Abstract
Spatial cues that mismatch the colour of a subsequent target have been shown to slow responses to targets that share their location. The source of this ‘same location cost’ (SLC) is currently unknown. Two potential sources are attentional signal suppression and object-file updating. Here, we tested a direct prediction of the suppression account using data from a spatial-cueing study in which we recorded brain activity using electroencephalography (EEG), and focusing on the event-related PD component, which is thought to index attentional signal suppression. Correlating PD amplitude with SLC magnitude, we tested the prediction that if attentional signal suppression is the source of the SLC, then the SLC should be positively correlated with PD amplitude. Across 48 participants, SLC and PD magnitudes were negatively correlated, in direct contradiction to a suppression account of the SLC. These results are compatible with an object-file updating account of the SLC in which updating is facilitated by reactive suppression of the to-be-updated stimulus information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human visual attention is remarkably flexible in the ways it can prioritise stimulus properties during task performance. Priority can be given to particular target features (e.g., red or green; Folk et al., 1992; Folk & Remington, 1998; Harris et al., 2013), feature relations (e.g., redder, smaller, etc.; Becker, 2010; Becker et al., 2010, 2013, 2014), feature singletons (e.g., Bacon & Egeth, 1994; Harris et al., 2015, 2019), or conjunctions of relations and/or features (e.g., Becker et al., 2017, 2020). Numerous studies have shown that when participants search for a target with a particular stimulus property, cues prior to the search array capture attention to their location if they possess the target property. Such attentional capture manifests as a ‘same location benefit’ (Carmel & Lamy, 2014), such that responses to a subsequent target are speeded if the target shares the location of the cue. However, many studies have also demonstrated a ‘same location cost’ (SLC) produced by cues that do not match the target properties (e.g., Carmel & Lamy, 2014, 2015; Harris et al., 2013, 2017; Lamy et al., 2015; Schoeberl et al., 2018; Travis et al., 2019). The mechanisms underlying the SLC are currently unclear, but two potential sources are object file updating (Carmel & Lamy, 2014) and attentional suppression of nontarget cues (Gaspar & McDonald, 2014; Gaspelin & Luck, 2018b).
Object file theory proposes that when a stimulus is presented, a temporary episodic representation is formed as an ‘object file’ (Kahneman et al., 1992), in which the properties of the object (colour, shape, position, etc.) are stored. The object-file updating account of the SLC (Carmel & Lamy, 2014) proposes that response delays for targets cued by non-target features are due to time costs involved in updating an object-file containing cue-related feature information with the features of the subsequent target. This initial proposal was supported by an experiment in which participants performed a spatial cueing task, either with or without placeholders highlighting possible stimulus locations. With placeholders creating continuity between the cues and targets, and thus theoretically favouring the creation of an object-file, a robust SLC was observed. Without placeholders, the SLC was smaller and less consistently different from zero. It was suggested that, without the placeholders, there was no consistent object to attach features to, and so nothing to be updated upon appearance of the target (Carmel & Lamy, 2014).
An alternative account of the SLC is that attention actively suppresses nontarget features (Gaspar & McDonald, 2014; Lamy et al., 2004). Recently, studies have shown that features that regularly appear as search distractors can be proactively suppressed (e.g., Chang & Egeth, 2019; Vatterott & Vecera, 2012), receiving less attention than irrelevant background items (Gaspelin & Luck, 2018b). Gaspelin and colleagues have shown that salient distractors presented during visual search are attentionally suppressed, as assessed via eye movements (Gaspelin et al., 2017) and via memory probes (Gaspelin et al., 2015). These examples of proactive feature suppression are complemented by examples in which a salient distractor first captures attention, and then is reactively suppressed (Geng, 2014; Luck et al., 2021). For example, Geng and DiQuattro (2010) required participants to ignore a salient distractor that was present on some trials and respond to a non-salient target. They found that when participants knew the salient item could not be the target it still attracted the first fixation on a majority of trials, indicating salience-based attentional capture. Interestingly, however, these fixations were very brief compared with those on non-salient distractors, or on salient distractors in an experiment in which it was possible for the salient item to be the target. Thus, it seems that although participants’ attention was caught by the salient distractor, they were able to rapidly suppress this item in a reactive manner to direct their attention elsewhere. On this account, presenting cues in nontarget colours would trigger salience-based attentional capture. This would in turn produce reactive attentional suppression at the location of the cue, which might carry over to the processing of the subsequent target, giving rise to the SLC.
One way of assessing attentional suppression directly in cueing tasks is by recording brain activity during task performance using electroencephalography (EEG). The PD component of the EEG signal is thought to provide an index of attentional suppression (Gaspar & McDonald, 2014; Hickey et al., 2009; Sawaki & Luck, 2010). The PD component is a positivity in posterior electrodes contralateral versus ipsilateral to the location of a suppressed stimulus (e.g., a distractor). PD components, for example, are produced contralateral to the location of a singleton distractor in visual search, and their amplitude is correlated with the extent of memory probe suppression (Gaspelin & Luck, 2018a). Thus, the PD component provides an index of attentional inhibition that can be related to behaviour.
Here we aimed to determine the source of the SLC by testing a prediction arising from the attentional suppression account. We used data from a previous spatial cueing study (Harris et al., 2017), in which participants reported the orientation of a target of a particular colour that was presented among coloured distractors. Nonpredictive cues were presented in target and non-target colours. We correlated each individual’s SLC with the magnitude of their PD component evoked by nontarget-coloured cues. If the SLC is produced by attentional suppression, then stronger suppression (indexed by a larger PD component) should be positively correlated with the magnitude of the SLC.
Methods
The current work reports a new analysis of data previously published by our group. The full details of that study, which focussed on the role of EEG oscillations in attentional capture, can be found in Harris et al. (2017). For the current study, the data from Harris et al. (2017) were combined across two near-identical experiments. A Bayes factor analysis for a between-subjects comparison across experiments revealed strong evidence for no behavioural difference between them (BF01 = 14.7). This study was not preregistered.
Participants
Forty-eight participants (aged 18–31 years, mean = 22.22 years, 26 females) completed the study and provided analysable data. All participants were right-handed, had normal or corrected-to-normal vision, provided written informed consent, and were monetarily compensated for their time. The study was approved by The University of Queensland Human Research Ethics Committee.
Behavioural task
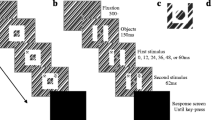
Participants completed a spatial cueing task (Folk et al., 1992) (Fig. 1). On each trial, participants fixated a central grey cross surrounded by four grey placeholder circles. A cue display followed, in which all four placeholders doubled in thickness and one changed colour (the cue). This colour cue was equally likely at each location, and could be red, green, blue, yellow, or grey (no cue), with equal likelihood. The fixation display was then presented again, followed by the target display, in which four ‘T’ stimuli appeared, one within each of the placeholder circles. Each ‘T’ was a different colour (red, blue, green, or yellow). Two of the ‘Ts’ were rotated 90° to the right and two were rotated 90° to the left. Participants made a speeded response to the orientation of the target-coloured ‘T’. In Experiment 1 (n = 24) the target was always red, whereas in Experiment 2, the target colour was counterbalanced between all four colours across participants (n = 24). Participants were then presented with the fixation display, during which they could make a response. Cue and target locations were uncorrelated, each being equally likely at all locations. Participants completed 1,040 experimental trials, plus 80 practice trials.
EEG recording and analysis
EEG data were recorded using a BioSemi Active Two system, digitized at a rate of 1,024 Hz. We recorded from 64 active Ag/AgCl scalp electrodes arranged according to the 10-10 system (Chatrian et al., 1985). Pre-processing was performed with the EEGLAB Toolbox (Delorme & Makeig, 2004). Data were high-pass filtered at 0.3 Hz and re-referenced to the average of all scalp electrodes. Trial epochs were extracted between -800 ms and 2,000 ms relative to cue onset. Trials contaminated by muscle artifacts or eye movements were rejected by visual inspection, resulting in an average loss of < 1% of trials per participant. The data were then further cleaned by means of Independent Components Analysis (ICA), using the SASICA plugin for EEGLAB (Chaumon et al., 2015).
For event-related potential (ERP) analysis, the pre-processed EEG data were contralateralised relative to the location of the cue, further epoched between -100 ms and 600 ms, and baseline-corrected by subtracting the average voltage between -100 ms and 0 ms. ERPs were calculated from symmetrical regions of interest, averaging data from electrodes P7, P9, and PO7, and electrodes P8, P10, and PO8. The two ERP components of interest were the N2pc and the PD components, with respective time windows of 160–260 ms and 260–460 ms, consistent with past studies (Harris et al., 2019; Mertes et al., 2016; Sawaki & Luck, 2013; Schönhammer et al., 2020; Travis et al., 2019). The N2pc is measured over posterior electrodes, and is characterised by a more negative deflection of the ERP in contralateral than ipsilateral electrodes. It is thought to index the allocation of attention (Eimer, 1996, 2014; Luck & Hillyard, 1994). The N2pc component from our data was reported in Harris et al. (2017), and is included here for the individual differences analyses.
Statistical analyses
All statistical analyses were performed in JASP (JASP Team, 2020). Significance level was set a priori to α = 0.05, giving us 0.8 power to detect correlations of r = .38 and above. Follow-up t-tests after ANOVAs were two-tailed. No correction for multiple comparisons was applied as comparisons were determined a priori. Specific tests are presented in the Results for brevity.
Results
Full behavioural and N2pc analyses can be found in Harris et al. (2017), separately for the two experiments. Here we combined the data from the two experiments to provide adequate statistical power for the individual differences analyses.
Reaction-time analyses
Reaction times (RTs) were analysed for trials in which participants responded to the target correctly (M = 97% of trials), excluding RTs faster than 250 ms and slower than 1,500 ms (< 1% of data).
A three-way mixed ANOVA was conducted on participants’ RTs, with the within-subjects’ factors of Cue Colour (Target Matching, Nontarget Matching) and Validity (Valid, Invalid), and the between-subjects’ factor of Experiment (Experiment 1, Experiment 2). As expected, there were no significant effects involving the factor of Experiment (all ps > .11). There was, however, a significant interaction between Validity and Cue Colour, F(1,46) = 283.82, p < .001, η2 = .09 (Fig. 2). Follow-up t-tests comparing the effect of Validity for each of the Cue types showed that, as expected, target-coloured cues produced significant attentional capture, M = 50 ms, t(47) = 15.08, p < .001, Cohen’s d = 2.18, whereas nontarget-coloured cues produced a significant SLC, M = -13ms, t(47) = 7.03, p < .001, Cohen’s d = 1.01.
Reaction times. Group level reaction times and individual data for the spatial cueing task. In a error bars represent within-participants SEM (Cousineau, 2005; Morey, 2008). In b density plots show the distribution of differences between valid and invalid trials across participants. Box plots show median and inter-quartile range. Small dots represent individual participant means. Blue data show the effect of target-coloured cues (i.e., attentional capture). Red data show the effect of nontarget-coloured cues (i.e., the same location cost (SLC))
N2pc analyses
A three-way mixed ANOVA was applied to EEG activity averaged within the N2pc timeframe, with within-subjects’ factors of Cue Colour (Target Matching, Nontarget Matching) and Electrodes (Contralateral, Ipsilateral), and a between-subjects’ factor of Experiment (Experiment 1, Experiment 2). This revealed a significant interaction between Cue Colour and Electrodes, F(1,46) = 36.70, p < .001, η2 < .01 (Fig. 3). Follow-up t-tests revealed a significant N2pc for target-coloured cues, t(47) = 5.86, p < .001, Cohen’s d = 0.85, and a non-significant trend for an N2pc for nontarget-coloured cues, t(47) = 1.94, p = .058, Cohen’s d = 0.28.
PD analyses
A comparable ANOVA conducted on EEG activity averaged within the PD timeframe revealed a significant interaction between Cue Colour and Electrodes, F(1,46) = 11.70, p = .001, η2 < .01 (Fig. 3). Follow-up analyses revealed significant PD components for both target-coloured cues, t(47) = 8.79, p < .001, Cohen’s d = 1.27, and nontarget-coloured cues, t(47) = 10.41, p < .001, Cohen’s d = 1.50. The interaction was driven by a larger PD component following target-coloured cues (M = 0.92μV, SD = 0.73μV) than nontarget-coloured cues (M = 0.59μV, SD = 0.39μV), t(47) = 3.45, p = .001, Cohen’s d = .50.
Individual differences
To test the two hypotheses about the source of the SLC, we correlated the magnitude of the PD component evoked by nontarget-coloured cues with the magnitude of the SLC in behavioural responses (Fig. 4). There was a significant negative correlation, r = -.41, p = .004, indicating that larger amplitude PD components were associated with smaller SLC effects in RTs. For completeness, we also calculated the correlation between the SLC and the magnitude of the N2pc, r = -.08, p = .573, and between the magnitude of the target-elicited attentional capture effect and both the N2pc, r = .01, p = .934, and the PD, r = .08, p = .585. None of these tests approached statistical significance.
Onset latency
An alternative perspective on the suppression account (see Discussion) posits that an earlier PD component is expected to correlate with more efficient termination of attentional allocation to the cue, and thus a reduced impact of the cue on the subsequent target response (i.e., a reduced SLC). To assess this perspective, we computed the cue-locked N2pc and PD onset latencies for each participant, in both the target-coloured and nontarget-coloured cue conditions, as the latency at which each individual’s ERP component reached 50% of its absolute maximum amplitude (Miller et al., 1998). These onset latencies were then correlated with the magnitude of the behavioural effect in each condition. In the target-coloured cue condition, N2pc onset latency (M = 179 ms) and PD onset latency (354 ms) did not correlate with the magnitude of attentional capture (N2pc: r = .11, p = .461, PD: r = .16, p = .269). In the nontarget-coloured cue condition, N2pc onset latency (183 ms) and PD onset latency (344 ms) did not correlate with the magnitude of the SLC (N2pc: r = -.01, p = .970, PD: r = -.07, p = .659). These results did not change if PD onset was calculated as 50% of maximum amplitude in the first half of the PD window, rather than over the whole window.
Discussion
We sought to examine the source of the same location cost (SLC) by testing the relationship between the magnitude of the SLC as indexed by participants’ RTs, and the amplitude of the PD component measured using EEG. The PD is commonly taken as an indicator of attentional suppression (Gaspar & McDonald, 2014; Gaspelin & Luck, 2018b; Hickey et al., 2009; Sawaki & Luck, 2010). We reasoned that if the SLC is due to attentional suppression of non-target features, there should be a positive correlation between the two. We reanalysed data from a prior study (Harris et al., 2017) in which we had observed an SLC at the group level but had not analysed the PD component. An individual differences analysis revealed a moderate negative correlation between the magnitude of the SLC and the PD component produced by nontarget-coloured cues, which is inconsistent with the attentional suppression account of the SLC.
An alternative account of our results is that the PD might not reflect ongoing suppression of the cued location, as we have suggested, but rather the termination of an attentional event at that location (Sawaki, Geng, & Luck, 2012). On this alternative account, a larger PD would reflect less attention being allocated to the cued item, and thus should correlate with reduced interference from that item. In this way, the SLC should correlate negatively with the PD component while still being consistent with the suppression account. We believe this explanation is unlikely for several reasons. First, in the nontarget-coloured cue condition we observed minimal, if any, attentional capture, as indicated by the nonsignificant N2pc in this condition. Although there was a trend for a very weak N2pc (likely only observed due to our large sample size), at best this implies a modest degree of salience-based capture (Awh et al., 2012; Theeuwes, 1992) on a small number of trials or in a small number of participants. This small amount of capture is logically inconsistent with a temporally subsequent attentional termination effect, as on most trials, for most participants, there was no capture to terminate. Second, if attentional termination was the basis for the correlation we observed, then PD latency should correlate with the SLC, as an earlier termination of attention should be associated with reduced stimulus processing and thus a reduced SLC. No such correlation was observed. Finally, the attentional termination account should also apply to target-coloured cue trials, as this cue: (a) captured attention, (b) cued a distractor on most trials, giving reason for its attentional termination, and (c) evoked a robust PD component. No such correlation was observed. Thus, this alternative conception of the suppression account is also not supported by our data.
An alternative explanation for the SLC is object-file updating (Carmel & Lamy, 2014), with which our findings are broadly compatible. The object-file updating account has received support from behavioural studies, which have shown that the magnitude of the behavioural SLC is reduced by task conditions that should evenly distribute the need for object-file updating across all stimulus locations (Schoeberl et al., 2018, 2020) and by conditions in which the need for updating is diminished by the absence of a consistently present object (Carmel & Lamy, 2014). While the object-file updating account does not explicitly predict the presence or absence of attentional suppression indexed by the PD, we may speculate that a negative correlation between the SLC and PD to non-target-coloured cues is plausible under this account. This is because attentional suppression of cue signals, if present, should weaken their representation, in turn making them easier to update (Harris et al., 2019).
Our use of a spatial cueing design with multiple distractor features was necessary to examine the SLC (Carmel & Lamy, 2015), but it limited our test of suppression to examining the role of reactive suppression (Geng, 2014) rather than proactive suppression. Proactive suppression is typically applied to a single, known, repeating distractor feature (Gaspelin & Luck, 2018b). There is little evidence for proactive suppression of multiple distractor features, as used in the current study. Our results suggest that reactive suppression does not contribute to the SLC. It is possible, however, that proactive suppression contributes to the SLC under other conditions, such as if a single distractor feature is used (although the SLC is not typically observed under single-distractor conditions; Carmel & Lamy, 2015).
A number of aspects of our results are worth mentioning. First, a PD component was present after all cues, with target-coloured cues leading to a larger PD relative to non-target-coloured cues. One explanation for this finding is that since all cues were non-predictive of target location and were effectively distractors, they all underwent reactive suppression (Sawaki & Luck, 2013). On this account, the most distracting cues (target-coloured, eliciting a large N2pc) required greater reactive suppression (indexed by the PD) than less distracting cues (non-target-coloured, eliciting a marginal N2pc). This account of PD magnitude as reflecting the degree of distraction raises a potential alternative explanation for our results, namely, that a larger PD in the nontarget-coloured cue condition is correlated with a smaller behavioural SLC because the SLC is cancelled out by a larger capture effect for more distracting cues (Carmel & Lamy, 2015). There are two reasons why we do not think this is the case. The first is that the N2pc results show little evidence that nontarget-coloured cues captured attention, as described above. The second is that such an account would apply equally to the target-coloured cue condition, but no correlation was observed in that condition. The most parsimonious explanation for a correlation between the PD and behaviour that is specific to the nontarget-coloured cue condition is that the PD interacts with a process that is specific to that condition, namely, the SLC.
Second, there was no significant correlation between the same-location cueing benefit and the amplitude of N2pc to target-coloured cues. This is surprising because the N2pc has traditionally been considered as a neural correlate of attentional selection (Eimer, 1996, 2014). It is interesting to note that a positive correlation between N2pc and cueing benefits is often assumed but rarely reported in this paradigm, and the absence of this correlation has been reported before (e.g., Harris et al., 2020; Matusz et al., 2019). While clearly outside the scope of the current study, the focus of which was to test the suppression account of the SLC, we speculate that the cue-elicited N2pc indexes an attentional process that is not sufficient to explain individual differences in the same-location cueing benefit, such as the tagging of a location for later attentional allocation (Yaron & Lamy, 2021).
Finally, it is worth noting that an alternative interpretation of the PD is that it reflects attentional enhancement of the cued target display element (Livingstone et al., 2017; but see Mertes et al., 2016, Mertes & Schneider, 2018, for evidence of cue-elicited PD components prior to target onset). On this account, one would predict a negative correlation between PD amplitude and the SLC for non-target-coloured cues, as observed here, as enhancement of cued targets should serve to counteract the SLC. However, this account also predicts an even stronger negative correlation between PD amplitude and the same-location benefit arising from target-coloured cues, as these cues produce the largest PD (note, the correlation should be negative here because the same-location benefit has been calculated as valid minus invalid RT, yielding a negative number. With capture coded as positive, the correlation would also be positive). No such correlation was observed here.
Conclusion
Various accounts of the SLC have been put forward (e.g., Carmel & Lamy, 2014; Gaspelin & Luck, 2018b; Schönhammer et al., 2020) and each has garnered evidence under different stimulus conditions. Our results indicate that participants with stronger attentional suppression of irrelevant features also show a smaller SLC, a finding that is inconsistent with the attentional suppression account. We speculate that an alternative mechanism, an SLC caused by the updating of object-file properties at the target location, may be influenced by reactive suppression of the to-be-updated object.
References
Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16(8), 437–443.
Bacon, W. F., & Egeth, H. E. (1994). Overriding stimulus-driven attentional capture. Perception & Psychophysics, 55(5), 485–496.
Becker, S. I. (2010). The role of target–distractor relationships in guiding attention and the eyes in visual search. Journal of Experimental Psychology: General, 139(2), 247.
Becker, S. I., Folk, C. L., & Remington, R. W. (2010). The role of relational information in contingent capture. Journal of Experimental Psychology: Human Perception and Performance, 36(6), 1460.
Becker, S. I., Folk, C. L., & Remington, R. W. (2013). Attentional capture does not depend on feature similarity, but on target-nontarget relations. Psychological Science, 24(5), 634–647.
Becker, S. I., Harris, A. M., Venini, D., & Retell, J. D. (2014). Visual search for color and shape: When is the gaze guided by feature relationships, when by feature values? Journal of Experimental Psychology: Human Perception and Performance, 40(1), 264.
Becker, S. I., Harris, A. M., York, A., & Choi, J. (2017). Conjunction search is relational: Behavioral and electrophysiological evidence. Journal of Experimental Psychology: Human Perception and Performance, 43(10), 1828.
Becker, S. I., Atalla, M., & Folk, C. L. (2020). Conjunction search: Can we simultaneously bias attention to features and relations? Attention, Perception, & Psychophysics, 82(1), 246–268.
Carmel, T., & Lamy, D. (2014). The same-location cost is unrelated to attentional settings: An object-updating account. Journal of experimental psychology: human perception and performance, 40(4), 1465.
Carmel, T., & Lamy, D. (2015). Towards a resolution of the attentional-capture debate. Journal of Experimental Psychology: Human Perception and Performance, 41(6), 1772.
Chang, S., & Egeth, H. E. (2019). Enhancement and suppression flexibly guide attention. Psychological Science, 30(12), 1724–1732.
Chatrian, G. E., Lettich, E., & Nelson, P. L. (1985). Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. American Journal of EEG Technology, 25(2), 83–92.
Chaumon, M., Bishop, D. V., & Busch, N. A. (2015). A practical guide to the selection of independent components of the electroencephalogram for artifact correction. Journal of Neuroscience Methods, 250, 47–63.
Cousineau, D. (2005). Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorial in Quantitative Methods for Psychology, 1(1), 42–45.
Delorme, A., & Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21.
Eimer, M. (1996). The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology, 99(3), 225–234.
Eimer, M. (2014). The neural basis of attentional control in visual search. Trends in Cognitive Sciences, 18(10), 526–535.
Folk, C. L., & Remington, R. (1998). Selectivity in distraction by irrelevant featural singletons: evidence for two forms of attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 24(3), 847.
Folk, C. L., Remington, R. W., & Johnston, J. C. (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance, 18(4), 1030.
Gaspar, J. M., & McDonald, J. J. (2014). Suppression of salient objects prevents distraction in visual search. Journal of Neuroscience, 34(16), 5658–5666.
Gaspelin, N., & Luck, S. J. (2018a). Combined electrophysiological and behavioral evidence for the suppression of salient distractors. Journal of Cognitive Neuroscience, 30(9), 1265–1280.
Gaspelin, N., & Luck, S. J. (2018b). The role of inhibition in avoiding distraction by salient stimuli. Trends in Cognitive Sciences, 22(1), 79–92.
Gaspelin, N., Leonard, C. J., & Luck, S. J. (2015). Direct evidence for active suppression of salient-but-irrelevant sensory inputs. Psychological Science, 26(11), 1740–1750.
Gaspelin, N., Leonard, C. J., & Luck, S. J. (2017). Suppression of overt attentional capture by salient-but-irrelevant color singletons. Attention, Perception, & Psychophysics, 79(1), 45–62.
Geng, J. J. (2014). Attentional mechanisms of distractor suppression. Current Directions in Psychological Science, 23(2), 147–153.
Geng, J. J., & DiQuattro, N. E. (2010). Attentional capture by a perceptually salient non-target facilitates target processing through inhibition and rapid rejection. Journal of Vision, 10(6), 5–5.
Harris, A. M., Remington, R. W., & Becker, S. I. (2013). Feature specificity in attentional capture by size and color. Journal of Vision, 13(3), 12–12.
Harris, A. M., Becker, S. I., & Remington, R. W. (2015). Capture by colour: Evidence for dimension-specific singleton capture. Attention, Perception, & Psychophysics, 77(7), 2305–2321.
Harris, A. M., Dux, P. E., Jones, C. N., & Mattingley, J. B. (2017). Distinct roles of theta and alpha oscillations in the involuntary capture of goal-directed attention. Neuroimage, 152, 171–183.
Harris, A. M., Jacoby, O., Remington, R. W., Travis, S. L., & Mattingley, J. B. (2019). Taking a closer look at visual search: Just how feature-agnostic is singleton detection mode? Attention, Perception, & Psychophysics, 81(3), 654–665.
Harris, A. M., Jacoby, O., Remington, R. W., Becker, S. I., & Mattingley, J. B. (2020). Behavioral and electrophysiological evidence for a dissociation between working memory capacity and feature-based attention. Cortex, 129, 158–174.
Hickey, C., Di Lollo, V., & McDonald, J. J. (2009). Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience, 21(4), 760–775.
JASP Team. (2020). JASP (Version 0.14.1)[Computer software].
Kahneman, D., Treisman, A., & Gibbs, B. J. (1992). The reviewing of object-files: Object-specific integration of information. Cognitive Psychology, 24(2), 175–219.
Lamy, D., Leber, A., & Egeth, H. E. (2004). Effects of task relevance and stimulus-driven salience in feature-search mode. Journal of Experimental Psychology: Human Perception and Performance, 30(6), 1019.
Lamy, D., Alon, L., Carmel, T., & Shalev, N. (2015). The role of conscious perception in attentional capture and object-file updating. Psychological Science, 26(1), 48–57.
Livingstone, A. C., Christie, G. J., Wright, R. D., & McDonald, J. J. (2017). Signal enhancement, not active suppression, follows the contingent capture of visual attention. Journal of Experimental Psychology: Human Perception and Performance, 43(2), 219.
Luck, S. J., & Hillyard, S. A. (1994). Electrophysiological correlates of feature analysis during visual search. Psychophysiology, 31, 291–308.
Luck, S. J., Gaspelin, N., Folk, C. L., Remington, R. W., & Theeuwes, J. (2021). Progress toward resolving the attentional capture debate. Visual Cognition, 29(1), 1–21.
Matusz, P. J., Turoman, N., Tivadar, R. I., Retsa, C., & Murray, M. M. (2019). Brain and cognitive mechanisms of top–down attentional control in a multisensory world: Benefits of electrical neuroimaging. Journal of Cognitive Neuroscience, 31(3), 412–430.
Mertes, C., & Schneider, D. (2018). Subtle distinctions: How attentional templates influence EEG parameters of cognitive control in a spatial cuing paradigm. Frontiers in Human Neuroscience, 12, 113.
Mertes, C., Wascher, E., & Schneider, D. (2016). From capture to inhibition: How does irrelevant information influence visual search? Evidence from a spatial cuing paradigm. Frontiers in Human Neuroscience, 10, 232.
Miller, J., Patterson, T. U. I., & Ulrich, R. (1998). Jackknife-based method for measuring LRP onset latency differences. Psychophysiology, 35(1), 99–115.
Morey, R. D. (2008). Confidence intervals from normalized data: A correction to Cousineau (2005). Tutorial in Quantitative Methods for Psychology, 4(2), 61–64.
Sawaki, R., & Luck, S. J. (2010). Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception, & Psychophysics, 72(6), 1455–1470.
Sawaki, R., & Luck, S. J. (2013). Active suppression after involuntary capture of attention. Psychonomic Bulletin & Review, 20(2), 296–301.
Sawaki, R., Geng, J. J., & Luck, S. J. (2012) A common neural mechanism for preventing and terminating the allocation of attention. Journal of Neuroscience, 32(31), 10725–10736.
Schoeberl, T., Ditye, T., & Ansorge, U. (2018). Same-location costs in peripheral cueing: The role of cue awareness and feature changes. Journal of Experimental Psychology: Human Perception and Performance, 44(3), 433.
Schoeberl, T., Goller, F., & Ansorge, U. (2020). The influence of display-to-display feature changes on net cueing effects: Evidence for a contribution of object-file updating. Quarterly Journal of Experimental Psychology, 73(6), 908–919.
Schönhammer, J. G., Becker, S. I., & Kerzel, D. (2020). Attentional capture by context cues, not inhibition of cue singletons, explains same location costs. Journal of Experimental Psychology: Human Perception and Performance, 46(6), 610.
Theeuwes, J. (1992). Perceptual selectivity for color and form. Perception & Psychophysics, 51(6), 599–606.
Travis, S. L., Dux, P. E., & Mattingley, J. B. (2019). Neural correlates of goal-directed enhancement and suppression of visual stimuli in the absence of conscious perception. Attention, Perception, & Psychophysics, 81(5), 1346–1364.
Vatterott, D. B., & Vecera, S. P. (2012). Experience-dependent attentional tuning of distractor rejection. Psychonomic Bulletin & Review, 19(5), 871–878.
Yaron, I., & Lamy, D. (2021). Spatial cueing effects are not what we thought: On the timing of attentional deployment. Journal of Experimental Psychology: Human Perception and Performance, 47(7), 946.
Funding
This work was supported by the Australian Research Council Centre of Excellence for Integrative Brain Function (ARC Centre Grant CE140100007). CB was supported by a University of Queensland post-doctoral researcher fellowship (UQ Research Stimulus Allocation Two (Fellowships)). AMH was supported by an ARC Discovery Early Career Researcher Award (DE220101019). JBM was supported by an NHMRC Investigator Grant (GNT2010141). Data available from lead author upon request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Open practices statement
Data available from lead author upon request. The analysis was not preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harris, A.M., Bradley, C., Yoo, S.Y. et al. Neurophysiological evidence against attentional suppression as the source of the same-location cost in spatial cueing. Atten Percept Psychophys 85, 284–292 (2023). https://doi.org/10.3758/s13414-022-02633-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-022-02633-w