Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.2040
Revised: April 23, 2003
Accepted: May 11, 2003
Published online: September 15, 2003
AIM: To investigate the effect of gadolinium chloride (GaCl3) and salvia miltiorrhiza compound (SMCo) on ischemia and reperfusion (I/R) injury in hepatocellular mitochondria.
METHODS: Wistar rats were randomly to divided into control group, GaCl3 group, SMCo group and GaCl3 + SMCo group (n = 15 each). GaCl3 (7 mg·kg-1) was injected into tail vein on d 1 and d 2 in contrast group. SMCo (2 mL·kg-1) was injected into muscle on d 1 and d 2 in SMCo group. GaCl3 + SMCo group received both GaCl3 (iv) and SMCo (im) injection. Control group received saline injection only. On d 3, all the rats were subjected to 2 h ischemia in the middle and left lobes of the liver, followed by reperfusion for 2 h, 6 h and 18 h respectively. The level of serum alanine aminotransferase (ALT) and malondialdehyde (MDA) in hepatocellular mitochondria was measured. Pathological changes in hepatic tissue and in hepatocellular mitochondria were determined with optical microscope and electronic microscope, respectively.
RESULTS: Remarkablly pathohistological and biochemical changes were detected after 6 h of I/R. Compared with control, the level of ALT was decreased in GaCl3, SMCo and GaCl3 + SMCo treated groups (1314.0 ± 278.7 vs 809.4 ± 196.1, 716.6 ± 242.8 and 837.2 ± 190.6 IU·L-1, respectively. P < 0.05). Similarly, the level of MDA was decreased in GaCl3, SMCo and GaCl3 + SMCo treated groups (293.1 ± 51.1 vs 190.8 ± 55.5, 214.3 ± 32.9 and 221.0 ± 47.3 nmol·g-1, respectively, P < 0.05). Accordingly, in control group, swelling, degeneration, focal necrosis, infiltration of leucocyte were found in reperfused tissue under an optical microscope, and mitochondria swelling, rupture and even breakdown were seen under an electronic microscope. These pathohistological and ultrastructural damages caused by I/R were greatly attenuated in GaCl3, SMCo and GaCl3 + SMCo treated groups. However, there was no additive effect observed when GaCl3 and SMCo were used together.
CONCLUSION: Both GaCl3 and SMCo can alleviate the I/R injury in hepatocellular mitochondria.
- Citation: Zhang WH, Wang JS, Zhou Y, Li JY. Gadolinium chloride and salvia miltiorrhiza compound ameliorate reperfusion injury in hepatocellular mitochondria. World J Gastroenterol 2003; 9(9): 2040-2044
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/2040.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.2040
Lipid peroxide is one of the main mechanisms that cause ischemia and reperfusion (I/R) injury in the liver[1,2]. The degree of change in lipid peroxide and configuration of hepatocellular mitochondria primarily determine the severity of I/R injury[3]. Previous studies have shown that the number of Kupffer cell as well as its function are activated in response to I/R in the liver, which can be specifically inhibited by gadolinium chloride (GdCl3)[4,5]. Salvia miltiorrhiza compound (SMCo) is widely used to treat many kinds of diseases in clinic, because it can scavenge oxygen free radicles, therefore reducing oxidative stress[6,7]. Reducing I/R injury constitutes a big clinical challenge and depends on the elucidation of the pathological and molecular process of I/R injury. The aim of the present study was to investigate the roles of lipid peroxide and configuration of hepatocellular mitochondria in response to I/R as well as the effect of GaCl3 and SMCo on the process.
Wistar rats (male, 8 wk old, weight 200-250 g) were randomly divided into control group, GaCl3 group, SMCo group, GaCl3 + SMCo group (n = 15 each).
Gadolinium chloride hexahydrate (Wako pure medicine, Japan) was dissolved in physiological saline (2 g·L-1), which was injected into tail vein at a dose of 7 mg·kg-1 on d 1 and d 2 in GaCl3 group. Salvia miltiorrhiza compound (First Shanghai pharmacy) was injected into hind limb muscle at a dose of 2 mL·kg-1 on d 1 and d 2 in SMCo group. The GaCl3 + SMCo group received both GaCl3 and SMCo injection. The control group received saline only on d 1 and d 2.
On d 3, after 12 h fasting, each rat was anesthetized with sodium thiopental at a dose of 50 mg·kg-1 (im). A midline incision was made to expose the liver vessels. The arteries to middle and left lobes of the liver were dissected and the blood flow was blocked with a pair of vessel clamps. The abdomen was then closed. The blood flow was restored by releasing the clamps after 2 h. The blood reperfusion in each group lasted for 2 h, 6 h and 18 h respectively (n = 5 for each time point).
The blood sample was taken from heart 2 h, 6 h and 18 h after reperfusion for ALT assay.
The liver was excised and washed with saline solution, cleaned with filter paper and then homogenized with 0.25 g·L-1 saccharose-EDTA solution. The homogenized solution was centrifugated for 10 min in 1000 g of centrifugal force, and the supernatant was taken out and centrifugated again for 15 min in 7000 g. The deposit after second centrifugation, which contained mitochondria, was reserved. All approaches were performed at 4 °C and each procedure was repeated three times to make mitochondria pure.
The separated mitochondria was fixed by entering 2 mL of 25 g·L-1-tris phosphoric acid glutaradehyde for 2 h and then dehydrated bleach routinely. After embedded with EPSONB for 48 h, an extreme thin slice was made and lead acetate-uranium double staining was performed. The ultrastructure of mitochondria was observed under electronic microscopy.
The separated mitochondria were mixed with 0.125 mol·L-1 kalium chloride up to 1 mL. The content of MDA in hepatocellular mitochondria was determined with biuret reaction at the 525 nm of wave.
The hepatic tissue fixed with formaldehyde was embedded into paraffine, slices were made and stained with HE. Pathological changes were observed under optical microscopy.
Analysis of variance and Student-newman-Keuls test were used to determine the difference between groups. The results were considered statistically significant when P < 0.05.
In control group, ALT became significantly activated 6 h after reperfusion. In GaCl3, SMCo and GaCl3 + SMCo treated groups, ALT levels were all significantly inhibited compared with control group (Table 1).
The dynamic changes of MDA were identical to the pattern of ALT (Table 2).
In the control group, the major change of hepatocytes after 2 h of I/R was swelling without necrosis. The swelling became more and more severe with some scattered and focal necrosis and deposition of fibrin in central lobular vein after 6 h of I/R. In addition to obvious swelling, denaturalization and necrosis were seen after 18 h of I/R, which were associated with destroyed hepatic sinuses and infiltration of numerous white blood cells. In GaCl3, SMCo and GaCl3 + SMCo groups, there was no necrosis 2 h and 6 h after I/R, and the hepatocyte swelling and denaturalization were much less than those in control group. In treated groups, the hepatic structure was maintained with less necrosis and infiltration of leucocyte 18 h after I/R.
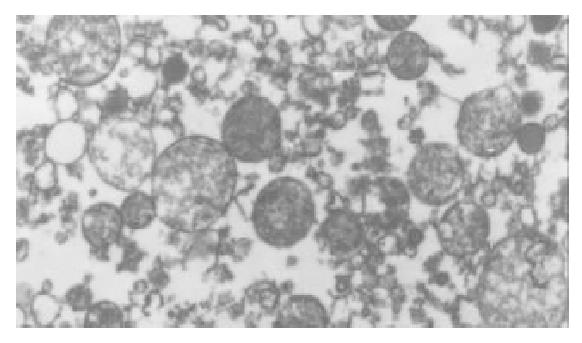
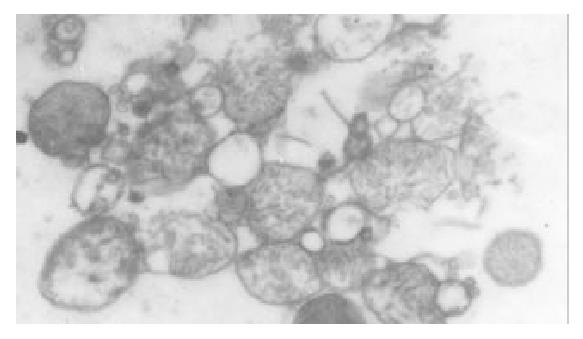
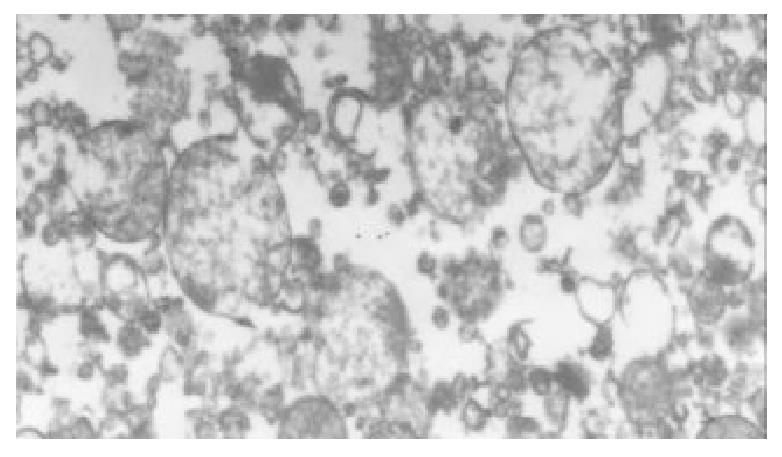
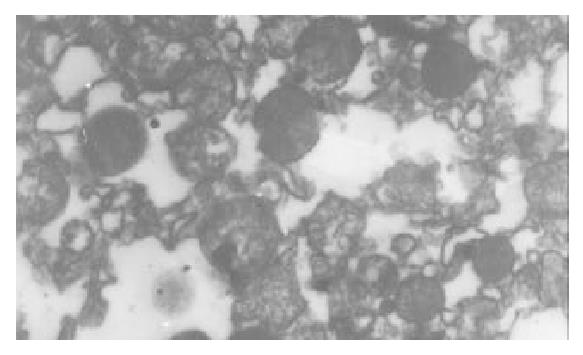
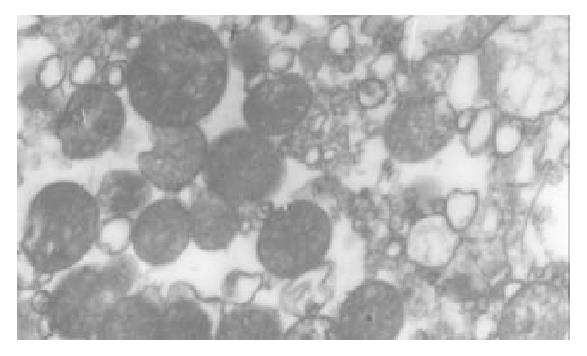
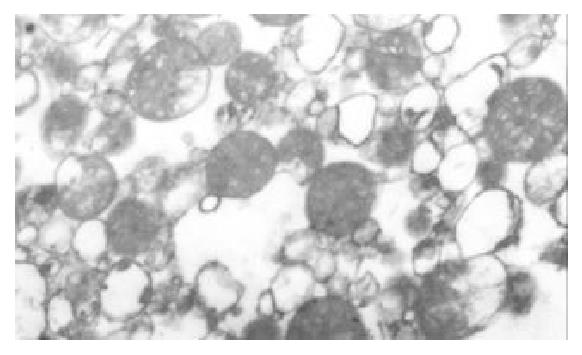
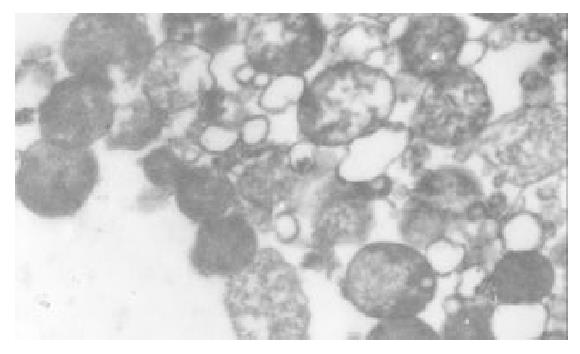
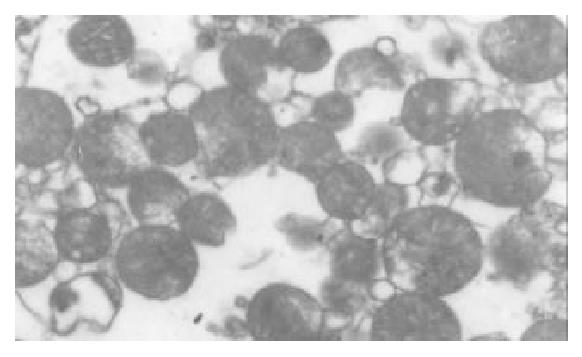
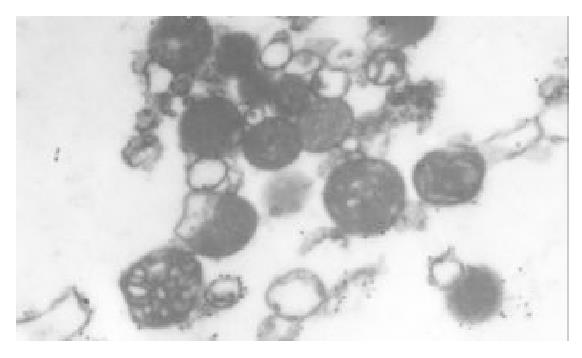
The I/R injury in hepatocellular mitochondria became more severe with the prolongation of I/R time in control group. It was obvious that swelling, rupture of inner membrane ridge, damage or even breakdown of mitochondrial structure with outline only (Figure 1, Figure 2, Figure 3). The changes in GaCl3 and SMCo group were less severe than those in control group. Except for slight swelling, the structure of mitochondria was unchanged. The protective effects of GaCl3 and SMCo against I/R damage on mitochondria were seen not only 2 h, 6 h, and 18 h after I/R (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). The effect of GaCl3 + SMCo combination on mitochondria was similar to that of either GaCl3 or SMCo alone.
Hepatocellular mitochondria are the place of energy metabolism in hepatocytes. In response to the process of I/R, the mitochondrion produces excessive oxidative free radicle, which in turn result in lipid peroxidation of the mitochondria membrane, therefore, causing mitochondria damage. Sammut observed that the degree of I/R injury in renal mitochondria of rabbit was associated with the preserved time of the kidney. The longer the cold preserved time was, the easier the I/R injury developed[8]. Leducq found that opening of mitochondrial permeability transition pore resulted in mitochondrial and energetic dysfunction of the liver during normothermic reperfusion[9]. These studies have directly or indirectly proved that I/R injury in hepatocellular mitochondria is closely related with the structure of hepatocellular mitochondria and the extent of damage in function[10].
The experimental results from the present study showed that ALT increased after 2 h of I/R, and reached the highest value after 6 h of I/R in control group, indicating that I/R injury in hepatic tissues is maximized 6 h after I/R. Previous studies have suggested that activation of Kupffer cells is involved in the injury process at the early stage of liver I/R. GaCl3 can effectively blockade the function of Kupffer cells and reduce the secretion of cytokines and reactive oxygen species from activated Kupffer cells, so that MDA in hepatocellular mitochondria is inhibited[11]. The fact that both ALT and MDA in mitochondria in GaCl3 group were markedly decreased because of the use of GaCl3 suggests that inhibition of Kupffer cells can protect the hepatic function[12]. This study also showed that there was no significant decrease in ALT and MDA 18 h after I/R, which was in agreement with the hypothesis that inhibition of Kupffer cells could only decrease the injury in the liver at the early stage of I/R. These biochemistry alterations are in parallel to the pathohistological changes. In control group, slight but detectable structural change was seen after 2 h, severe changes including necrosis and denaturalization were seen after 6 h, and more severe changes were seen after 18 h following I/R. In the mean time, by inhibiting Kupffer cells, GaCl3 remarkably ameliorated those detrimental changes, in particular, 6 h after I/R. It should be pointed out that the role played by Kupffer cells is only one of the factors that is involved I/R injury in the liver. In addition to GaCl3, other endogenous and exogenous compounds have also shown protective effects. For example, hepatocyte growth factor could reduce the impairment of both hepatocytes and sinusoidal endothelial cells[13]. Administration of epoprostenol could decrease hepatocellular I/R injury in transplanted liver[14], and nonselective endothelin-1 receptor antagonists could improve hepatic microcirculatory impairment[15]. Special neutrophil eastase inhibitor[16], tissue factor pathway inhibitor[17], adenosine[18], precondition of low-dose TNF-α[19] or ischemic precondition[20,21], etc, could alleviate I/R injury. All of these suggest that I/R is a complicated process. Although the potential role of Kupffer cells in I/R injury is still controversial[22,23], our data provide the strongest evidence in favor of its important role because blockade of Kupffer cells greatly reduces I/R injury in hepatocellular mitochondria.
Our data showed that SMCo treatment decreased the content of MDA in mitochondria in SMCo group. As a scavenger of oxygen free radicls, the SMCo can effectively inhibit lipid peroxidation in hepatocellular mitochondria[24,25], apoptosis of cells[26], and adhesion of neutrophil[27], etc, and accordingly protect hepatocellular mitochondria. Whereas simple inhibition of lipid peroxidation is not enough to prevent entirely the occurrence of I/R injury since a lot of factors are involved. Our results imply that SMCo can protect the function and structure of mitochondria, maintain energy metabolism of hepatocytes, and thereby reduce significantly I/R injury in the liver. Because a lot of factors are involved in I/R injury, it is understandable that inhibition of lipid peroxidation by SMCo can improve, but not prevent I/R injury.
Although both GaCl3 and SMCo are able to alleviate I/R injury in hepatocellular mitochondria when used separately, there is no additive effect when used in combination. We speculate that the two compounds act at different level in a same mechanism. While GaCl3 inhibits mainly the functions of Kupffer cells, decreases the production of oxygen free radicls, SMCo is mainly to promote microcirculation and to restrain lipid peroxidation. Therefore, the effect of SMCo will become insignificant when oxygen free radicls are reduced when GaCl3 is used.
In conclusion, both GaCl3 and SMCo can ameliorate I/R injury in hepatocellular mitochondria at some extent.
Edited by Su Q and Wang XL
| 1. | Nguyen WD, Kim DH, Alam HB, Provido HS, Kirkpatrick JR. Polyethylene glycol-superoxide dismutase inhibits lipid peroxidation in hepatic ischemia/reperfusion injury. Crit Care. 1999;3:127-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 28] [Reference Citation Analysis (0)] |
| 2. | Rhoden E, Pereira-Lima L, Lucas M, Mauri M, Rhoden C, Pereira-Lima JC, Zettler C, Petteffi L, Belló-Klein A. The effects of allopurinol in hepatic ischemia and reperfusion: experimental study in rats. Eur Surg Res. 2000;32:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Jeon BR, Lee SM. S-adenosylmethionine protects post-ischemic mitochondrial injury in rat liver. J Hepatol. 2001;34:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kojima Y, Suzuki S, Tsuchiya Y, Konno H, Baba S, Nakamura S. Regulation of pro-inflammatory and anti-inflammatory cytokine responses by Kupffer cells in endotoxin-enhanced reperfusion injury after total hepatic ischemia. Transpl Int. 2003;16:231-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lázár G, Paszt A, Kaszaki J, Duda E, Szakács J, Tiszlavicz L, Boros M, Balogh A, Lázár G. Kupffer cell phagocytosis blockade decreases morbidity in endotoxemic rats with obstructive jaundice. Inflamm Res. 2002;51:511-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sugiyama A, Zhu BM, Takahara A, Satoh Y, Hashimoto K. Cardiac effects of salvia miltiorrhiza/dalbergia odorifera mixture, an intravenously applicable Chinese medicine widely used for patients with ischemic heart disease in China. Circ J. 2002;66:182-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Peng B, Du J, Jia Q, Qiao A, Wu Y, Liu X, Qiang Q. [The effect of salvia miltiorrhiza and shengmai on inflammatory mediator and renal function of post-operative patients with obstructive jaundice]. Huaxi Yike Daxue Xuebao. 2001;32:587-589. [PubMed] [Cited in This Article: ] |
| 8. | Sammut IA, Burton K, Balogun E, Sarathchandra P, Brooks KJ, Bates TE, Green CJ. Time-dependent impairment of mitochondrial function after storage and transplantation of rabbit kidneys. Transplantation. 2000;69:1265-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Leducq N, Delmas-Beauvieux MC, Bourdel-Marchasson I, Dufour S, Gallis JL, Canioni P, Diolez P. Mitochondrial and energetic dysfunctions of the liver during normothermic reperfusion: protective effect of cyclosporine and role of the mitochondrial permeability transition pore. Transplant Proc. 2000;32:479-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Grattagliano I, Vendemiale G, Lauterburg BH. Reperfusion injury of the liver: role of mitochondria and protection by glutathione ester. J Surg Res. 1999;86:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] [Cited in This Article: ] |
| 12. | von Frankenberg M, Golling M, Mehrabi A, Nentwich H, Thies J, Schaeffer F, Jahnke C, Bud O, Gebhard MM, Otto G. Destruction of Kupffer's cells increases total liver blood flow and decreases ischemia reperfusion injury in pigs. Transplant Proc. 1999;31:3253-3254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Takeda Y, Arii S, Kaido T, Niwano M, Moriga T, Mori A, Hanaki K, Gorrin-Rivas MJ, Ishii T, Sato M. Morphologic alteration of hepatocytes and sinusoidal endothelial cells in rat fatty liver during cold preservation and the protective effect of hepatocyte growth factor. Transplantation. 1999;67:820-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Klein M, Geoghegan J, Wangemann R, Böckler D, Schmidt K, Scheele J. Preconditioning of donor livers with prostaglandin I2 before retrieval decreases hepatocellular ischemia-reperfusion injury. Transplantation. 1999;67:1128-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Mitsuoka H, Suzuki S, Sakaguchi T, Baba S, Miwa M, Konno H, Nakamura S. Contribution of endothelin-1 to microcirculatory impairment in total hepatic ischemia and reperfusion injury. Transplantation. 1999;67:514-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Soejima Y, Yanaga K, Nishizaki T, Yoshizumi T, Uchiyama H, Sugimachi K. Effect of specific neutrophil elastase inhibitor on ischemia/reperfusion injury in rat liver transplantation. J Surg Res. 1999;86:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Yoshimura N, Kobayashi Y, Nakamura K, Yamagishi H, Oka T. The effect of tissue factor pathway inhibitor on hepatic ischemic reperfusion injury of the rat. Transplantation. 1999;67:45-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Chen XH, Bao MS, Li ZZ. Effects of adenosine and its mecha-nism in ischemic preconditioning in rat liver in vivo. Shijie Huaren Xiaohua Zazhi. 1999;7:298-299. [Cited in This Article: ] |
| 19. | Teoh N, Leclercq I, Pena AD, Farrell G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology. 2003;37:118-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kumamoto Y, Suematsu M, Shimazu M, Kato Y, Sano T, Makino N, Hirano KI, Naito M, Wakabayashi G, Ishimura Y. Kupffer cell-independent acute hepatocellular oxidative stress and decreased bile formation in post-cold-ischemic rat liver. Hepatology. 1999;30:1454-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Schauer RJ, Gerbes AL, Vonier D, op den Winkel M, Fraunberger P, Bilzer M. Induction of cellular resistance against Kupffer cell-derived oxidant stress: a novel concept of hepatoprotection by ischemic preconditioning. Hepatology. 2003;37:286-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Tsukamoto S, Ohkohchi N, Fukumori T, Orii T, Asakura T, Takayama J, Shibuya H, Kato H, Satomi S. Elimination of Kupffer cells and nafamostat mesilate rinse prevent reperfusion injury in liver grafts from agonal non-heart-beating donors. Transplantation. 1999;67:1396-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Jiang SL, Yao XX, Lu T. Inhibitory effect of Danshen on lipid peroxidation in mitochondria of hepatic fibrosis in rats. Shijie Huaren Xiaohua Zazhi. 2002;10:1253-1256. [Cited in This Article: ] |
| 25. | Liu P, Hu Y, Liu C, Zhu D. Effects of salviainolic acid A (SA-A) on liver injury: SA-A action on hepatic peroxidation. Liver. 2001;21:384-390. [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Zhang XL, Liu L, Jiang HQ. Salvia miltiorrhiza monomer IH764-3 induces hepatic stellate cell apoptosis via caspase-3 activation. World J Gastroenterol. 2002;8:515-519. [PubMed] [Cited in This Article: ] |
| 27. | Ren DC, Du GH, Zhang JT. Inhibitory effect of the water-soluble extract of Salvia miltiorrhiza on neutrophil-endothelial adhesion. Jpn J Pharmacol. 2002;90:276-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |