Abstract
Oculomotor inhibition of return (IOR) is believed to facilitate scene scanning by decreasing the probability that gaze will return to a previously fixated location. This “foraging” hypothesis was tested during scene search and in response to sudden-onset probes at the immediately previous (one-back) fixation location. The latencies of saccades landing within 1º of the previous fixation location were elevated, consistent with oculomotor IOR. However, there was no decrease in the likelihood that the previous location would be fixated relative to distance-matched controls or an a priori baseline. Saccades exhibit an overall forward bias, but this is due to a general bias to move in the same direction and for the same distance as the last saccade (saccadic momentum) rather than to a spatially specific tendency to avoid previously fixated locations. We find no evidence that oculomotor IOR has a significant impact on return probability during scene search.
Similar content being viewed by others
When searching a cluttered scene for a hard-to-find object, we need to focus our eyes on each part of the scene in turn. The sequence of fixations (when the eyes are still) and saccades (ballistic movements of the eyes) provides insight into how a scene is processed in visual search (Findlay, 2004; Henderson, Chanceaux & Smith, 2009; Henderson & Smith, 2009; Malcolm & Henderson, 2010; Yarbus, 1967; Zelinsky, Rao, Hayhoe & Ballard, 1997). Each saccadic eye movement requires that the next candidate location be selected from a range of potential target locations. An optimum search strategy would be to visit each candidate location only once to check whether the search target is present, before moving on to the next candidate location (Melcher & Kowler, 2001; Najemnik & Geisler, 2005). This strategy would require a mechanism for keeping track of visited locations (Peterson, Kramer, Wang, Irwin & McCarley, 2001). One mechanism proposed to fulfill this purpose is inhibition of return (IOR).
Classically, IOR has been defined as an increase in reaction time (RT) to a target appearing at a previously attended location (Posner & Cohen, 1984). IOR is traditionally observed in attentional cuing paradigms, and both its behavioral and neural properties have been extensively studied (see Klein, 2000, and Wang & Klein, 2010, for reviews). Overt return of attention via eye movements has also been shown to experience delay (Klein & Hilchey 2011). Oculomotor IOR is thought to delay the programming of saccades to the previous fixation location (one back; Hooge, Over, van Wezel & Frens, 2005; Klein & MacInnes, 1999; MacInnes & Klein, 2003; Smith & Henderson, 2009) and to the penultimate fixation location (two back; Dodd, Van der Stigchel & Hollingworth, 2009; Klein & MacInnes, 1999), and may even extend as far back as four fixations (four back; Dodd et al., 2009).
It has been proposed that the delay in returning attention to previously attended locations (both overt and covert) may be functional. Posner and Cohen (1984) proposed that IOR “reduce[s] the effectiveness of a previously active area of space in summoning attention and serve[s] as a basis for favoring fresh areas” (p. 550). This novelty-seeking mechanism was later investigated by Klein (1988), who confirmed the presence of IOR during array search. The results suggested that the temporal influence of IOR may have a spatial consequence: decreasing the probability of orienting to previous locations, and as a consequence increasing the probability of attending to new locations, thus facilitating foraging (Klein, 1988; Klein & MacInnes, 1999).
Early formulations of the foraging facilitator hypothesis described IOR as “preventing attention from returning to the same stimulus” (Klein, 1988, p. 430) or “repelling attention” (Klein & MacInnes, 1999, p. 346). This absolute interpretation of the function of IOR encouraged other theorists to adopt IOR as a simple and robust mechanism for ensuring that attention does not return to recently attended locations (e.g., Itti & Koch, 2001; Navalpakkam & Itti, 2005; Parkhurst, Law & Niebur, 2002; Pomplun, Reingold & Shen, 2003; Rao, Zelinsky, Hayhoe & Ballard, 2002; Sun, Fisher, Wang & Gomes, 2008; Zelinsky, 2008). Computational models of visual attention typically use a saliency map computed from stimulus features such as luminance, color, and orientation to make predictions about where a viewer will attend. This saliency map indicates the conspicuity of each location in a search array or scene, and attention is assumed to be driven through the scene by progressing in rank order through the conspicuous locations (Itti & Koch, 2001). However, such models of attention require an extra mechanism to ensure that attention does not return to a highly conspicuous region immediately after leaving it, creating an infinite cycle between the two most salient regions. IOR is believed to serve this function.
However, the absolute functional interpretation of IOR adopted by some is an extreme position that fails to take into consideration other factors that may necessitate return to a previously attended location. Klein and colleagues have suggested that IOR is not an “all or none system. Rather, it is simply another source of input to the [activation] map” (Klein & Hilchey, 2011, MS p. 18) that controls saccade programming. This map is also influenced by other factors, such as process monitoring—that is, the need to reinspect locations that have previously received insufficient processing (Henderson, 1992)—the relevance of a region to the current viewing task (Henderson, Malcolm & Schandl, 2009), and search strategies (Gilchrist & Harvey, 2006; Peterson et al., 2001). Under complex viewing conditions, such as search of a real-world scene, these competing factors may outweigh the influence of IOR. However, when all other factors are controlled, IOR is still believed to discourage reorienting (Klein & Hilchey, in press; Wang & Klein, 2010).
The evidence that return to previous fixation locations is discouraged during naturalistic viewing tasks is currently mixed. The most compelling evidence of oculomotor IOR during scene search was put forward by Klein and MacInnes (1999) in a Where’s Waldo study. In this study, participants searched cluttered cartoon scenes for a character known as Waldo (Handford, 2008). While participants were searching the scene, a probe (a small black ring) appeared at the previous fixation location (one back), the penultimate fixation location (two back), or the same distance away at new locations. Participants were instructed to fixate the probe as soon as it appeared. Saccadic RTs to these probes indicated that saccades back to prior fixation locations took longer than saccades in the opposite direction (although only the delay relative to the two-back location reached significance). Since Klein and MacInnes’s seminal study, the delay experienced by voluntary saccades to probes during scene search has been replicated at the one-back location by MacInnes and Klein (2003) and at the two- and four-back locations by Dodd et al. (2009).
Along with temporal evidence of oculomotor IOR during scene search, Klein and MacInnes (1999) also reported what appeared to be evidence for a spatial effect, with saccades throughout the search period being biased away from prior fixation locations. They interpreted the observed low frequency of return saccades as evidence that oculomotor IOR facilitates foraging during scene search (Klein & MacInnes, 1999). Similar evidence that saccades are biased away from prior fixation locations has also been reported during search of simple visual arrays (Gilchrist & Harvey, 2000; Keech & Resca, 2010; Peterson et al., 2001). However, the evidence for a bias away from prior fixation locations is less clear in more complex search arrays. As the complexity of the search targets or the task increases, so does the frequency of immediate return fixations. For example, immediate returns occur more than would be predicted by chance during searches of large object arrays by monkeys (Motter & Belky, 1998) and humans (Peterson, Beck & Vomela, 2007; Peterson et al., 2001) and during free-view search (Hooge et al., 2005) and memorization (Smith & Henderson, 2009) of photographs of real-world scenes.
To test the generality of the functional interpretation of oculomotor IOR during real-world scene viewing, Smith and Henderson (2009) conducted two scene-viewing experiments in which the spatial and temporal evidence for IOR was examined during both voluntary and involuntary eye movements. In each experiment, viewers were asked to view photographs of scenes in preparation for an upcoming memory test. As in Klein and MacInnes (1999), localized onset probes (small pink boxes) were presented in the visual periphery at one of four locations relative to the current fixation location: the last location fixated, a directly forward location equal in distance to the last fixation location, and two locations equal in distance to the last location but perpendicular to the direction of the last saccade. Viewers were instructed to ignore the onset probes. This instruction was used to ensure that onset-induced saccades were involuntary and distinct from normal, voluntary saccades. Saccades were analyzed both during normal viewing and in response to the onset probes. There were three main results. First, the time taken to initiate a saccade was inversely proportional to the angular difference between the direction of that saccade and the last saccade, regardless of amplitude. Saccades that completely reversed direction from the last saccade (i.e., went directly backward) had the greatest latencies. These results are consistent with what Smith and Henderson (2009) termed saccadic momentum: the tendency for the eyes to continue moving in the same direction from one saccade to the next.
Second, the increased latency due to reversing saccade direction was supplemented by localized inhibition for saccades landing within 2º of the previous fixation location. The spatial extent of IOR was similar to the region reported in attentional cuing IOR studies (Bennett & Pratt, 2001; Berlucchi, Tassinari, Marzi & di Stefano, 1989; Dorris, Taylor, Klein & Munoz, 1999; Hooge & Frens, 2000; Maylor & Hockey, 1985; Tassinari, Aglioti, Chelazzi, Marzi & Berlucchi, 1987). These results suggested a highly localized influence of IOR on saccade latencies independent from saccadic momentum.
Third, and most significantly, eye movements back to the last fixation location were no less likely (and in some cases were more likely) than those to the distance-matched control locations in other directions. These results were not consistent with the hypothesis that IOR decreased the probability of return saccades. The results were consistent with other reports of above-chance levels of return saccades across a variety of tasks (Hooge et al., 2005; Motter & Belky, 1998; Peterson et al., 2001).
Following on from their precise analysis of the probability of return saccades during scene memorization, Smith and Henderson (2011) applied the same analysis to Where’s Waldo search. The delay for return saccades shown by Klein and MacInnes (1999) was replicated in this study, but Smith and Henderson (2011) did not find any evidence that IOR had a significant effect on the probability of return. Precise analysis of the distribution of fixations during search revealed one- and two-back return probabilities as high as those for the distance-matched locations and higher than an a priori fixation probability (Smith & Henderson, 2011). The forward bias in saccades demonstrated by Klein and MacInnes was attributed to saccadic momentum (Smith & Henderson, 2009), not to oculomotor IOR. Return saccades during Where’s Waldo search exhibit a temporal effect of oculomotor IOR but no spatial effect.
Present study
The results from Smith and Henderson (2009) suggest that oculomotor IOR does not have a significant influence on the probability of return in at least one scene-viewing task: memorization. However, it could be argued that scene memorization is an idiosyncratic task because object memory benefits from multiple fixations during encoding (Castelhano, Mack & Henderson, 2009; Williams, Henderson & Zacks, 2005). Consistent with the idea that IOR may be greater during search than during memorization, Dodd et al. (2009) reported that temporal oculomotor IOR was observed in scene search but not in other scene-viewing tasks. On the other hand, Hooge et al. (2005) found that return fixations were delayed but more likely than would be expected by chance in both free viewing and search, and Smith and Henderson (2011) demonstrated the same dissociation during pseudoscene Where’s Waldo search. Finding that IOR acts as a foraging facilitator under some conditions and not others undermines the idea that it is a general mechanism for keeping the eyes moving forward (Itti & Koch, 2001; Navalpakkam & Itti, 2005; Parkhurst et al., 2002; Pomplun et al., 2003; Rao et al., 2002; Sun et al., 2008; Zelinsky, 2008).
In the present study, we investigated whether oculomotor IOR has a significant influence on the probability of returning gaze to the previous fixation location during scene search. The experimental paradigm was similar to that of Smith and Henderson (2009), except that visual search replaced scene memorization. Viewers searched for and distinguished a small T or L embedded in full-color photographs of real-world scenes (Brockmole & Henderson, 2006). Eye movements during search and in response to sudden-onset probes were examined. In Experiment 1a, participants were instructed to “ignore” onsets, and in Experiment 1b they were instructed to “fixate” onsets. This manipulation was included in order to dispel concern that the relevance of the onset might influence the expression of oculomotor IOR (Klein & Hilchey, in press). The prevalence and latencies of saccades to the immediately previous fixation location were examined.
Method
Participants
A group of 32 members of the Edinburgh University community participated for payment. All participants had normal or corrected-to-normal vision, were naïve with respect to the purposes of the study, and were informed of their rights of participation according to the British Psychological Society’s ethical guidelines. Participants were randomly allocated to one of two groups, depending on whether they were instructed to “ignore” (Exp. 1a) or “fixate” (Exp. 1b) the onset probe.
Apparatus
Eye movements were monitored by an SR Research EyeLink 1000 eyetracker. Fixation position was sampled at 1000 Hz, and saccades prior to critical fixations were detected using a 17-sample saccade detection model with a velocity threshold of 30º/s, an acceleration threshold of 8000º/s2, and a minimum amplitude of 0.5º. Viewing was binocular, but only the right eye was tracked. The images were presented on a 21-in. CRT monitor at a viewing distance of 90 cm with a refresh rate of 140 Hz. The experiment was controlled with SR Research Experiment Builder software.
Stimuli
Participants were presented 152 unique full-color 800 × 600 pixel 24-bit photographs of real-world scenes (subtending a visual angle of 25.7º × 19.4º) from a variety of scene categories. A gray letter T or L was superimposed on 52 of the scenes (see Fig. 1). The letter subtended 0.3º horizontally and vertically. Scenes containing the search target were taken from Brockmole and Henderson (2006). The scenes containing the target were used as fillers and were not analyzed. The remaining 100 critical scenes were identical to those used in our previous investigation of oculomotor IOR during scene memorization (Smith & Henderson, 2009) and did not contain the target. The absence of the target ensured that participants searched for the full presentation time on all critical scenes and that any observed effects were not due to fixating the target itself.
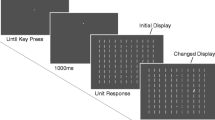
Sequence of events in each trial. Participants were instructed to search each scene for a small T or L superimposed on the scene (indicated here by a circle and arrow, which were not visible during trial). They terminated a trial as soon as they identified the target letter. After 1,000 ms of scene presentation (white circles indicate fixations), an onset (gray square) was presented at one of four locations around the current fixation at relative angular deviations from the previous saccade of 0º (previous fixation), 90º, 180º, or 270º. The onset was either presented for 250 ms and then removed (Exp. 1a) or remained on the screen until fixated (Exp. 1b). Participants were given a further 5,000 ms to locate the target before the trial terminated
Procedure
Participants were instructed to search the scenes for a small gray T or L superimposed on the scene. At the beginning of the experimental session, they were shown two example scenes with targets highlighted and then were given three practice trials. Each trial began with a fixation cross that was presented in the center of the display. When participants were registered as fixating within 1º of the fixation cross, the experimenter initiated the presentation of the search scene. If participants were more than 1º away from the fixation cross, a recalibration was performed. As soon as the scene appeared, participants were free to begin searching the scene for the search target. After 1,000 ms of scene presentation, the program controlling the experiment identified a critical fixation and presented an onset probe (a bright pink square of 1º of visual angle) about 40 ms into the critical fixation at one of four locations. The locations were defined to be on the circumference of a circle with its origin at the current fixation point and its radius equal to the distance between the current fixation location and the immediately previous fixation location (see Fig. 1). The angular deviation of the onset probe from the previous saccade was 0º (exactly backward toward the location of the immediately previous fixation), 90º, 270º, or 180º from that location. The 90º and 270º probe locations were at the endpoints of vectors perpendicular to the vector of the previous saccade, and the 180º probe location was at the end of the vector directly forward. If any of these points fell off the display screen or the amplitude of the previous saccade was less than 1º, the computer waited until the next suitable fixation before presenting the onset probe. The onset probe always appeared 40 ms into the critical fixation. Each participant saw an equal number of onset probes at each of the four locations, randomly ordered, with onset location within each scene counterbalanced across participants. Half of the participants (Exp. 1a) were instructed to ignore the onset, and the other half were instructed to fixate the onset (Exp. 1b). In the ignore condition, the onset probe was removed from the display after 250 ms. In the fixate condition, the onset remained on the screen until fixated or the trial timed out. In all conditions, the scene remained in view for a total of 5,000 ms. If the participant located the search target before the trial ended, he or she could terminate the trial by pressing the appropriate button on a joypad (Microsoft Sidewinder) to indicate whether the target was a T or an L. Only 52 out of the 152 trials contained a search target, but participants were told the target was present in all trials to ensure that search would continue for the full presentation time. Only the 100 target-absent trials were used in the analysis.
Results
Search performance
Before investigating the presence and effect of oculomotor IOR on eye movements, performance in the T/L search task was assessed. As intended, participants found locating the small T/L embedded in the scenes very difficult, only locating the target in 51.9% of the target-present scenes and taking 3.61 s on average. The search accuracy and RTs did not vary significantly across trials with different onset locations or instructions, suggesting that search behavior was consistent across participant groups and conditions. The low frequency of target-present trials (only a third of all trials) and short presentation times (5 s) meant that participants only located targets in 17.8% of trials overall. Such low performance could have disheartened participants and encouraged them to lapse into a nonsearch mode of viewing by the end of the experiment. However, analysis of hit rates and RTs over the course of the experiment indicated that performance did not drop off (Trials 1–50: hits = 53%, RT = 3.59 ms; Trials 51–100: hits = 50.3%, RT = 3.61 ms; Trials 101–152: hits = 52.4%, RT = 3.48 ms). This performance suggests that participants continued searching throughout the experiment. We have confidence that the T/L search task used in this study elicited naturalistic but difficult scene search behavior.
Time taken to return
To investigate the influence of oculomotor IOR on the distribution of fixations during scene search, the existence of a delay in orienting to the previously attended location needs to be established. The traditional measure of oculomotor IOR is a temporal delay experienced by saccades directed back to the immediately previous (one-back) fixation location. Saccadic RT for voluntary eye movements during scene search cannot be directly measured, because there is no way to know precisely when saccade programming begins in a fixation (Nuthmann, Smith, Engbert & Henderson, 2010). However, differences in the durations of fixations preceding critical saccades provide a reasonable estimate of differences in saccade programming time (Dodd et al., 2009; Hooge et al., 2005; Klein & MacInnes, 1999; MacInnes & Klein, 2003; Smith & Henderson, 2009). Therefore, mean fixation durations preceding saccades landing within 1º of the four locations 0º, 90º, 180º, and 270º away from the current fixation location following an onset and during normal viewing were calculated in both instruction conditions (Exps. 1a and 1b). Fixation durations are presented in Fig. 2. Data were averaged across the two perpendicular locations (90º and 270º) in order to look for a linear effect of angular deviation on preceding fixation duration. Saccades during the normal search period had to meet two criteria to be entered into the analysis: Saccade amplitude had to be greater than 1º, and although these were not probe saccades, all potential onset probe locations (the 0º, 90º, 180º, and 270º directions relative to one back) had to lie on the display screen (see Fig. 1 for a map of angular deviations relative to the previous saccade). After all exclusions, 47,183 saccades remained for analysis.
A repeated measures ANOVA of fixation durationsFootnote 1 with the factors fixation type (normal viewing vs. following an onset) and location (0º, 90/270º, and 180º) and the between-participants cross-experiment factor instruction (ignore vs. fixate) revealed no main effect of fixation type, F(1, 29) = 2.378, MSE = 1,792, p = .134, no main effect of instruction, F < 1, a main effect of location, F(2, 58) = 11.097, MSE = 1,118, p < .001, and no interactions. Across all conditions, the main effect of location was due to fixations of longer duration preceding return saccades (0º: M = 266 ms, SD = 60.8) than preceding saccades in all other directions (90º/270º: M = 255 ms, SD = 41.1, p < .088; 180º: M = 238 ms, SD = 39.3, p < .001; Bonferroni corrected for multiple comparisons). Saccades to the perpendicular locations (90º/270º) were also preceded by significantly longer fixations than saccades to 180º (p < .001; Bonferroni corrected).
Adopting the convention of calculating temporal IOR as the difference between the time taken to return to the one-back location and the 180º location (Klein & Hilchey, in press; Klein & MacInnes, 1999; Wang & Klein, 2010), our results indicate that we observed a delay of 28 ms for saccades returning to the one-back location. This delay is comparable to the delay for one-back return saccades previously reported during search (Hooge et al., 2005; Klein & MacInnes, 1999; Thomas et al., 2006) and memorization (Smith & Henderson, 2009).
Identifying the zone of oculomotor IOR
The delay experienced by returns to the one-back location could be interpreted as evidence of oculomotor IOR. However, given the linear effect of angular deviation on preceding fixation durations (i.e., saccades back to 90º/270º were also preceded by fixations longer than 180º), a competing explanation could be that reversing the direction of a saccade relative to the previous saccade might introduce a delay. This decreased latency for moving forward versus backward has previously been referred to as saccadic momentum (Smith & Henderson, 2009). In order to look for spatially specific inhibition around the one-back location (Bennett & Pratt, 2001; Hooge & Frens, 2000), general direction effects due to saccadic momentum would need to be controlled. This can be achieved by conducting location-specific analyses. In particular, we can compare the difference between the time taken to program backward saccades landing close to the one-back location and saccades over- or undershooting this location. Performing such an analysis during scene memorization has previously identified a ±2º region around the one-back location in which return saccades are delayed more than saccades in the same direction not landing in this region (Smith & Henderson, 2009). This localized delay was interpreted as temporal oculomotor IOR.
To determine whether there is evidence of spatially specific temporal oculomotor IOR in the present data, all saccades during the normal viewing period from Experiments 1a and 1b were classified in terms of their angular deviation from a saccade back to the previous fixation (collapsed into 45º bins clockwise and counterclockwise; see the lines in Fig. 3) and of the difference between the amplitudes of the next and previous saccades (collapsed into 2º bins and restricted to ±6º; x-axis of Fig. 3). This classification was then used to test whether return saccades (0º angular deviation + 0º difference in saccade amplitude) were preceded by longer fixations than were either saccades away from the previous fixation location or saccades in the same direction but with different amplitudes.
Mean fixation durations (in milliseconds) preceding saccades with a particular angular deviation from the previous saccade (0º–180º; lines) and difference in saccade amplitude (next – prior; x-axis). The data represent means across all normal fixations (i.e., without onset) collapsed across instruction conditions (Exps. 1a and 1b). 0º angular deviation + 0º difference in saccade amplitude = return saccades
A repeated measures ANOVA on preceding fixation durations with the factors angular deviation (0º, 45º, 90º, 135º, and 180º) and difference in saccade amplitude (−6, –4, –2, 0, 2, 4, 6) revealed a main effect of angular deviation, F(4, 124) = 39.906, MSE = 477.9, p < .001, a main effect of difference in saccade amplitude, F(6, 186) = 58.45, MSE = 405.9, p < .001, and a significant interaction, F(24, 744) = 1.902, MSE = 320.4, p < .05. The effect of angular deviation can be attributed to a linear increase in the preceding fixation duration as angular deviation decreased from 180º (M = 231 ms, SD = 35.14) to 45º (M = 254 ms, SD = 34.16; p < .001, Bonferroni corrected), although the trend ended with angular deviations of 0º, which were preceded by significantly shorter fixations (M = 244 ms, SD = 37.3) than were deviations of 45º (p < .001, Bonferroni corrected).Footnote 2
The main effect of difference in saccade amplitude was due to a linear increase in the preceding fixation duration as the difference decreased from 6º (M = 227 ms, SD = 35.9) to –6º (M = 260 ms, SD = 39.46). This indicates that when long saccades (i.e., prior) are followed by shorter saccades (i.e., next), the fixation in between is greater in duration than when short saccades are followed by long saccades. This relationship is well established during scene viewing (Tatler & Vincent, 2008; Unema, Pannasch, Joos & Velichovsky, 2005).
For most angular deviations, the main effect of the difference in saccade amplitudes was linear and highly significant (180º: R 2 = .802, p < .01; 135º: R 2 = .952, p < .001; 90º: R 2 = .984, p < .001; 45º: R 2 = .978, p < .001). However, return saccades (0º angular deviation) showed a characteristic peak for saccades landing within ±1º of the one-back location that deviates from the simple linear trend (see Fig. 3). To examine whether this peak constituted a significant deviation from the predicted fixation duration given the linear trend, a regression line was fit to all points except 0º/0º (R 2 = .982, p < .001). The predicted fixation duration at 0º/0º was 243 ms, which was less than the actual value, 254 ms (one-sample t test, t(32) = 1.98, p = .052). Given the noise intrinsic in sampling fixations during free search of a natural scene, it is likely that this marginal effect would reach significance given a larger corpus of eye movements. This peak in fixation durations at ±1º of the one-back location is suggestive of highly localized temporal IOR independent of saccadic momentum.
Probability of return
If the oculomotor IOR reported above functions as a foraging facilitator, eye movements during scene scanning should then be biased away from the last fixation location. Klein and MacInnes (1999) analyzed the angular deviation of all saccades relative to the previous saccade during normal search (i.e., excluding the saccade after the onset) and reported a clear forward bias. However, their analysis collected saccades into large, 60º angular deviation bins and did not identify the probability of returning to the precise one-back location (see Hooge et al., 2005, and Smith & Henderson, 2011, for similar criticisms). During scene memorization and Where’s Waldo search, the same forward bias in saccades has been observed, but with an accompanying significant tendency to return to the previous fixation location (Smith & Henderson, 2009, 2011). If oculomotor IOR facilitates foraging, then (1) there should be a forward bias in saccade distributions, (2) participants should avoid the one-back location during free search, and (3) participants should be less likely to experience oculomotor capture by onsets at the one-back location relative to other distance-matched locations. Each of these predictions will be examined in turn.
Distribution of saccades
To examine the angular bias of saccades during the present study, the angular deviation of every regular saccade relative to the previous saccade was calculated (excluding the saccade immediately following an onset). Only saccades in which the onset could have been presented were used: The distance to the last fixation location had to be greater than 1º, and all potential onset locations (0º, 90º, 180º, and 270º) had to lie on the screen (see Fig. 1 for a map of angular deviations relative to the previous saccade). Figure 4 represents the average probability for each participant that a saccade would have a particular angular deviation relative to the previous saccade (10º bins; 0º/350º is a return in the direction of the one-back fixation). Figure 4 shows a clear tendency for saccades to maintain a trajectory similar to that of the previous saccade—that is, 160º–200º angular deviation. This forward tendency has previously been characterized as saccadic momentum (Smith & Henderson, 2009). There was no difference in the distribution of saccades from Experiments 1a and 1b. The linear relationship between angular deviation and saccade probability only held between ~45º/135º and 180º, as there was also a smaller but significant tendency for saccades to return in the direction of the previous fixation (0º–30º and 330º–360º). For example, the probability of a saccade moving back in the direction of the previous fixation location (0º/340º) was significantly greater (M = .031, SD = .006) than the probability of saccades directed perpendicular to the last saccade (20º–110º or 240º–340º; all means between .022 and .025, all ps < .001, Bonferroni corrected). Note that this tendency to return cannot be due to proximity to the screen edge, because only saccades for which all four potential onset locations fell on the screen were used in the analysis.
Distribution of saccades relative to the previous saccade during normal search (i.e., all saccades except those immediately following an onset) collapsed across instruction conditions (Exps. 1a and 1b). Saccades are grouped into 10º angular deviation bins, with 0º/340º constituting a return saccade. The data are split according to onset fixation instructions: ignore = solid black, fixate = dotted gray. Error bars represent one standard error
Distribution of fixations
Even though saccades during normal search exhibited a tendency to be directed back toward the previous fixation location more than perpendicular to this location, oculomotor IOR might still have influenced the probability of landing at the precise one-back location, with most return saccades over- or undershooting the one-back location. To examine the probability of return to the precise one-back location, all saccades during normal search across Experiments 1a and 1b were further classified in terms of the difference between their amplitude and the amplitude of the previous saccade (next minus previous). This classification was combined with the angular deviations in order to produce a map of fixation probabilities at locations relative to the one-back location (see Fig. 5, inspired by scatterplots reported by Motter & Belky, 1998, and heat maps by Hooge et al., 2005). Precise return to the previous fixation location has a value of 0º/0º. The same forward tendency displayed in Fig. 4 can be observed in Fig. 5, with most saccades landing within an arc 130º–230º (centered at 180º) away from the previous fixation location and with amplitudes similar to the amplitude of the previous saccade (light color cells to the right of Fig. 5). However, there was also a distinct population of return saccades visible as a peak in the distribution at the previous fixation location (0º/0º; lighter cell on the left of Fig. 5). This analysis suggests that saccades tend to return fixation very precisely to the previous fixation location and that these returns occur more often than saccades in the same direction with different amplitudes.
Distribution of next fixation locations relative to the previous fixation location during normal search (i.e., all saccades except those immediately following an onset), collapsed across instruction conditions (Exps. 1a and 1b). Locations are classified by the difference between the angles of the next saccade and the previous saccade (circumference, 10º bins) and the difference in amplitude of the next saccade minus the previous saccade (radial values, 2º bins). 0º + 0º indicates a saccade returning to the previous fixation location. Colors indicate fixation probability
Fixation probability
In order to statistically compare the fixation probabilities at various points of the distribution relative to the one-back location (Fig. 5), an analysis similar to that performed on preceding fixation durations (Fig. 2) was performed for fixation probability. The probability of fixating onsets presented at the four locations with (Exp. 1b) and without (Exp. 1a) the instruction to fixate onsets was also analyzed to determine whether oculomotor IOR affected oculomotor capture. The probabilities that the next fixation would land within 1º of the previous fixation location (0º) and the other distance-matched control locations (90º/270º and 180º) were calculated for normal fixations and fixations following onset probes across both instruction conditions. Fixation probabilities are presented in Fig. 6. A repeated measures ANOVA for fixation probability revealed main effects of fixation type, F(1, 30) = 274.3, MSE = .013, p < .001, location, F(2, 60) = 5.115, MSE = .004, p < .01, and instruction, F(1, 30) = 50.7, MSE = .012, p < .001, and all interactions except type x location (ps < .05). As can clearly be seen from Fig. 6, the presence of the onset probe significantly increased the probability that the next saccade would land at all locations, and there seemed to be a greater tendency to fixate the straight-ahead (180º) location than any of the other locations in all conditions except when instructed to fixate the onset.
Mean probability of fixating target locations (0º, 90º, 180º, or 270º angular deviation from the previous saccade) during normal viewing (solid lines) and in the fixation following an onset at the peripheral location (dashed lines). Error bars represent one standard error. Participants were instructed either to ignore (a) or to fixate (b) the onset
Within the onset conditions, there was a marginal main effect of location, F(2, 60) = 2.995, MSE = .008, p = .058, a significant effect of instruction, F(1, 30) = 48.77, MSE = .024, p < .001, and a significant interaction, F(2, 60) = 5.677, MSE = .008, p < .01. When participants were instructed to fixate the onset, there were no significant differences between any of the onset locations (F = 1.025), although the probability of fixating onsets was numerically greater at 0º (M = .43, SD = .12) than at 90º/270º (M = .39, SD = .10) or 180º (M = .39, SD = .13), contrary to what would be expected if oculomotor IOR were influencing the probability of capture at the one-back location. By comparison, location of the onset had a large effect on fixation probability when participants were instructed to ignore the onset, F(2, 30) = 8.840, MSE = .007, p < .001. Onsets at the forward (180º) location were more likely to be fixated (M = .252, SD = .15) than onsets at any of the other locations (0º: M = .14, SD = .11, p < .01; 90º/270º: M = .152, SD = .083, p < .01; all comparisons Bonferroni corrected). There was no difference between fixation probability for onsets at 0º or 90º/270º.
The forward bias was also observed during normal viewing (i.e., every saccade except immediately following the onset). There was a main effect of location, F(2, 60) = 30.10, MSE = .0009, p < .001, but no effect of instruction or interaction (Fs < 1). The absence of an effect of instruction confirms that eye movement behavior was similar across instruction conditions during most of the viewing time, and only differed in the saccade immediately following the onset. As in the ignore onset condition, the effect of location was due to an increase in fixation probability at the forward (180º) location (M = .037, SD = .017) relative to all other locations (0º: M = .02, SD = .006, p < .001; 90/270º: M = .018, SD = .005, p < .001; both comparisons Bonferroni corrected). There was no significant difference between the probabilities of fixating 0º and 90º/270º (p = .137), although the probability was numerically greater at 0º than at 90º/270º.
In summary, this statistical analysis of fixation probabilities confirms that return fixations to the previous fixation location during normal search or following onsets were no less likely than fixations to most other distance-matched locations. The only location that was fixated significantly more often than the previous location was the straight-ahead (180º) location. This forward bias seems to be due to a general tendency to repeat the direction (Fig. 4) and amplitude (Fig. 5) of the previous saccade and not to a decrease in the probability of returning to the one-back location. The increase in hits for onsets at 180º during the ignore instruction condition is probably due to oculomotor capture for preprogrammed forward saccades; when participants are instructed to fixate the onsets, this forward bias disappears and may even reverse (as was observed during scene memorization; Smith & Henderson, 2009).
A priori fixation probability
The fixation probabilities reported above suggest that oculomotor IOR does not significantly decrease the probability of fixating previous fixation locations as compared to distance-matched controls. However, it has been argued that distance-matched controls may not be the best way of identifying an a priori baseline fixation probability for a previously attended location (Wang & Klein, 2010). The distance-matched locations control for systematic biases in saccade amplitudes (Tatler & Vincent, 2008) but do not control for the visual content at each location. It has been argued that the baseline probability of fixating each location may vary depending on the relevance of that location to the search task, so any measure of the impact of IOR on refixation probability should be calculated relative to this initial baseline (Wang & Klein, 2010). One option for calculating the baseline would be to use a simple model based only on low-level visual saliency to predict base fixation probabilities (see, e.g., Itti & Koch, 2001; Navalpakkam & Itti, 2005; Parkhurst et al., 2002; Pomplun et al., 2003; Rao et al., 2002; Sun et al., 2008; Zelinsky, 2008). However, such models have been shown to be inaccurate in active tasks such as visual search (Cristino & Baddeley, 2009; Einhäuser, Spain & Perona, 2008; Foulsham & Underwood, 2008; Henderson, Brockmole, Castelhano & Mack, 2007; Henderson et al., 2009b; Tatler, 2007; Tatler, Baddeley & Gilchrist, 2005). An alternate method for generating a baseline fixation probability for each location in a scene is to consider how often that location is actually fixated, irrespective of the order in which the fixations occur. If the fixation probability for a location is very high, attention should return to that location once any effects of IOR have worn off. This baseline fixation probability can be calculated by shuffling the order of fixations in a scene multiple times, removing order effects such as IOR (see Hooge et al., 2005, for a similar method). If IOR decreases the probability of immediate refixation, the one-back refixation probability should be significantly less than the probability of landing in the same location when that location is not one back.
A shuffled fixation probability baseline was created for all normal saccades collapsed across both instruction conditions (i.e., excluding the saccade following the onset) by randomly shuffling each participant’s fixations on each scene 50 times. The shuffled baseline fixation probability at one back (M = .0141, SD = .009) was significantly greater than the probability of landing within 1º of a randomly selected point on the screen (M = .0063, SD = .0014), t(62) = 4.640, p < .001, confirming that fixations cycle through a limited set of locations in the scene during search. Critically, the actual probability of returning to the one-back location was significantly greater (M = .020, SD = .014) than the one-back probability in the shuffled data (M = .0141, SD = .009), t(62) = 2.70, p < .01. This result indicates that the probability of fixating a location is significantly greater when the eyes have just left that location than when they have not. This finding is contrary to what would be expected if oculomotor IOR facilitates foraging.
Discussion
The experiments presented here investigated whether oculomotor inhibition of return influences the distribution of fixations during scene search. Many current computational models of eye movements assume that IOR drives attention through a scene by acting as a foraging facilitator—that is, by decreasing the probability that fixation will return to recently fixated locations (Itti & Koch, 2001; Navalpakkam & Itti, 2005; Parkhurst et al., 2002; Rao et al., 2002; Sun et al., 2008; Zelinsky, 2008). However, the evidence for this spatial effect of IOR on eye movements has been unclear (Hooge et al., 2005; Smith & Henderson, 2009, 2011). In the present study, we investigated the foraging facilitator hypothesis during visual search in real-world scenes. We examined normally occurring eye movements as well as eye movements in response to sudden-onset probes. The probes appeared at four possible locations: the previous fixation location, a forward location equal in distance to the previous location, or two perpendicular locations equal in distance to the previous location. In Experiment 1a, participants were instructed to ignore the onset probes, and in Experiment 1b, they were instructed to fixate the onset probes.
The results were not consistent with the foraging facilitator hypothesis. Saccades returning to the one-back location were delayed both during normal search and in response to onsets, consistent with oculomotor IOR. However, the distribution of fixations showed no evidence that oculomotor IOR discouraged return of fixation to the one-back location. The probability that a saccade would bring the eyes back to the last fixation location was equal to the probability for distance-matched perpendicular locations, and greater than the a priori probability of fixating the same location when it was not one back. Returns occurred both during normal eye movements and following sudden-onset probes. Similarly greater oculomotor capture by onsets at previously attended locations has also been reported during scene memorization (Smith & Henderson, 2009).
In the present data, the only location with consistently greater fixation probability than the one-back location was the forward location in which the eyes moved with the same direction and amplitude as during the last saccade. This forward bias was clearly visible in the angular distribution of saccades (Fig. 4) and the distribution of fixations (Fig. 5). We have interpreted this effect as saccadic momentum, or the tendency to repeat the previous saccade program (Smith & Henderson, 2009, 2011). Previous analyses of saccade and fixation distributions relative to the one-back location have used coarse bins that masked the precise return fixations evident in the present data, and instead contrasted backward against forward biases (Klein & MacInnes, 1999; MacInnes & Klein, 2003). Finer analysis of fixation probabilities has revealed a significant tendency for fixation to return to the one-back location during scene memorization (Smith & Henderson, 2009), Where’s Waldo search (Smith & Henderson, 2011), and free viewing and search of scenes (Hooge et al., 2005).
Taken together, the results suggest that whereas IOR may delay return of fixation, it does not facilitate foraging during scene search, in the sense that it does not reduce the likelihood that the eyes will return to a previously fixated location. These results are consistent with previous evidence from normal eye movements during scene viewing and visual search (Hooge et al., 2005), Where’s Waldo search (Smith & Henderson, 2011), scene memorization (Smith & Henderson, 2009), and in response to sudden-onset probes during scene memorization (Smith & Henderson, 2009). They are also consistent with previous results showing that eye movements back to the immediately preceding fixation location are greater than would be expected by chance during difficult array search (Motter & Belky, 1998; Peterson et al., 2001).
Klein and MacInnes (1999) argued that return saccades to the immediately preceding fixation location might be caused by temporal and spatial proximity. For example, they suggested that the brief time that elapses between a current fixation and the immediately preceding fixation (around 300 ms of fixation time on average) may be too short for attention to have fully shifted away from the last and to the current fixation location. If this is true, they suggest that a better test of the foraging facilitator hypothesis might be at the location fixated two saccades prior to the current fixation: that is, two back (Dodd et al., 2009; Klein & MacInnes, 1999).
We see two problems with this argument. First, there is no evidence that attention remains behind when the eyes move to a new location. Instead, current evidence strongly suggests that covert attention shifts to the upcoming fixation location before, not after, a saccadic eye movement (e.g., Deubel & Schneider, 1996; Findlay & Gilchrist, 2003; Henderson, Pollatsek & Rayner, 1989; Hoffman & Subramaniam, 1995; Kowler, Anderson, Dosher & Blaser, 1995; Peterson, Kramer & Irwin, 2004; Shepard, Findlay & Hockey, 1986). A further implication of this fact is that prior to a saccade away from the current fixation position, attention will have been disengaged from the last fixation location for the duration of the current fixation plus the difference in time between when attention shifted to the current location and when the eyes landed on that location. Estimates of the covert shift are based on saccade programming time and are typically well over 100 ms. So, the amount of time that attention has been disengaged from the last fixation location prior to a new saccade is underestimated in the analysis that was offered by Klein and MacInnes (1999), and instead is clearly within the time window that IOR is thought to be maximal (Posner & Cohen, 1984).
Second, and perhaps more importantly, the foraging facilitator hypothesis is significant because it provides a possible mechanism for ensuring that the eyes move forward to new scene areas rather than backward to a previously fixated area. The strong form of this hypothesis, adopted in the computational attention literature, is that IOR prevents the eyes from oscillating between two salient locations: the present and one-back locations (e.g., Itti & Koch, 2001; Navalpakkam & Itti, 2005; Parkhurst et al., 2002; Pomplun et al., 2003; Rao et al., 2002; Sun et al., 2008; Zelinsky, 2008). Support of this strong hypothesis should be evident in a decreased return probability at one back. Greater expression of temporal and spatial oculomotor IOR at two back would be inadequate to stop attention oscillating between the present and the one-back location, and therefore would not provide support for the strong form of the foraging facilitator hypothesis. The present one-back results and those of other related studies (Hooge et al., 2005; Smith & Henderson, 2009, 2011) demonstrate that this strong hypothesis is incorrect. A weaker version of the hypothesis states that IOR reduces rather than eliminates the probability that the eyes will return to the last location (Klein & Hilchey, in press; Wang & Klein, 2010). The present results also provide evidence against this version of the hypothesis, since the likelihood of fixating the immediately previous fixation location was greater than the baseline fixation probability calculated by eliminating order effects.
Oculomotor IOR
The primary interest of the present study was the foraging facilitator hypothesis, a proposed consequence of the application of IOR at previous fixation locations. However, the more traditional measure of IOR in the eye movement literature is a temporal one involving increased saccade latencies (or, in the case of free viewing, fixation durations) prior to saccades that move the eyes back to the previous fixation location versus other locations. In our previous memorization study, we found evidence for an effect of IOR on fixation durations in the absence of evidence for a reduction in fixation likelihood (Smith & Henderson, 2009). The present study confirmed the presence of IOR on fixation duration at one back. During normal search and in response to onsets, saccades that moved the eyes back to the previous fixation location were preceded by significantly longer fixation durations than saccades that moved the eyes forward the same distance.
However, our results also demonstrate that direct interpretation of the delay experienced by return saccades as oculomotor IOR is misguided. Changing saccade direction relative to the last saccade results in a general delay that may be due to saccadic momentum (Smith & Henderson, 2009) and so be independent from the delay caused by IOR.
Saccadic momentum
The most prominent characteristic of saccade behavior observed in these experiments was saccadic momentum: the tendency for the eyes to continue moving in the direction of the previous saccade. Related evidence that attention is biased toward continuing in the same direction as the last attentional shift can be found in saccade trajectories during array search (Gilchrist & Harvey, 2006; Hooge & Erkelens, 1996), RTs during covert attentional shifts (Bennett & Pratt, 2001; Pratt, Adam & McAuliffe, 1998; Pratt, Spalek & Bradshaw, 1999), and saccade initiation times in a variety of tasks, including cuing (Anderson, Yadav & Carpenter, 2008; Ro, Pratt & Rafal, 2000), double step (Hou & Fender, 1979; Komoda, Festinger, Phillips, Duckman & Young, 1973), search (Hooge et al., 2005), free viewing of scenes (Hooge et al., 2005; Tatler & Vincent, 2008, 2009), and scene memorization (Smith & Henderson, 2009).
Is saccadic momentum responsible for oculomotor IOR? Dodd et al. (2009) argued that the “oculomotor compatibility” between a saccade returning to the one-back location and a saccade that brought the eyes from that location may mask any influence of IOR (a similar argument was also put forward by Klein & MacInnes, 1999). This is a valid concern. Residual activation of the previous saccade program could result in a delay in programming saccades in the opposite direction, and so in a tendency to produce forward saccades. This would account for the temporal and spatial patterns observed here and in previous studies (Hooge et al., 2005; Klein & MacInnes, 1999; Smith & Henderson, 2009). However, studies in which forward momentum has been dissociated from IOR have shown that temporal IOR exists even in the absence of momentum (Machado & Rafal, 2004; MacInnes & Klein, 2003; Snyder, Kingstone & Schmidt, 2001). For instance, MacInnes and Klein (2003) ruled out the possibility that a forward bias was responsible for the delay in orienting to the immediately preceding fixation location by presenting onset probes only after search had stopped. MacInnes and Klein argued that by waiting until fixation had been voluntarily held on an object of interest for 500 ms before presenting the probes, all residual activation of saccade programs should have dissipated, and the observed delay in reorienting to a previous location could be attributed to IOR. This finding was supported by Smith and Henderson (2009), who showed that spatially specific IOR at the last fixated location could be dissociated from the delay associated with saccadic momentum (see Fig. 4 of Smith & Henderson, 2009).
In the present data, the distinction between saccadic momentum and spatially specific IOR was evident once saccades that over- and undershot the previous fixation location were analyzed. All return saccades were preceded by longer fixations than forward saccades, but saccades landing close to the previous fixation location (±1º) experienced an extra delay that might be attributed to oculomotor IOR, although the marginal effect suggests that caution should be taken in drawing this conclusion. A similar zone of IOR has been identified in cuing tasks (Bennett & Pratt, 2001; Berlucchi et al., 1989; Dorris et al., 1999; Hooge & Frens, 2000; Maylor & Hockey, 1985; Tassinari et al., 1987) and for eye movements during scene memorization (Smith & Henderson, 2009). In order to accurately identify the delay associated with oculomotor IOR, the spatially specific effects of IOR must be extracted from the overall delay associated with changing saccade direction. Future studies will attempt to empirically dissociate IOR from saccadic momentum in order to answer this question.
A proliferation of processes?
Rather than one simple process—IOR—influencing both the timing of saccades and their probability, the present interpretation of our findings (and of other findings: Hooge et al., 2005; Klein & MacInnes, 1999; MacInnes & Klein, 2003; Smith & Henderson, 2009, 2011) seems to lead to a proliferation of processes: (1) Return saccades are delayed by oculomotor inhibition of return. (2) Forward saccades are quicker and more likely than other saccade programs due to saccadic momentum. (3) The probability of return saccades is greater than chance due to top-down factors such as process monitoring (referred to as facilitation of return [FOR]; Smith & Henderson, 2009, 2011). This might seem a rather unparsimonious interpretation, but it is currently unclear whether these three processes are independent, with independent neural substrates, or are different manifestations of a single process or subset of processes. For example, Ivanoff and Klein (2001, 2004) showed that increased RTs to cued locations could be accompanied by a decrease in false alarms at the cued location in a no-go task. They argued that this was evidence that IOR could delay responses while increasing accuracy, a dissociation similar to IOR and FOR. However, accuracy in their no-go task was defined as a failure to respond. Therefore, a delay in activating a saccade program back to an inhibited location would increase the probability that the top-down no-go instruction could be issued, increasing the accuracy. This is a distinctly different definition of accuracy than is found in most other saccade tasks, in which an accurate response requires a saccade to a target.
Typically, models of saccade programming couple latencies with response probability. Saccade generation is often conceptualized as an accumulator model of decision making (e.g., the LATER model; Carpenter, 1981; Carpenter & Williams, 1995). Evidence in favor of discrete, competing saccade programs is accumulated until a program reaches threshold and is initiated. As the model is a race between competing saccade programs, the program with the shortest latency also has the highest probability of being chosen. Such a linear accumulation model has been shown to account for various aspects of saccade execution, including contextual variability in the expression of temporal oculomotor IOR (Farrell, Ludwig, Ellis & Gilchrist, 2011; Ludwig, Farrell, Ellis & Gilchrist, 2009).
The time to reach threshold can be influenced either by variation in the baseline level of activation (effectively equivalent to a variation in the threshold) or variation in the rate of accumulation (see Ludwig et al., 2009, for discussion of the distinction). Both of these influences could be a consequence of bottom-up factors such as IOR and residual activation from a prior saccade program (i.e., saccadic momentum) or top-down factors such as process monitoring. For example, the CRISP model of fixation durations during scene viewing accounts for a direct influence of processing difficulty within a fixation by decreasing the accumulation rate of evidence, thus increasing the time between saccades (Nuthmann et al., 2010). Within such an accumulation model of saccade programming, IOR is simply one factor influencing the accumulation of evidence. This view is shared by Klein and Hilchey (in press): IOR, which “temporarily reduces activity for previously inspected locations in [the activation] map, is not likely an all or none system. Rather, it is simply another source of input to the aforementioned map” (MS p. 18). Within such a model of saccade programming, there is no need to conceptualize IOR, saccadic momentum, and facilitation of return as discrete processes. Rather, they are classifications of behaviors manifest by a complex system receiving a multitude of inputs from both bottom-up and top-down sources. Some inputs may be a bottom-up consequence of prior saccade programs, such as inhibition of return saccades (IOR) and facilitation of forward saccades (saccadic momentum), whereas others may be due to direct top-down control, such as a belief that a location requires further processing (FOR). The pattern of saccade latencies and probabilities observed during a complex scene-viewing task is the result of the combined influences of these and many other factors. Further investigation is required to understand what these factors are during naturalistic scene viewing, how they interact, and whether behaviors such as IOR and saccadic momentum are the result of independent factors or the negative and positive consequences of a single factor.
Conclusion
This study investigated whether oculomotor IOR facilitates foraging during scene search. Saccades back to the previous fixation location were delayed relative to saccades of the same amplitude but directly away from the previous location and to return saccades that over- and undershot the one-back location, consistent with oculomotor IOR. However, despite this delay, there was no evidence that eye movements back to the previous fixation location were less likely than eye movements to perpendicular control locations or an a priori baseline, either for normal free-viewing eye movements or for eye movements generated in response to onsets. Instead, the probability of immediately returning to a location was greater than the probability of fixating the same location if it had not just been visited. It does not appear that IOR operates as an effective mechanism for driving attention through real-world scenes.
Notes
In the ignore condition, only 15 out of 16 participants were entered into the analysis, due to 1 participant failing to produce valid hits in all of the onset conditions. Analysis with and without this participant produced the same pattern of results.
The shorter fixations preceding saccades with 0º angular deviation as compared with 45º are surprising, considering previous evidence of a linear increase in preceding fixation duration as angular deviation increases (Smith & Henderson, 2009). It is unclear why this pattern occurs during search and not memorization. It may indicate that return saccades during search differ from saccades in other directions along one or more dimensions, such as amplitude, direction relative to the screen, or proximity to the screen edge, all of which have been shown to influence the preceding fixation duration (e.g. Tatler & Vincent, 2008). The influence of these factors on the expression of temporal IOR will be studied in subsequent studies.
References
Anderson, A. J., Yadav, H., & Carpenter, R. H. S. (2008). Directional prediction by the saccadic system. Current Biology, 18, 614–618.
Bennett, P. J., & Pratt, J. (2001). The spatial distribution of inhibition of return. Psychological Science, 12, 76–80. doi:10.1111/1467-9280.00313
Berlucchi, G., Tassinari, G., Marzi, C. A., & di Stefano, M. (1989). Spatial distribution of the inhibitory effect of peripheral non-informative cues on simple reaction time to non-fixated visual targets. Neuropsychologia, 27, 201–221. doi:10.1016/0028-3932(89)90172-3
Brockmole, J. R., & Henderson, J. M. (2006). Using real-world scenes as contextual cues for search. Visual Cognition, 13, 99–108. doi:10.1080/13506280500165188
Carpenter, R. H. S. (1981). Oculomotor procrastination. In D. F. Fisher, S. A. Monty, & J. W. Senders (Eds.), Eye movements: Cognition and visual perception (pp. 237–246). Hillsdale: Erlbaum.
Carpenter, R. H. S., & Williams, M. L. L. (1995). Neural computation of log likelihood in control of saccadic eye movements. Nature, 377, 59–62.
Castelhano, M. S., Mack, M., & Henderson, J. M. (2009). Viewing task influences eye movement control during active scene perception. Journal of Vision, 9(3), 6:1–15. doi:10.1167/9.3.6
Cristino, F., & Baddeley, R. (2009). The nature of visual representations involved in eye movements when walking down the street. Visual Cognition, 17, 880–903. doi:10.1080/13506280902834696
Deubel, H., & Schneider, W. X. (1996). Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Research, 36, 1827–1837.
Dodd, M. D., Van der Stigchel, S., & Hollingworth, A. (2009). Novelty is not always the best policy: Inhibition of return and facilitation of return as a function of visual task. Psychological Science, 20, 333–339. doi:10.1111/j.1467-9280.2009.02294.x
Dorris, M. C., Taylor, T. L., Klein, R. M., & Munoz, D. P. (1999). Influence of previous visual stimulus or saccade on saccadic reaction times in monkey. Journal of Neurophysiology, 81, 2429–2436.
Einhäuser, W., Spain, M., & Perona, P. (2008). Objects predict fixations better than early saliency. Journal of Vision, 8(14), 18:11–26. doi:10.1167/8.14.18
Farrell, S., Ludwig, C. L., Ellis, L. A., & Gilchrist, I. D. (2011). The influence of environmental statistics on inhibition of saccadic return. Proceedings of the National Academy of Sciences, 107, 929–934.
Findlay, J. M. (2004). Eye scanning and visual search. In J. M. Henderson & F. Ferreira (Eds.), The interface of language, vision, and action: Eye movements and the visual world (pp. 134–159). New York: Psychology Press.
Findlay, J. M., & Gilchrist, I. D. (2003). Active vision: The psychology of looking and seeing. Oxford: Oxford University Press.
Foulsham, T., & Underwood, G. (2008). What can saliency models predict about eye movements? Spatial and sequential aspects of fixations during encoding and recognition. Journal of Vision, 8(2), 6:1–17. doi:10.1167/8.2.6
Gilchrist, I. D., & Harvey, M. (2000). Refixation frequency and memory mechanisms in visual search. Current Biology, 10, 1209–1212. doi:10.1016/S0960-9822(00)00729-6
Gilchrist, I. D., & Harvey, M. (2006). Evidence for a systematic component within scan paths in visual search. Visual Cognition, 14, 704–715. doi:10.1080/13506280500193719
Handford, M. (2008). Where’s Wally? The solid gold collection. London: Walker.
Henderson, J. M. (1992). Visual attention and eye movement control during reading and picture viewing. In K. Rayner (Ed.), Eye movements and visual cognition: Scene perception and reading (pp. 260–283). New York: Springer.
Henderson, J. M., Brockmole, J. R., Castelhano, M. S., & Mack, M. (2007). Visual saliency does not account for eye movements during visual search in real-world scenes. In R. van Gompel, M. Fischer, W. Murray, & R. Hill (Eds.), Eye movements: A window on mind and brain (pp. 537–562). Amsterdam: Elsevier.
Henderson, J. M., Chanceaux, M., & Smith, T. J. (2009). The influence of clutter on real-world scene search: Evidence from search efficiency and eye movements. Journal of Vision, 9(1), 32:1–8. doi:10.1167/9.1.32
Henderson, J. M., Malcolm, G. L., & Schandl, C. (2009b). Searching in the dark: Cognitive relevance drives attention in real-world scenes. Psychonomic Bulletin & Review, 16, 850–856. doi:10.3758/PBR.16.5.850
Henderson, J. M., Pollatsek, A., & Rayner, K. (1989). Covert visual attention and extrafoveal information use during object identification. Perception & Psychophysics, 45, 196–208. doi:10.3758/BF03210697
Henderson, J. M., & Smith, T. J. (2009). How are eye fixation durations controlled during scene viewing? Further evidence from a scene onset delay paradigm. Visual Cognition, 17, 1055–1082. doi:10.1080/13506280802685552
Hoffman, J. E., & Subramaniam, B. (1995). The role of visual attention in saccadic eye movements. Perception & Psychophysics, 57, 787–795. doi:10.3758/BF03206794
Hooge, I. T. C., & Erkelens, C. J. (1996). Control of fixation duration in a simple search task. Perception & Psychophysics, 58, 969–976. doi:10.3758/BF03206825
Hooge, I. T. C., & Frens, M. A. (2000). Inhibition of saccade return (ISR): Spatio-temporal properties of saccade programming. Vision Research, 40, 3415–3426. doi:10.1016/S0042-6989(00)00184-X
Hooge, I. T. C., Over, E. A. B., van Wezel, R. J. A., & Frens, M. A. (2005). Inhibition of return is not a foraging facilitator in saccadic search and free viewing. Vision Research, 45, 1901–1908. doi:10.1016/j.visres.2005.01.030
Hou, R. L., & Fender, D. H. (1979). Processing of direction and magnitude by the saccadic eye-movement system. Vision Research, 19, 1421–1426.
Itti, L., & Koch, C. (2001). Computational modelling of visual attention. Nature Reviews Neuroscience, 2, 194–203. doi:10.1038/35058500
Ivanoff, J., & Klein, R. (2001). The presence of a nonresponding effector increases inhibition of return. Psychonomic Bulletin & Review, 8, 307–314.
Ivanoff, J., & Klein, R. M. (2004). Stimulus–response probability and inhibition of return. Psychonomic Bulletin & Review, 11, 542–550. doi:10.3758/BF03196608
Keech, T. D., & Resca, L. (2010). Eye movement trajectories in active visual search: Contributions of attention, memory, and scene boundaries to pattern formation. Attention, Perception, & Psychophysics, 72, 114–141. doi:10.3758/APP.72.1.114
Klein, R. (1988). Inhibitory tagging system facilitates visual search. Nature, 334, 430–431. doi:10.1038/334430a0
Klein, R. M. (2000). Inhibition of return. Trends in Cognitive Sciences, 4, 138–147. doi:10.1016/S1364-6613(00)01452-2
Klein, R. M., & Hilchey, M. D. (2011). Oculomotor inhibition of return. In S. Liversedge, I. D. Gilchrist & S. Everling (Eds.), The Oxford handbook of eye movements. Oxford: Oxford University Press
Klein, R. M., & MacInnes, W. J. (1999). Inhibition of return is a foraging facilitator in visual search. Psychological Science, 10, 346–352. doi:10.1111/1467-9280.00166
Komoda, M. K., Festinger, L., Phillips, L. J., Duckman, R. H., & Young, R. A. (1973). Some observations concerning saccadic eye movements. Vision Research, 13, 1009–1020.
Kowler, E., Anderson, E., Dosher, B., & Blaser, E. (1995). The role of attention in the programming of saccades. Vision Research, 35, 1897–1916. doi:10.1016/0042-6989(94)00279-U
Ludwig, C. L., Farrell, S., Ellis, L. A., & Gilchrist, I. D. (2009). The mechanism underlying inhibition of saccadic return. Cognitive Psychology, 59, 180–202.
Machado, L., & Rafal, R. D. (2004). Inhibition of return generated by voluntary saccades is independent of attentional momentum. Quarterly Journal of Experimental Psychology, 57, 789–796.
MacInnes, W. J., & Klein, R. M. (2003). Inhibition of return biases orienting during the search of complex scenes. The Scientific World Journal, 3, 75–86.
Malcolm, G. L., & Henderson, J. M. (2010). Combining top-down processes to guide eye movements during real-world scene search. Journal of Vision, 10(2), 4:1–11. doi:10.1167/10.2.4
Maylor, E. A., & Hockey, R. (1985). Inhibitory component of externally controlled covert orienting in visual space. Journal of Experimental Psychology. Human Perception and Performance, 11, 777–787. doi:10.1037/0096-1523.11.6.777
Melcher, D., & Kowler, E. (2001). Visual scene memory and the guidance of saccadic eye movements. Vision Research, 41, 3597–3611. doi:10.1016/S0042-6989(01)00203-6
Motter, B. C., & Belky, E. J. (1998). The guidance of eye movements during active visual search. Vision Research, 38, 1805–1815. doi:10.1016/S0042-6989(97)00349-0
Najemnik, J., & Geisler, W. S. (2005). Optimal eye movement strategies in visual search. Nature, 434, 387–391. doi:10.1038/nature03390
Navalpakkam, V., & Itti, L. (2005). Modeling the influence of task on attention. Vision Research, 45, 205–231. doi:10.1016/j.visres.2004.07.042
Nuthmann, A., Smith, T. J., Engbert, R., & Henderson, J. M. (2010). CRISP: A computational model of fixation durations in scene viewing. Psychological Review, 117, 382–405. doi:10.1037/a0018924
Parkhurst, D., Law, K., & Niebur, E. (2002). Modeling the role of salience in the allocation of overt visual attention. Vision Research, 42, 107–123. doi:10.1016/S0042-6989(01)00250-4
Peterson, M. S., Beck, M. R., & Vomela, M. (2007). Visual search is guided by prospective and retrospective memory. Perception & Psychophysics, 69, 123–135. doi:10.3758/BF03194459
Peterson, M. S., Kramer, A. F., & Irwin, D. E. (2004). Covert shifts of attention precede involuntary eye movements. Perception & Psychophysics, 66, 398–405.
Peterson, M. S., Kramer, A. F., Wang, R. F., Irwin, D. E., & McCarley, J. S. (2001). Visual search has memory. Psychological Science, 12, 287–292. doi:10.1111/1467-9280.00353
Pomplun, M., Reingold, E. M., & Shen, J. (2003). Area activation: A computational model of saccadic selectivity in visual search. Cognitive Science, 27, 299–312. doi:10.1016/S0364-0213(03)00003-X
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. In H. Bouma & D. Bouwhuis (Eds.), Attention and performance X (pp. 531–556). Hillsdale: Erlbaum.
Pratt, J., Adam, J. J., & McAuliffe, J. (1998). The spatial relationship between cues and targets mediates inhibition of return. Canadian Journal of Experimental Psychology, 52, 213–216. doi:10.1037/h0087294
Pratt, J., Spalek, T. M., & Bradshaw, F. (1999). The time to detect targets at inhibited and noninhibited locations: Preliminary evidence for attentional momentum. Journal of Experimental Psychology. Human Perception and Performance, 25, 730–746. doi:10.1037/0096-1523.25.3.730
Rao, R. P. N., Zelinsky, G. J., Hayhoe, M. M., & Ballard, D. H. (2002). Eye movements in iconic visual search. Vision Research, 42, 1447–1463. doi:10.1016/S0042-6989(02)00040-8
Ro, T., Pratt, J., & Rafal, R. D. (2000). Inhibition of return in saccadic eye movements. Experimental Brain Research, 130, 264–268.
Shepard, M., Findlay, J. M., & Hockey, R. J. (1986). The relationship between eye movements and spatial attention. Quarterly Journal of Experimental Psychology, 38A, 475–491.
Smith, T. J., & Henderson, J. M. (2009). Facilitation of return during scene viewing. Visual Cognition, 17, 1083–1108.
Smith, T. J., & Henderson, J. M. (2011). Looking back at Waldo: Oculomotor inhibition of return does not prevent return fixations. Journal of Vision, 11(1), 3:1–11. doi:10.1167/11.1.3
Snyder, J. J., Kingstone, A., & Schmidt, W. C. (2001). Attentional momentum does not underlie the inhibition of return effect. Journal of Experimental Psychology. Human Perception and Performance, 27, 1420–1432.
Sun, Y., Fisher, B., Wang, H., & Gomes, M. (2008). A computer vision model for visual-object-based attention and eye movements. Computer Vision and Image Understanding, 112, 126–142.
Tassinari, G., Aglioti, S., Chelazzi, L., Marzi, C. A., & Berlucchi, G. (1987). Distribution in the visual field of the costs of voluntarily allocated attention and of the inhibitory after-effects of covert orienting. Neuropsychologia, 25, 55–71.
Tatler, B. W. (2007). The central fixation bias in scene viewing: Selecting an optimal viewing position independently of motor biases and image feature distributions. Journal of Vision, 7(14), 4:1–17. doi:10.1167/7.14.4
Tatler, B. W., Baddeley, R. J., & Gilchrist, I. D. (2005). Visual correlates of fixation selection: Effects of scale and time. Vision Research, 45, 643–659. doi:10.1016/j.visres.2004.09.017
Tatler, B. W., & Vincent, B. T. (2008). Systematic tendencies in scene viewing. Journal of Eye Movement Research, 2, 1–18.
Tatler, B. W., & Vincent, B. T. (2009). The prominence of behavioural biases in eye guidance. Visual Cognition, 17, 1029–1054. doi:10.1080/13506280902764539
Thomas, L. E., Ambinder, M. S., Hsieh, B., Levinthal, B., Crowell, J. A., Irwin, D. E., et al. (2006). Fruitful visual search: Inhibition of return in a virtual foraging task. Psychonomic Bulletin & Review, 13, 891–895.
Unema, P. J. A., Pannasch, S., Joos, M., & Velichovsky, B. M. (2005). Time course of information processing during scene perception: The relationship between saccade amplitude and fixation duration. Visual Cognition, 12, 473–494. doi:10.1080/13506280444000409
Wang, Z., & Klein, R. M. (2010). Searching for inhibition of return in visual search: A review. Vision Research, 50, 220–228. doi:10.1016/j.visres.2009.11.013
Williams, C. C., Henderson, J. M., & Zacks, R. T. (2005). Incidental visual memory for targets and distractors in visual search. Perception & Psychophysics, 67, 816–827. doi:10.3758/BF03193535
Yarbus, A. L. (1967). Eye movements and vision. New York: Plenum Press.
Zelinsky, G. J. (2008). A theory of eye movements during target acquisition. Psychological Review, 115, 787–835. doi:10.1037/a0013118
Zelinsky, G. J., Rao, R. P. N., Hayhoe, M. M., & Ballard, D. H. (1997). Eye movements reveal the spatiotemporal dynamics of visual search. Psychological Science, 8, 448–453. doi:10.1111/j.1467-9280.1997.tb00459.x
Author Note
Thanks to Antje Nuthmann, the Edinburgh University Visual Cognition Lab, and the Eye Movement User Group for their feedback. We also thank Raymond Klein and Matt Hilchey for comments on an early version of this article. This project was supported by a grant from the Economic and Social Research Council U.K. (RES-062-23-1092) to J.M.H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, T.J., Henderson, J.M. Does oculomotor inhibition of return influence fixation probability during scene search?. Atten Percept Psychophys 73, 2384–2398 (2011). https://doi.org/10.3758/s13414-011-0191-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-011-0191-x