting intracerebral theta-band current density for each subject (Pascual-Marqui et al., 1994; http://www.unizh.ch/keyinst/NewLORETA). First, spectral analyses were performed on artifact-free 2,048-ms epochs, using a discrete Fourier transform and boxcar windowing.Footnote 4 Next, LORETA computed current density for each voxel within the solution space (i.e., 3-D space where the inverse EEG problem is solved) as a linear weighted sum of scalp electrical potentials. The LORETA solution space was restricted to the gray matter and hippocampus in the Talairach atlas and was divided into 2,394 7-mm-wide cubic voxels. Finally, for each voxel, current density was computed as the squared magnitude of the intracerebral current density within each frequency band (μA/mm2). In order to probe the specificity of the expected findings for the theta band, we also estimated the current density in neighboring frequency bands (i.e., delta, alpha). For each participant and band, LORETA values were normalized to a total power of 1 and then log10 transformed before statistical analyses (Pizzagalli et al., 2006). In addition to whole-brain analyses, region of interest (ROI) analyses were run for current density within the following subdivisions of the ACC (Bush, Luu, & Posner, 2000): the more ventral/rostral ACC (rACC), “affective” subregions, including BA 25 (17 voxels, 5.83 cm3), BA 24 (12 voxels, 4.12 cm3), and BA 32 (25 voxels, 8.57 cm3), and the more dorsal, “cognitive” subregions of the ACC (dACC), including BA 32′ (20 voxels, 6.86 cm3) and BA 24′ (48 voxels, 16.46 cm3). Location and extent of the ROI ACC subdivisions were based on structure probability maps and anatomical landmarks, as previously described in detail (Pizzagalli et al., 2006). ROI analyses were carried out using SPAMalize (http://brainimaging.waisman.wisc.edu/ ~oakes).
Statistical data analysis
For the Pz - Fz scores, we used unpaired t-tests to investigate the aE effect. For LORETA images, the statistical nonparametric mapping method (Nichols & Holmes, 2002) was used. This approach uses a randomization strategy to determine a critical probability threshold t-value for the observed statistic corrected for multiple testing. Using voxel-by-voxel unpaired t-tests for log-transformed LORETA activities, this method yields a difference map for high versus low aE, showing differences in localization for each frequency band. Probability threshold for identifying significant voxels was set to p < .01. To assess the relationship between aE, Pz - Fz score, and theta current density in the ACC ROIs, we ran a series of regression and correlation analyses.
Results
Posterior versus anterior theta
Replicating previous observations, high and low aE differed in their Pz - Fz difference scores, t(76) = -6.61, d = 1.52, p < .0001. High aE showed more posterior EEG theta (M = -.10, SE = 0.016), whereas low aE presented a frontally distributed EEG theta activity (M = -.249, SE = 0.016). Scalp topographies for high and low aE and a difference map are provided in Fig. 1. The extraversion effect was also evident for the delta band, t(76) = 3.27, d = 0.75, p < .001, but not for broad alpha and the beta bands, ps ≥ .24. As is displayed in Fig. 2, aE-related effects were significant in the range of 1–9.5 Hz (i.e., from delta to low alpha), peaking within the theta band. The test–retest correlation of the Pz - Fz difference in the theta band for an interval of 5 hr was high, r(20) = .85.
Topographic maps of theta activity in high and low aE. Stronger theta activity is coded by darker red. The map on the right displays the difference between high and low aE, using t-values. Regions where low aE exhibited stronger theta activity than did high aE are coded blue; regions where high aE exhibited stronger theta activity than did low aE are coded red. All t-values are not significant at p = .05
LORETA
Whole-brain analyses
Computation of voxel-by-voxel differences between high and low aE revealed significant effects in voxels clustering to the rACC for theta current density. For delta, alpha, and beta current densities, no significant findings emerged.Footnote 5 Figure 3a and b display the difference maps between high and low aE for theta current density. Low aE exhibited stronger theta current densities in the rACC, BA 24 (peak voxel MNI coordinates: x = 4, y = 31, z = 22; peak t(76) = -4.34; cluster size = 4) and BA 32 (peak voxel MNI coordinates: x = 11, y = 31, z = 22; peak t(76) = -4.36; cluster size = 10). Differences between high and low aE extended to neighboring voxels within medial frontal gyri, BA 9 (peak voxel MNI coordinates: x = -10, y = 38, z = 29; peak t(76) = -4.12; cluster size = 3), dACC BA 32′ (peak voxel MNI coordinates: x = 11, y = 24, z = 29; peak t(76) = -4.05; cluster size = 2), and dACC BA 24′ (peak voxel MNI coordinates: x = 4, y = 24, z = 22; peak t(76) = -4.27; cluster size = 4). To illustrate the convergence of the present EEG findings with prior neuroimaging work, peak activations from prior PET and fMRI studies are provided in Fig. 3c.
LORETA-based statistical nonparametric maps illustrating the differences at p < .01 (corrected for whole-brain testing) of LORETA theta current density between high and low aE. All t-statistics are negative (the mean of the high-aE group is smaller than the mean of the low-aE group) and are displayed in gray. Displayed are the horizontal (left), sagittal (middle), and coronal (right) sections through the voxel with maximal | t |-statistic (a local maximum for BA 32; b local maximum for BA 24). In the horizontal and coronal sections, the left of the figure represents the left of the brain. In the sagittal section, the anterior part of the brain is on the left of the figure. L = left; R = right; A = anterior; P = posterior. c Approximating and projecting peak activations (x-coordinates for all peaks within X ± 1 cm) from prior positron emission tomography (PET; Johnson et al., 1999; Keightley et al., 2003), single photon emission computed tomography (SPECT; Sugiura et al., 2000), continuous arterial spin labeling (CASL; Hermes, 2007), and functional magnetic resonance imaging studies (fMRI; Canli et al., 2004; Canli et al., 2001; Eisenberger et al. 2005; Fruehholz et al. 2010; Gray & Braver, 2002; J. R. Gray et al., 2005; Haas, Omura, Amin, Constable, & Canli, 2006; Kumari, ffytche, Williams, & Gray, 2004; Rapp et al., 2008) investigating extraversion effects under resting conditions (circles), while emotional/motivational tasks (triangles) and cognitive tasks (squares) were performed, on the sagittal slice of the present LORETA solution running through the BA 24 peak voxel (MNI-coordinates x = 4, y = 31, z = 22). For all studies, sample size, the extraversion scale employed, and the direction of the extraversion–ACC relation are provided (+ = positive correlation; - = negative correlation). BAS = BAS scale of Carver and White (1994); EPQ E = eysenck personality questionnaire–extraversion; EPQ–R E = revised eysenck personality questionnaire–extraversion; EPQ–RK E = revised eysenck personality questionnaire–extraversion, german short version; NEO-FFI E = NEO five-factor inventory–extraversion; NEO-PI–R E= revised NEO personality inventory–extraversion; STCI = state–trait–cheerfulness inventory; TCI–NS = temperament and character inventory novelty seeking
ROI analyses
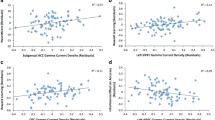
Results of the series of t-tests performed on the five ROIs are documented in Fig. 4. Significant differences in theta current density were observed for ROIs rostral BA 32 and 24, as well as for dorsal BA 32′. An inspection of ROI intercorrelations revealed that the information between rACC areas BA 32 and BA 24, as well as between dACC areas BA 32′ and BA 24′, was redundant, rs(78) ≥ .90, p < .0001. Thus, we ran all further analyses with rACC and dACC compounds (averaging dorsal and rostral BA 32 and BA 24, respectively). BA 25 correlated significantly with the rostral cluster, r(77) = .41, p < .001, and marginally with the dorsal cluster, r(77) = .21, p = .06. The average test–retest correlations computed for the rACC and dACC compounds indicated moderate to high stability for theta current density for the rACC, r(20) = .78, and for the dACC, r(20) = .68.
Posterior–anterior theta index and rACC activity
Average rACC theta current density (average BA 24/32) was the strongest predictor of Pz - Fz scores (see Table 1 for correlations and Fig. 5 for a scatterplot). Both the dACC and rACC predicted Pz - Fz, but when partialling dACC activity, rACC still significantly predicted Pz - Fz scores, r partial = -.40, p < .01, whereas after partialling rACC, the relation between dACC and Pz - Fz scores dropped to nonsignificance, r partial = -.17, p = .13.
To clarify the individual contributions of Fz and Pz to the effects observed for the difference score, we computed Fz and Pz theta activity adjusted for whole-head theta power to remove variance due to anatomical factors, such as scalp thickness, while preserving the topographic pattern within participants (Davidson et al., 2000; Wheeler, Davidson, & Tomarken, 1993). Subsequent computation of a multiple regression model revealed that both Fz and Pz (each whole-head residualized) were significant and independent predictors of rACC theta activity (β = .69, p < .0001 for Fz; β= -.19, p = .05 for Pz; R 2 = .42). The 5-hr test–retest correlations computed for the whole-head residualized Fz and Pz scores were r(20) = .82 and .79, respectively.
Supplementary analyses
Correlations of posterior–anterior theta index and rACC activity with other personality scales
As is shown in Table 1, the lower order facets of aE, well-being, and achievement, as well as the EPQ E scale measuring broad extraversion, showed moderately negative correlations with rACC, r > -.27, ps < .05, and positive correlations with the Pz - Fz index, r > .37, ps < .05. Unxepectedly, this pattern was not significant for social potency, the third lower order facet of aE. Neuroticism/negative emotionality showed an inverse correlational pattern; affiliative extraversion (measured with the MPQ–SC scale) and psychoticism (measured with EPQ P) did not correlate with dACC, rACC, or Pz - Fz theta. Note that the correlations observed for the aE scales MAE Total Score, MAE Achievement, and MAE Well-Being remained significant even when partialling neuroticism, r partial = .26, p ≤ .05.
Comparison of the power of various measures of EEG theta activity for predicting aE
A series of stepwise regressions with Pz - Fz, rACC, residualized Fz, and residualized Pz as predictors of aE revealed that Pz - Fz was the best single predictor (β = -.55, p < .0001), accounting for 31% of aE variance. The best two-predictor model accounted for 32% of aE variance and entered residualized Fz and residualized Pz (β = -.578, p < .0001 for Fz; β = .406, p < .001 for Pz). The signs of the beta weights obviously justify the formula for the Pz - Fz index. After entering Pz - Fz, none of the other predictors significantly improved the prediction.
Is rACC mediating the aE/posterior–anterior theta activity relationship?
Using the SAS macro provided by Preacher and Hayes (2004), we tested whether rACC mediates the association between aE and Pz - Fz. Prior to mediator analysis, all variables were z-standardized to yield standardized regression weights. The significance of the mediator effect was evaluated with the Sobel test and a bootstrap approach using 10,000 bootstrap resamples (Preacher & Hayes, 2004). As is summarized in Fig. 6, we indeed found a significant partial mediation effect, z = 2.45, p = .015 (CI 95 = 0.046–0.244).
Results of mediator analysis (N = 78). Coefficients are standardized regression weights (*p < .05, **p < .01, ***p < .001, two-tailed). The mediating effect of rACC was significant (see the text for details). After controlling for the mediator effect, the direct association between Pz - Fz scores and aE was still significant
Discussion
Replicating six prior studies, we again observed an association of aE with more posterior versus anterior resting EEG theta activity. More important, we showed, for the first time, (1) that the individual differences captured by the simple Pz - Fz difference score can be at least partly localized to the rACC and (2) that aE is associated with resting theta activity in the rACC, an area that receives dense dopaminergic projections and that has been linked to extraversion spectrum traits in previous neuroimaging studies. In addition, we found that of all the EEG variables investigated, the Pz - Fz difference score demonstrated both the highest retest stability and the strongest association with aE, further strengthening the potential of posterior–anterior theta as a biological trait signature of aE.
Anterior cingulate and agentic extraversion: Converging evidence from EEG and neuroimaging
Colocalization of the present findings with the results of recent fMRI/PET studies that investigated the relation between extraversion and resting or task brain activity reveals that most of these studies reported significant results for ACC, peaking in or adjacent to the significant ACC voxels reported here (see Fig. 3c). Studies that showed more dorsally located ACC activations that correlated with extraversion/BAS mostly used highly demanding working memory tasks (J. R. Gray & Braver, 2002; J. R. Gray et al., 2005; Kumari et al., 2004). This corresponds to the segregation of ACC into affective-ventral and cognitive-dorsal subdivisions (Bush et al., 2000). Despite the spatial convergence of fMRI studies on the ACC, they report both positive and negative associations of ACC activity and extraversion/BAS. Whereas Kumari et al. (2004) reported a positive correlation for activation during a three-back working memory task, Gray and colleagues found a negative correlation for a similar paradigm (J. R. Gray & Braver, 2002; J. R. Gray et al., 2005). Eisenberger et al. (2005) observed a positive correlation in a more rostral ACC locus during an oddball paradigm, which is less demanding than are three-back working memory tasks. Using emotional-motivational tasks (e.g., emotional Stroop, emotion induction), most studies showed a positive correlation (e.g., Canli et al., 2004; Canli et al., 2001; Haas et al., 2006; Keightley et al., 2003; for a negative correlation, see Fruehholz et al., 2010; Rapp et al., 2008). During resting state, positive (e.g., Ebmeier et al., 1994; Sugiura et al., 2000), as well as negative (e.g., Hermes, 2007), associations were detected. Thus, although the region is rather consistent (but see, e.g., Fischer, Wik, & Fredrikson, 1997; O'Gorman et al., 2006), the direction of the ACC–extraversion relation seems to depend on the experimental task and/or other unknown moderators. Differences in neuroimaging modalities, sample composition (gender, range of participants' extraversion scores), and applied personality questionnaires may account for the disparity of findings regarding the relationship between extraversion and brain activity. In addition, the extent of sensory stimulation could be an important factor moderating the direction of correlational findings. However, in the present study, the theta rACC current density was negatively related to aE — in line with the report by Hermes, but contrary to other resting neuroimaging paradigms. Theta Pz - Fz has already been demonstrated to be a robust correlate of BAS/aE traits (Wacker et al., 2010), but this robustness remains to be shown for the theta rACC–aE relationship. Currently, we cannot rule out the possibility that some moderators influencing the ACC–aE relation in neuroimaging studies might also apply to the theta rACC–aE relation reported here (e.g., experimenters' gender or personality).
The relation of resting theta activity and resting cortical activity in the ACC is currently not well understood. Pizzagalli, Oakes, and Davidson (2003) found a positive association between EEG theta activity (6.5–8 Hz) and glucose metabolism in the rACC. Because the firing of both excitatory and inhibitory neurons needs glucose (Ackermann, Finch, Babb, & Engel, 1984), the stronger rACC current density observed in low aE, as compared with high aE, in the present study might indicate increased neuronal firing of excitatory or inhibitory neurons in the rACC. However, Pizzagalli et al. (2004) observed a negative association between resting delta/theta activity (1–6 Hz) and glucose metabolism in the subgenual ACC. Thus, increased theta activity (4–7.5 Hz) in low-aE participants may also suggest weak neuronal activity within the ACC. For a more definitive resolution of these ambiguities, further research is needed to elucidate how different oscillatory signals from the ACC and other brain regions relate to the hemodynamic measures obtained by fMRI and PET in various situational contexts (e.g., task vs. rest; eyes open vs. eyes closed).
Functional significance of posterior versus anterior theta: A default hypothesis
The present study not only represents the seventh replication of the association between aE and posterior versus anterior theta, but also extends prior findings by showing that this index demonstrates a high short-term stability, predicts aE better than do a number of alternative resting EEG measures, including estimates of rACC theta activity, and is associated with aE even after controlling for the correlated trait of neuroticism. Because the present research was conducted in an all-male student sample, it remains to be demonstrated that our observations also generalize to more heterogeneous samples including females. However, the association of posterior versus anterior theta and extraversion seems to be similar across both genders and across several labs differing in the specifics of EEG recording methodology (Koehler et al., 2010; Wacker et al., 2010; Wacker & Gatt, 2010). As was noted above, previous work demonstrated that resting posterior versus anterior theta activity is also (1) sensitive to D2 antagonist-induced changes in dopamine actvity (Wacker et al., 2006) and (2) associated with genetic polymorphisms implicated in the modulation of prefrontal dopamine levels (Wacker & Gatt, 2010) and DRD2 receptor sensitivity (Koehler et al., 2010).Taken together, these findings strongly attest to the utility of resting posterior versus anterior theta activity as a brain-based intermediate endophenotype for investigating the dopaminergic basis of aE.
The suggestion of Wacker et al. (2010) that the theta Pz - Fz score may be related to the default network (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001) is also supported by the present observations: The rACC as a brain source of posterior versus anterior theta figures prominently in the default network (Buckner et al., 2008). Linking the present data to the default network research seems particularly appealing for the following reasons. First, the default network is active during the resting state, and the data reported here were obtained under resting conditions. Second, the default network is currently the only account that suggests what might happen under resting conditions: It assumes that individuals who are not engaging in goal-directed behavior focus on self-relevant activities, such as envisioning and anticipating future events (Buckner et al., 2008). Third, future-oriented mentation processes aligned to the default network should be of relevance for individual differences in reward processing, because an essential feature of BFS activation is that the individual is motivated to want or get something that is not immediately present. This means that aE is about incentive and approach motivation that propels individuals to move toward positive reinforcements that are not directly accessible within the immediate context (Wahlstrom, Collins, White, & Luciana, 2010) and, hence, focuses on anticipatory processes (i.e., motivation and ability to image and imagine a desired outcome) that can be conceptualized as wanting and the experience of desire, in distinction to the consummatory processes associated with liking, satiation, and pleasure (Berridge & Kringelbach, 2008). In addition, recent reviews concluded that dopamine — the neurotransmitter most prominently related to reward processes — modulates default network activity (e.g., Broyd et al., 2009). Fourth, recent evidence by Scheeringa et al. (2008) supports the idea that resting state networks are related to frontal midline theta. They reported a negative correlation between frontal midline theta and activity in the default network. Therefore, it seems reasonable to assume that less frontal (and more posterior) theta, such as we have found in high aE individuals, might be associated with more activity in the default network and could go along with states of relaxation, anticipation, and evaluation of upcoming events before they happen and other self-referential mental activities. More frontally located, presumably rACC-generated theta, as reported here in low-aE individuals, might be associated with less activity in the default network and an alert and attentive scanning of the external or internal environment. Note that frontal theta has also been observed during various cognitive activities that require attention or short-term memory (e.g., Onton et al., 2005).
Summing up, we hypothesize that tonic individual differences in functional dopamine activity are associated with trait differences in default network activity characterized by variation in resting rACC theta activity and resting posterior versus frontal theta activity. These variations in functional brain activity underlie individual differences in positive future-oriented mentation that are captured by aE scales on the level of self-report.
Note that this hypothesis does not rule out an involvement of other brain regions apart from the strongly dopaminergically innervated rACC. As we have shown in the present study, the rACC only partly mediates the association between posterior versus anterior theta and aE, and, as Depue and Collins (1999) already pointed out, the mesocorticolimbic system, which is the presumed basis of aE, is associated with broad functions. It would be naive to propose that the posterior versus anterior theta index reflects a single function, instead of assuming that it rather mirrors broad functional effects of the mesocorticolimbic dopamine system. This system is related to various processes integrated within the target structures and their associated networks. Dopaminergic projections innervate approximately 30 structures; thus, the rACC is probably only one of several sources contributing to differences in EEG theta scalp distributions.
It may therefore be more accurate to view posterior versus anterior theta activity as an economic and reliable indicator of trait-like differences in a cortico-striato-thalamic circuit. Especially, the ventral striatum is an additional area of interest for aE theta effects. The rACC projects to the medial part of the ventral striatum (Kunishio & Haber, 1994), and recent findings suggest a role of the ACC and the ventral striatum in aE theta effects (see, e.g., O'Gorman et al., 2006, for effects within the caudate). Moreover, studies assessing dopamine receptor D2/3 availability have reported that the left ventral striatum correlates strongly with measures of extraversion (e.g., Tomer, Goldstein, Wang, Wong, & Volkow, 2008). Individual differences in ventral striatum activity may not only be related to resting delta/theta activity in the rACC, as previously shown (Wacker et al., 2009), but also contribute to the EEG posterior–anterior difference by modulating, for example, the insula and the posterior medial default network (Di Martino et al., 2008).Footnote 6 Taking the strong and consistent association between aE and resting posterior versus anterior theta activity observed here and in several other large samples as a reliable starting point, future research combining EEG/fMRI techniques may provide valuable insights into the relationship between extraversion, topographic variation in EEG theta activity, the ventral striatum, and the rACC.
Notes
We prefer aE, rather than BAS, as a label for the trait resulting from individual differences in reward and incentive salience processing, because Gray originally linked the BAS to impulsivity, as well as to a mixture of extraversion and neuroticism (Gray, 1994). In Depue’s model, impulsivity (or constraint) is nonaffective in nature and relates to serotonin (Depue & Collins, 1999).
Even large population effects of r = .50 require a sample size of N = 42 for detection with a power of .95. PET or fMRI studies that aim to identify personality correlates in localized brain activity and use samples of this size are still quite rare. Only Hermes (2007), J. R. Gray et al. (2005), and Ebmeier et al. (1994) analyzed extraversion and brain activity on the basis of samples with N > 35. Replications of personality associations across two or more samples of this size are missing.
All analyses of EEG Pz - Fz data were also done with an offline computed average mastoid reference. The results and head plots were nearly identical and even more pronounced with a mastoid, as compared with an average, reference. Because most LORETA studies use an average reference (for examples with 28 scalp electrodes, see Pizzagalli et al., 2003; Pizzagalli et al., 2004) for estimating current density, we decided to report both Pz - Fz and LORETA data using an average reference.
Note that for technical reasons, different windows were applied to the EEG data in Pz - Fz and LORETA analyses. Brain Vision Analyzer 2 provides Hamming and Hanning windowing, whereas LORETA uses a boxcar window as a default. If anything, the use of identical windows for both analyses paths should further strengthen the association between the two.
Lowering probability threshold to p < .05 (corrected for whole-brain testing) yielded a significant cluster of rACC activity for the delta band (see supplementary Fig. A). For alpha and beta bands, all voxels remained nonsignificant. Even relaxing the probability threshold to p < .1 (corrected for whole-brain testing) did not produce any significant voxel for either the broad alpha or the three beta bands. However, it should be noted that relaxing the significance threshold to uncorrected p = .05 for theta current density yielded significant voxels in left inferior parietal cortex BA 40 extending to the postcentral and supramarginal gyrus (peak voxel MNI coordinates: x = -45, y = -53, z = 50; t(76) = 2.53; cluster size = 57), the insular cortex BA 13 (peak voxel MNI coordinates: x = -38, y = -25, z = 22; t(76) = 2.75; cluster size = 11), and the superior temporal gyrus (BA 22/29; peak voxel MNI coordinates: x = -45, y = -39, z = 15; t(76) = 2.91; cluster size = 12). For those posterior voxels, theta current density was more pronounced in high aE than in low aE (see supplementary material Fig. B).
This hypothesis is also strengthened by our own explorative observation of aE differences in theta current density located to the insula and inferior parietal cortex and temporal cortex in the present study (see supplementary material Fig. B). These regions are also part of the posterior default network (see Buckner et al., 2008).
References
Ackermann, R. F., Finch, D. M., Babb, T. L., & Engel, J., Jr. (1984). Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. The Journal of Neuroscience, 4, 251–264.
Aftanas, L. I., & Golocheikine, S. A. (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neuroscience Letters, 310, 57–60.
Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E., & Hof, P. (2001). The anterior cingulate cortex: The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences, 935, 107–117.
Asada, H., Fukuda, Y., Tsunoda, S., Yamaguchi, M., & Tonoike, M. (1999). Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neuroscience Letters, 274, 29–32.
Barros-Loscertales, A., Ventura-Campos, N., Sanjuan-Tomas, A., Belloch, V., Parcet, M. A., & Avila, C. (2010). Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Social Cognitive and Affective Neuroscience, 5, 18–28.
Beaver, J. D., Lawrence, A. D., van Ditzhuijzen, J., Davis, M. H., Woods, A., & Calder, A. J. (2006). Individual differences in reward drive predict neural responses to images of food. The Journal of Neuroscience, 26, 5160–5166.
Berridge, K. C., & Kringelbach, M. L. (2008). Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology, 199, 457–480.
Broyd, S. J., Demanuele, C., Debener, S., Helps, S. K., James, C. J., & Sonuga-Barke, E. J. (2009). Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 33, 279–296.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4, 215–222.
Canli, T., Amin, Z., Haas, B., Omura, K., & Constable, R. T. (2004). A double dissociation between mood states and personality traits in the anterior cingulate. Behavioral Neuroscience, 118, 897–904.
Canli, T., Zhao, Z., Desmond, J. E., Kang, E., Gross, J., & Gabrieli, J. D. (2001). An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience, 115, 33–42.
Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333.
Cohen, M. X., Young, J., Baek, J. M., Kessler, C., & Ranganath, C. (2005). Individual differences in extraversion and dopamine genetics predict neural reward responses. Cognitive Brain Research, 25, 851–861.
Davidson, R. J., Jackson, D. C., & Larson, C. L. (2000). Human electroencephalography. In J. T. Cacioppo, L. G. Tassinary, & G. G. Bernston (Eds.), Handbook of psychophysiology (2nd ed., pp. 27–51). Cambridge: Cambridge University Press.
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21.
Depue, R. A. (1995). Neurobiological factors in personality and depression. European Journal of Personality, 9, 413–439.
Depue, R. A., & Collins, P. F. (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. The Behavioral and Brain Sciences, 22, 491–569.
Depue, R. A., Luciana, M., Arbisi, P., Collins, P., & Leon, A. (1994). Dopamine and the structure of personality: Relation of agonist-induced dopamine activity to positive emotionality. Journal of Personality and Social Psychology, 67, 485–498.
Depue, R. A., & Morrone-Strupinsky, J. V. (2005). A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. The Behavioral and Brain Sciences, 28, 313–350.
Di Martino, A., Scheres, A., Margulies, D. S., Kelly, A. M., Uddin, L. Q., Shehzad, Z., et al. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18, 2735–2747.
Ebmeier, K. P., Deary, I. J., O'Caroll, R. E., Prentice, N., Moffoot, A. P. R., & Goodwin, G. M. (1994). Personality associations with the uptake of the cerebral boold flow marker 99mTcexametazime estimated with single photon emission tomography. Personality and Individual Differences, 17, 587–595.
Eisenberger, N. I., Lieberman, M. D., & Satpute, A. B. (2005). Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective & Behavioral Neuroscience, 5, 169–181.
Fischer, H., Wik, G., & Fredrikson, M. (1997). Extraversion, neuroticism and brain function: A PET study of personality. Personality and Individual Differences, 23, 345–352.
Fruehholz, S., Prinz, M., & Herrmann, M. (2010). Affect-related personality traits and contextual interference processing during perception of facial affect. Neuroscience Letters, 469, 260–264.
Gevins, A., Smith, M. E., McEvoy, L., & Yu, D. (1997). High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex, 7, 374–385.
Gray, J. A. (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8, 249–266.
Gray, J. A. (1994). Personality dimensions and emotion systems. In P. Ekman & R. J. Davidson (Eds.), The nature of emotion: Fundamental questions (pp. 329–331). New York: Oxford University Press.
Gray, J. R., & Braver, T. S. (2002). Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cognitive, Affective & Behavioral Neuroscience, 2, 64–75.
Gray, J. R., Burgess, G. C., Schaefer, A., Yarkoni, T., Larsen, R. J., & Braver, T. S. (2005). Affective personality differences in neural processing efficiency confirmed using fMRI. Cognitive, Affective & Behavioral Neuroscience, 5, 182–190.
Haas, B. W., Omura, K., Amin, Z., Constable, R. T., & Canli, T. (2006). Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Social Neuroscience, 1, 16–24.
Harmon-Jones, E., & Allen, J. J. (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology, 106, 159–163.
Hermes, M. (2007). Personality and affect: A psychophysiological approach. Unpublished dissertation. Trier: University of Trier.
Hewig, J., Hagemann, D., Seifert, J., Naumann, E., & Bartussek, D. (2004). On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. Journal of Personality and Social Psychology, 87, 926–939.
Hewig, J., Hagemann, D., Seifert, J., Naumann, E., & Bartussek, D. (2006). The relation of cortical activity and BIS/BAS on the trait level. Biological Psychology, 71, 42–53.
Ishii, R., Shinosaki, K., Ukai, S., Inouye, T., Ishihara, T., Yoshimine, T., et al. (1999). Medial prefrontal cortex generates frontal midline theta rhythm. NeuroReport, 10, 675–679.
Jasper, H. H. (1958). The ten–twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology, 10, 371–375.
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. The European Journal of Neuroscience, 15, 1395–1399.
Johnson, D. L., Wiebe, J. S., Gold, S. M., Andreasen, N. C., Hichwa, R. D., Watkins, G. L., et al. (1999). Cerebral blood flow and personality: A positron emission tomography study. The American Journal of Psychiatry, 156, 252–257.
Keightley, M. L., Seminowicz, D. A., Bagby, R. M., Costa, P. T., Fossati, P., & Mayberg, H. S. (2003). Personality influences limbic-cortical interactions during sad mood induction. Neuroimage, 20, 2031–2039.
Knyazev, G. G. (2009). Is cortical distribution of spectral power a stable individual characteristic? International Journal of Psychophysiology, 72, 123–133.
Koehler, S., Wacker, J., Odorfer, T., Reif, A., Fallgatter, A. J., & Herrmann, M. J. (2010). Resting posterior versus frontal EEG slow wave activity is associated with extraversion and DRD2 genotype. Manuscript submitted for publication.
Korb, A. S., Hunter, A. M., Cook, I. A., & Leuchter, A. F. (2009). Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clinical Neurophysiology, 120, 1313–1319.
Kumari, V., Ffytche, D. H., Williams, S. C., & Gray, J. A. (2004). Personality predicts brain responses to cognitive demands. The Journal of Neuroscience, 24, 10636–10641.
Kunishio, K., & Haber, S. N. (1994). Primate cingulostriatal projection: Limbic striatal versus sensorimotor striatal input. The Journal of Comparative Neurology, 350, 337–356.
Luu, P., Tucker, D. M., & Makeig, S. (2004). Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology, 115, 1821–1835.
Morrone, J. V., Depue, R. A., Scherer, A. J., & White, T. L. (2000). Film-induced incentive motivation and positive activation in relation to agentic and affiliative components of extraversion. Personality and Individual Differences, 29, 199–216.
Morrone-Strupinsky, J. V., & Depue, R. A. (2004). Differential relation of two distinct, film-induced positive emotional states to affiliative and agentic extraversion. Personality and Individual Differences, 36, 1109–1126.
Morrone Strupinsky, J. V., & Lane, R. D. (2007). Parsing positive emotion in relation to agentic and affiliative components of extraversion. Personality and Individual Differences, 42, 1267–1278.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15, 1–25.
O'Gorman, R. L., Kumari, V., Williams, S. C. R., Zelaya, F. O., Connor, S. E., Alsop, D. C., et al. (2006). Personality factors correlate with regional cerebral perfusion. Neuroimage, 31, 489–495.
Onton, J., Delorme, A., & Makeig, S. (2005). Frontal midline EEG dynamics during working memory. Neuroimage, 27, 341–356.
Pascual-Marqui, R. D., Michel, C. M., & Lehmann, D. (1994). Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. International Journal of Psychophysiology, 18, 49–65.
Pizzagalli, D. A., Oakes, T. R., & Davidson, R. J. (2003). Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology, 40, 939–949.
Pizzagalli, D. A., Oakes, T. R., Fox, A. S., Chung, M. K., Larson, C. L., Abercrombie, H. C., et al. (2004). Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Molecular Psychiatry, 9, 393–405.
Pizzagalli, D. A., Peccoralo, L. A., Davidson, R. J., & Cohen, J. D. (2006). Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Human Brain Mapping, 27, 185–201.
Preacher, K. J., & Hayes, A. F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36, 717–731.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98, 676–682.
Rapp, A. M., Wild, B., Erb, M., Rodden, F. A., Ruch, W., & Grodd, W. (2008). Trait cheerfulness modulates BOLD response in lateral cortical but not limbic brain areas—A pilot fMRI study. Neuroscience Letters, 445, 242–245.
Reuter, M., & Hennig, J. (2005). Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. NeuroReport, 16, 1135–1138.
Ruch, W. (1999). Die revidierte Fassung des Eysenck Personality Questionnaire und die Konstruktion des deutschen EPQ-R bzw. EPQ-RK [The Eysenck Personality Questionnaire–Revised and the construction of the German standard and short versions (EPQ-R and EPQ-RK)]. Zeitschrift für Differentielle und Diagnostische Psychologie, 20, 1–24.
Schacter, D. L. (1977). EEG theta waves and psychological phenomena: A review and analysis. Biological Psychology, 5, 47–82.
Scheeringa, R., Bastiaansen, M. C., Petersson, K. M., Oostenveld, R., Norris, D. G., & Hagoort, P. (2008). Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology, 67, 242–251.
Smillie, L. D., Cooper, A. J., Proitsi, P., Powell, J. F., & Pickering, A. D. (2009). Variation in DRD2 dopamine gene predicts extraverted personality. Neuroscience Letters, 468, 234–237.
Stemmler, G., & Wacker, J. (2010). Personality, emotion, and individual differences in physiological responses. Biological Psychology, 84, 541–551.
Sugiura, M., Kawashima, R., Nakagawa, M., Okada, K., Sato, T., Goto, R., et al. (2000). Correlation between human personality and neural activity in cerebral cortex. Neuroimage, 11, 541–546.
Sutton, S. K., & Davidson, R. J. (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8, 204–210.
Tellegen, A., & Waller, N. G. (2008). Exploring personality through test construction: Development of the multidimensional personality questionnaire. In G. J. Boyle, G. Matthews, & D. H. Saklofske (Eds.), The Sage handbook of personality and assessment (Vol. 2, pp. 161–292). London: Sage.
Tomer, R., Goldstein, R. Z., Wang, G. J., Wong, C., & Volkow, N. D. (2008). Incentive motivation is associated with striatal dopamine asymmetry. Biological Psychology, 77, 98–101.
Völlm, B. A., de Araujo, I. E., Cowen, P. J., Rolls, E. T., Kringelbach, M. L., Smith, K. A., et al. (2004). Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology, 29, 1715–1722.
Wacker, J., Chavanon, M.-L., & Stemmler, G. (2006). Investigating the dopaminergic basis of extraversion in humans: A multilevel approach. Journal of Personality and Social Psychology, 91, 171–187.
Wacker, J., Chavanon, M. L., & Stemmler, G. (2010). Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality, 44, 167–179.
Wacker, J., Dillon, D. G., & Pizzagalli, D. A. (2009). The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage, 46, 327–337.
Wacker, J., & Gatt, J. M. (2010). Resting posterior versus frontal delta/theta EEG activity is associated with extraversion and the COMT VAL158MET polymorphism. Neuroscience Letters, 478, 88–92.
Wahlstrom, D., Collins, P., White, T., & Luciana, M. (2010). Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain and Cognition, 72, 146–159.
Wheeler, R. E., Davidson, R. J., & Tomarken, A. J. (1993). Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology, 30, 82–89.
Wise, R. A. (2004). Dopamine, learning and motivation. Nature Reviews. Neuroscience, 5, 483–494.
Acknowledgments
The reported research was conducted with the help of the Deutsche Forschungsgemeinschaft (Grant Ste 405/9-3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. A
LORETA-based statistical nonparametric maps illustrating the differences at p < .05 (corrected for whole brain testing) of LORETA delta current density between high and low aE in theta. All t-statistics are negative and displayed in blue (the mean of high aE group is smaller than the mean of the low aE group). Displayed are the horizontal (left), sagittal (middle), and coronal (right) sections through the voxel with highest t-statistic (local minimum for BA 24; peak voxel MNI- coordinates x = -3, y = 24, z = 22; clustersize = 16). In the horizontal and coronal sections, left of the figure represents left of the brain. In the sagittal section, the anterior part of the brain is on the left of the figure. L = left; R = right; A = anterior; P = posterior. (PDF 478 kb)
Fig. B
LORETA maps illustrating the differences at p < .05 (uncorrected for whole brain testing) of LORETA theta current density between high and low aE in theta. Displayed are the sagittal (left) and coronal (right) sections through the voxel with maximal t-statistic (local maximum for BA 29; -45, y = -35, z = 15). Only positive t-values at p < .05 (uncorrected for whole brain testing) are displayed (the mean of high aE group is higher than the mean of the low aE group). In the coronal section, left of the figure represents left of the brain. In the sagittal section, the anterior part of the brain is on the left of the figure. L = left; R = right; A = anterior; P = posterior. (PDF 146 kb)
Rights and permissions
About this article
Cite this article
Chavanon, ML., Wacker, J. & Stemmler, G. Rostral anterior cingulate activity generates posterior versus anterior theta activity linked to agentic extraversion. Cogn Affect Behav Neurosci 11, 172–185 (2011). https://doi.org/10.3758/s13415-010-0019-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-010-0019-5