Published online Aug 15, 2002. doi: 10.3748/wjg.v8.i4.724
Revised: April 22, 2002
Accepted: April 27, 2002
Published online: August 15, 2002
AIM: To investigate the relationship between hyposmotic membrane stretch and muscarinic receptor agonist-induced depolarization of membrane potential in antral gastric circular myocytes of guinea-pig.
METHODS: Using whole cell patch-clamp technique recorded membrane potential and current in single gastric myocytes isolated by collagenase.
RESULTS: Hyposmotic membrane stretch hyperpolarized membrane potential from -60.0 mV ± 1.0 mV to -67.9 mV ± 1.0 mV. TEA (10 mmol/L), a nonselective potassium channel blocker significantly inhibited hyposmotic membrane stretch-induced hyperpolarization. After KCl in the pipette and NaCl in the external solution were replaced by CsCl to block the potassium current, hyposmotic membrane stretch depolarized the membrane potential from -60.0 mV ± 1.0 mV to -44.8 mV ± 2.3 mV (P < 0.05), and atropine (1 μmol/L) inhibited the depolarization of the membrane potential. Muscarinic receptor agonist Carbachol depolarized membrane potential from -60.0 mV ± 1.0 mV to -50.3 mV ± 0.3 mV (P < 0.05) and hyposmotic membrane stretch potentiated the depolarization. Carbachol induced muscarinic current (Icch) was greatly increased by hyposmotic membrane stretch.
CONCLUSION: Hyposmotic membrane stretch potentiated muscarinic receptor agonist-induced depolarization of membrane potential, which is related to hyposmotic membrane stretch-induced increase of muscarinic current.
- Citation: Li L, Jin NG, Piao L, Hong MY, Jin ZY, Li Y, Xu WX. Hyposmotic membrane stretch potentiated muscarinic receptor agonist-induced depolarization of membrane potential in guinea-pig gastric myocytes. World J Gastroenterol 2002; 8(4): 724-727
- URL: https://www.wjgnet.com/1007-9327/full/v8/i4/724.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i4.724
In gastrointestinal smooth muscle, stretch is a very important physiological stimulation. However, the first quantitative electrophysiological evidence about such a myogenic response emerged until an experiment was done that the recordings of tension and intracellular membrane potential were simultaneously carried out in the rabbit taenia coli[1,2,4]. It has been demonstrated that when the muscle was stretched the tension relates closely to the depolarization of the membrane potential and the frequency of the action potential[1-4], but the mechanism is not very clear that how stretch excited cell membrane and caused smooth muscle contraction. It is well known that muscarinic current is nonselective cation current which can depolarize membrane potential[5,6] and contribute to calcium influx[7]. In our previous study we found that mechanical stretch increased the voltage-dependent calcium current[8-10], and Kirber et al[11,12]. reported that stretch activated ion channel (SACs) existed in gastric myocytes. In gut smooth muscle muscarinic current is very important for regulating motility of smooth muscle, but the relationship is not clear between muscarinic current and membrane stretch. In 1997, Waniishi et al[13] reported that hyposmotic membrane stretch increased muscarinic current in guinea-pig ileum. But the relationship of mechanical stretch and muscarinic receptor agonist-induced depolarization of membrane potential is not clear in gastric smooth muscle. Thus in this study, the relationship between hyposmotic membrane stretch and muscarinic receptor agonist-induced depolarization of membrane potential was investigated in antral gastric circular myocytes of guinea-pig.
Guinea-pigs (obtained from the Experimental Animal Department of Norman Bethune University, Certificate No 10-6004) of either sex weighing 300-350 g were anaesthetized by urethane (50 mg/kg). The antral part of the stomach was promptly excised and equilibrated in a Ca2+-free solution which was oxygenated, then the circular muscle layer was separated from the muscle layer and dissected into small segments (1 × 4 mm). These segments were kept in a modified Kraft-Bruhe (K-B) medium at 4 °C for 15 min. Then they were incubated at 36 °C in 4 mL digestion medium (Ca2+-free PSS) containing 0.1% collagenase (II), 0.1% dithioerythreitol, 0.15% trypsin inhibitor and 0.2% bovine serum albumin for 25-35 min. Then the softened muscle segments were transferred into the modified K-B medium, and single cells were dispersed by gentle agitation with a wide-bored fire-polished glass pipette. Isolated gastric myocytes were kept in modified K-B medium at 4 °C until use.
Isolated cells were transferred to a small chamber (0.1 mL) on the stage of an inverted microscope (IX-70 Olympus, Japan) for 10-15 min to settle down, and continuously superfused with isosmotic physiologic salt solution (PSS) by gravity (2-3 mL/min). Glass pipettes with a resistance of 2-5 MΩ were used to make a giga seal of 5-10 GΩ. Whole-cell currents and Membrane potential were recorded with an Axopatch 1-D patch-clamp amplifier (Axon Instrument, USA) polygraph (RM 6200, Nihon Kohden, Tokyo, Japan).
Ca2+-free PSS containing (in mmol/L) NaCl 134.8, KCl 4.5, glucose 5, and N-[2-hydroxyethyl] piperazine-N-[2-ethanesulphonic acid] (HEPES) 10 was adjusted to pH7.4 with Tris [hydroxymethyl] aminomethane (TRIZMA). Modified K-B solution containing (in mmol/L) L-glutamate 50, KCl 50, taurine 20, KH2PO4 20, MgCl2. H2O3, glucose 10, HEPES 10 and egtazic acid 0.5 was adjusted to pH7.40 with KOH. Isosmotic solution containing (in mmol/L) NaCl 80, KCl 4.5, MgCl2. H2O 1, CaCl2. H2O 2, glucose 5, HEPES 10, sucrose 110, was adjusted to pH7.4 with Tris; CsCl 99, HEPES 10, Sucrose 90, was adjusted to pH7.40 with tris. Hyposmotic solution containing (in mmol/L) NaCl 80, KCl 4.5, MgCl2. H2O 1, CaCl2. H2O 2, glucose 5, HEPES 10 was adjusted to pH7.40 with tris; CsCl 99, HEPES 10, was adjusted to pH7.40 with Tris. Pipette solution containing (in mmol/L) K-aspartic acid 110, Mg-ATP 5, MgCl2. H2O 1, KCl 20, EGTA 10, di-tris-creatine phosphate 2.5, disodium-creatine phosphate 2.5, HEPES 5, pH was adjusted to 7.30 with KOH; CsCl 135, Na2ATP 3, MgCl2 3, di-tris-creatine phosphate 2.5, disodium-creatine phosphate 2.5, HEPES 5, EGTA 0.5 was adjusted to pH7.30 with tris. Tetraethylammonium (TEA), Carbachol (CCh) and Atropine were made up as stock solutions (1 mol/L, 10 mmol/L and 1 μmol/L respectively). All drugs in this experiment were purchased from Sigma (USA).
Cells were superfused with normal and hypotonic solution.
Data were expressed as ¯x ± s. Statistical significance was estimated by paired t-test. P < 0.05 were considered to be statistically significant.
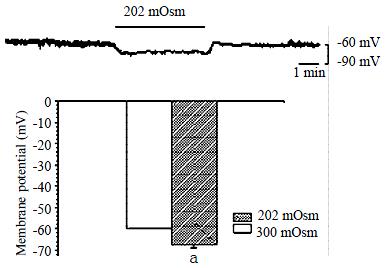
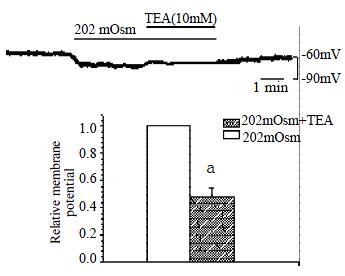
Membrane current was clamped by the conventional whole-cell patch clamp configuration and membrane potential was modulated to about -60.0 mV, so that it is close to resting potential of the gastric myocyte. After being superfused with hyposmotic solution (202 mOsm), the membrane potential hyperpolarized from -60.0 ± 1.0 mV to -67.9 ± 1.0 mV in a few seconds,and the amplitude of polarization was increased by 13.4% ± 1.6% (Figure 1, n = 10, P < 0.05). TEA, a nonspecific potassium channel blocker (10 mmol/L) significantly inhibited the hyperpolarization induced by hyposmotic membrane stretch, the membrane potential depolarized from -70.9 ± 2.2 mV to ± 65.7 ± 1.6 mV, inhibited from 100% of control to 47.8% ± 6.6% (n = 7, P < 0.05, Figure 2). In the condition of KCl in the pipette and NaCl in the external solution were replaced by CsCl to block the potassium current, hyposmotic membrane stretch depolarized membrane potential from -60.0 ± 1.0 mV to -44.8 ± 2.3 mV (n = 5, P < 0.05, Figure 3).
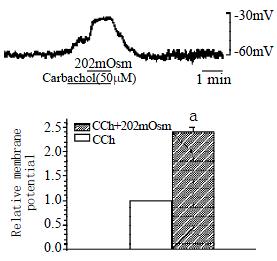
Potassium current was still blocked by CsCl, muscarinic receptor agonist carbachol depolarized membrane potential from -60.0 ± 1.0 mV to -50.3 ± 0.3 mV and hyposmotic membrane stretch potentiated the depolarization of the membrane potential from -50.3 ± 0.3 mV to -37.3 ± 1.8 mV, the potentiated percentage is 239.4% ± 10.6% (n = 3, P < 0.05, Figure 4). When the membrane potential depolarized by hyposmotic membrane stretch, atropine (1 μmol/L) inhibited the depolarization, the membrane potential hyperpolarized from -47.3 ± 2.4 mV to -53.0 ± 1.9 mV, inhibited from 100% of control to 47.6% ± 4.7% (n = 4, P < 0.05, Figure 5).
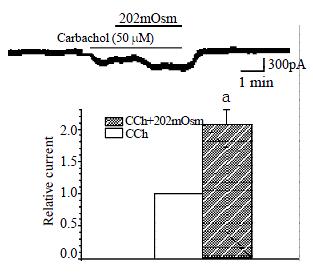
In conventional whole-cell patch clamp configuration, membrane potential was clamped at -20 mV, inward current was induced by carbachol and the mean value was 183.3 ± 30.7 pA. Hyposmotic membrane stretch significantly increased muscarinic current induced by carbachol from 183.3 ± 30.7 pA to 383.3 ± 73.8 pA, and the increased percentage is 206.9% ± 23.4% (n = 4, P < 0.05, Figure 6).
The simple and main method of stretching cell membrane is hyposmotic cell swelling, in this study, we applied hyposmotic cell swelling to investigated the relationship between hyposmotic membrane stretch and muscarinic receptor agonist-induced depolarization of membrane potential. In present study, we observed that hyposmotic membrane stretch hyperpolarized membrane potential; TEA, a nonselective potassium channel blocker significantly inhibited hyposmotic membrane stretch-induced hyperpolarization. After KCl in the pipette and NaCl in the external solution were replaced by CsCl to block the potassium current, hyposmotic membrane stretch depolarized the membrane potential, and atropine inhibited the depolarization of the membrane potential. Muscarinic receptor agonist carbachol depolarized membrane potential and hyposmotic membrane stretch potentiated the depolarization; carbachol induced muscarinic current (Icch) was greatly increased by hyposmotic membrane stretch.
It is must be mentioned here that we have applied hyposmotic membrane stretch which is not same as mechanical stretch under physiological condition, but it is the common method for stretching cell membrane now. It is well known that almost every kind of cells are able to regulate their volume so that they are still in the normal physiological condition[14,15]. In this experiment, hyposmotic cell swelling is inevitable to elicit the reaction resist to volume increase, so calcium sensitive potassium channels were activated and have potassium and chloride ion efflux to decrease cell volume, our previous studies have proved it[16,17]. In this work, hyposmotic membrane stretch includes two kinds of stimulation, one is cell volume increase; the other is cell membrane stretch, so it is inevitable to activate potassium channel. However, the focus of our study is the relationship between membrane stretch and muscarinic receptor agonist-induced depolarization of membrane potential, so we blocked potassium current to observe depolarization induced by muscarinic receptor agonist clearly. When the cell superfused with hyposmotic solution, the membrane potential was hyperpolarized, but in the condition of KCl in the pipette and NaCl in the external solution were replaced by CsCl to block the potassium current, hyposmotic membrane stretch depolarized membrane potential. Therefore the hyperpolarization induced by hyposmotic stress related to activation of potassium channel which is very important mechanism in cell volume regulation. In gut smooth muscle, stretch is very important physiological stimulation, the stretch caused smooth muscle contraction. There are two theories about the mechanism of stretch-induced smooth muscle contraction, one is nervous mechanism; the other is muscular mechanism. But the ionic channel event in muscular mechanism is not very clear. It is reported that stretch activated channel (SACs) were activated by membrane stretch in gastric myocyte, and this channel is not voltage dependent and nonselective cation[18,19]. Our previous study found that hyposmotic membrane stretch increased L-type calcium current and it is related to cytoskeleton in gastric myocytes of guinea-pig[8,9]. Waniishi et al[13]. observed that hyposmotic membrane stretch increase carbachol-induced muscarinic nonselective cation current obviously in ileum smooth muscle of guinea-pig. In present study, hyposmotic membrane stretch potentiated carbachol-induced depolarization of membrane potential and M receptor blocker, atropine significantly inhibited hyposmotic membrane stretch-induced depolarization. Since in the condition of clamping membrane potential, hyposmotic membrane stretch significantly increased carbachol-induced muscarinic current, so hyposmotic membrane stretch via increasing muscarinic current potentiated carbachol-induced depolarization of membrane potential.
Acetylcholine is very important for regulating gastrointestinal smooth muscle motility. When the muscarinic receptor is activated by acetylcholine, it not only releases the calcium from the calcium store through the G-protein path way[20], but also activates a nonselective cation channel which can depolarize the membrane potential. Activation of the muscarinic receptor operated channel is followed by the entry of the cations such as calcium, and it induces the contraction of the muscle eventually. Our results demonstrated that in the gut myocytes, the mechanical stretch is probably one of the important mechanisms of regulating the muscarinic receptor operated channel. Therefore when the stomach is distended by food in physiological condition, gastric tonic contraction is potentiated via long and short- vagus nerve reflex, in another way, stretch may be potentiated the effect of vagus nerve on gastric tonic contraction through increasing muscarinic current. Since hyposmotic membrane stretch potentiats carbachol-induced depolarization through increasing muscarinic current, it may be also one mechanism in smooth muscle contraction caused by stretch.
Edited by Zhao M
| 1. | BUELBRING E, KURIYAMA H. THE EFFECT OF ADRENALINE ON THE SMOOTH MUSCLE OF GUINEA-PIG TAENIA COLI IN RELATION TO THE DEGREE OF STRETCH. J Physiol. 1963;169:198-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Coburn RF. Stretch-induced membrane depolarization in ferret trachealis smooth muscle cells. J Appl Physiol (1985). 1987;62:2320-2325. [PubMed] [Cited in This Article: ] |
| 3. | Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 292] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Harder DR, Gilbert R, Lombard JH. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am J Physiol. 1987;253:F778-F781. [PubMed] [Cited in This Article: ] |
| 5. | Inoue R, Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990;424:57-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 104] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Chen S, Inoue R, Ito Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br J Pharmacol. 1993;109:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Kim SJ, Koh EM, Kang TM, Kim YC, So I, Isenberg G, Kim KW. Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes. J Physiol. 1998;513:749-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Xu WX, Kim SJ, Kim SJ, So I, Kang TM, Rhee JC, Kim KW. Effect of stretch on calcium channel currents recorded from the antral circular myocytes of guinea-pig stomach. Pflugers Arch. 1996;432:159-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Xu WX, Kim SJ, So I, Kim KW. Role of actin microfilament in osmotic stretch-induced increase of voltage-operated calcium channel current in guinea-pig gastric myocytes. Pflugers Arch. 1997;434:502-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Xu WX, Li Y, Wu LR, Li ZL. Effects of different kinds of stretch on voltage-dependent calcium current in antrial circular smooth muscle cells of the guinea-pig. Shengli Xuebao. 2000;52:69-74. [PubMed] [Cited in This Article: ] |
| 11. | Kirber MT, Walsh JV, Singer JJ. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988;412:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 167] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Kirber MT, Guerrero-Hernández A, Bowman DS, Fogarty KE, Tuft RA, Singer JJ, Fay FS. Multiple pathways responsible for the stretch-induced increase in Ca2+ concentration in toad stomach smooth muscle cells. J Physiol. 2000;524 Pt 1:3-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Waniishi Y, Inoue R, Ito Y. Preferential potentiation by hypotonic cell swelling of muscarinic cation current in guinea pig ileum. Am J Physiol. 1997;272:C240-C253. [PubMed] [Cited in This Article: ] |
| 14. | Baumagar CM, Feher JJ. Osmosis and the regulation of cell volume. In: Sperelakis N (ed) Cell Physiol Source Book 1995; 194-211. [Cited in This Article: ] |
| 15. | Hoffmann EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989;69:315-382. [PubMed] [Cited in This Article: ] |
| 16. | Piao L, Li Y, Li L, Jin NG, Li ZL, Xu WX. The involvement of calcium mobilization in the calcium-activated potassium currents activated by hyposmotic swelling in gastric antral circular myocytes of the guinea-pig. Jpn J Physiol. 2001;51:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Piao L, Li Y, Li L, Xu WX. Increment of calcium-activated and delayed rectifier potassium current by hyposmotic swelling in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2001;22:566-572. [PubMed] [Cited in This Article: ] |
| 18. | Xu WX, Kim SJ, So I, Kang TM, Rhee JC, Kim KW. Volume-sensitive chloride current activated by hyposmotic swelling in antral gastric myocytes of the guinea-pig. Pflugers Arch. 1997;435:9-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992;262:C1083-C1088. [PubMed] [Cited in This Article: ] |
| 20. | Komori S, Kawai M, Takewaki T, Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992;450:105-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |