Published online Mar 7, 2022. doi: 10.3748/wjg.v28.i9.933
Peer-review started: September 19, 2021
First decision: November 7, 2021
Revised: November 8, 2021
Accepted: January 27, 2022
Article in press: January 27, 2022
Published online: March 7, 2022
Few studies have been conducted on sex differences in the incidence, pathophysiology, and prognosis of gastric cancer (GC).
To analyze the differences in GC characteristics according to sex in patients who underwent surgical treatment for GC.
A total of 2983 patients diagnosed with gastric adenocarcinoma who received surgical treatment at the Seoul National University Bundang Hospital between 2003 and 2017 were included. Baseline clinicopathological characteristics, histologic type of GC, overall and GC-specific survival rates, and associated risk factors were analyzed.
Among the 2983 patients, 2005 (67.2%) and 978 (32.8%) were males and females, respectively. The average age of the female group (59.36 years) was significantly younger than that of the male group (61.66 years; P < 0.001). Cancer of the gastric body (P < 0.001) and diffuse-type histology (P < 0.001) were more common in females than in males. This trend was more prominent in females younger than 60 years of age, with a significantly higher proportion of diffuse-type cancer than in the male group. Regardless of sex, diffuse-type GC was more common in younger patients, and the proportion of intestinal-type GC increased with age. The overall survival rate was significantly higher in females (P < 0.001). However, this difference disappeared for GC-specific survival (P = 0.168), except for the poor GC-specific survival rate in advanced-stage cancer (stage III or above) in females (P = 0.045). The risk factors for GC-related mortality were older age, upper location of GC, and diffuse- or mixed-type histology. In terms of comorbidities, more males died from diseases other than GC, including other malignancies such as lung cancer, hepatocellular carcinoma, and pancreatic cancer, and respiratory diseases such as interstitial lung disease and chronic obstructive pulmonary disease, while there were relatively more cardiovascular or cerebrovascular deaths in females.
Sex-based differences in GC were observed in clinicopathological features, including age at diagnosis, tumor location, histologic type, survival rate, and comorbidities.
Core Tip: In the analyses of sex differences in gastric cancer (GC), the sex ratio between males and females was 2:1, but the incidence of diffuse-type cancer was higher in females until the age of 60 years. The average age of the female group was significantly younger, and cancer of the gastric body and diffuse-type histology were more common than those in the males. In addition, there was poor GC-specific survival rate in advanced-stage cancer in females, while comorbidities including cancers of other organs and respiratory diseases were more common in males.
- Citation: Choi Y, Kim N, Kim KW, Jo HH, Park J, Yoon H, Shin CM, Park YS, Lee DH, Oh HJ, Lee HS, Park YS, Ahn SH, Suh YS, Park DJ, Kim HH, Kim JW, Kim JW, Lee KW, Chang W, Park JH, Lee YJ, Lee KH, Kim YH. Sex-based differences in histology, staging, and prognosis among 2983 gastric cancer surgery patients. World J Gastroenterol 2022; 28(9): 933-947
- URL: https://www.wjgnet.com/1007-9327/full/v28/i9/933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i9.933
Gastric cancer (GC) is the fifth most common cancer and the third most common cause of cancer-related deaths worldwide[1]. Age-standardized incidence rates are approximately twice as high in males than in females[2]. Major risk factors for developing GC include Helicobacter pylori (H. pylori) infection, family history of GC, dietary habits, ionizing radiation, smoking, alcohol, and pernicious anemia, and the difference in incidence between males and females is likely due to the difference in exposure to these risk factors[3-5]. However, these factors alone do not fully explain the different characteristics of GC between the sexes. Recent research has revealed the role of sex hormones in various diseases and the resulting sex differences. It is well known that sex differences exist in the location and prognosis of various cancers, including colorectal cancer[6,7], renal cell carcinoma[8], and bladder cancer[9]. In addition, sex differences are also known in central nervous system diseases such as cognitive disorders, Alzheimer’s disease[10], Parkinson’s disease[11], and autoimmune diseases[12]. However, the role of sex hormones such as testosterone and estrogen in the etiology, response to therapy, and survival of patients with GC, and the involved mechanisms and pathways remain unclear. Also, few studies to date have detailed the epidemiological and prognostic differences in GC between males and females.
Lauren classification is an independent prognostic factor in patients with GC[13]. That is, intestinal-type GC shows better clinicopathological characteristics and prognosis than diffuse-type GC. Diffuse-type cancer exhibits a higher recurrence rate than intestinal-type cancer, and the clinical appearance and survival of mixed-type cancers are known to be similar to those of diffuse-type GC[14]. Environmental factors reportedly play an important role in the development of intestinal-type versus diffuse-type GC[15]. There is also a sex-based difference in the histologic type of GC. That is, there is a marked predominance of older age and male sex in intestinal-type GC and a younger female predominance in diffuse-type GC. Younger female patients seem to exhibit a higher percentage of diffuse-type GC, resulting in more aggressive tumor behavior[14]; therefore, treatment methods may vary according to the Lauren type[16]. From this point of view, we hypothesized that an accurate analysis of sex-based differences in GC is possible in a well-designed surgical cohort with regular follow-up observations, clear histologic results, accurate information on family history, and social history such as smoking and alcohol consumption. Based on this background, the aim of this study was to analyze the sex-based differences in clinicopathological features and staging in GC, and to investigate prognostic factors including survival and death.
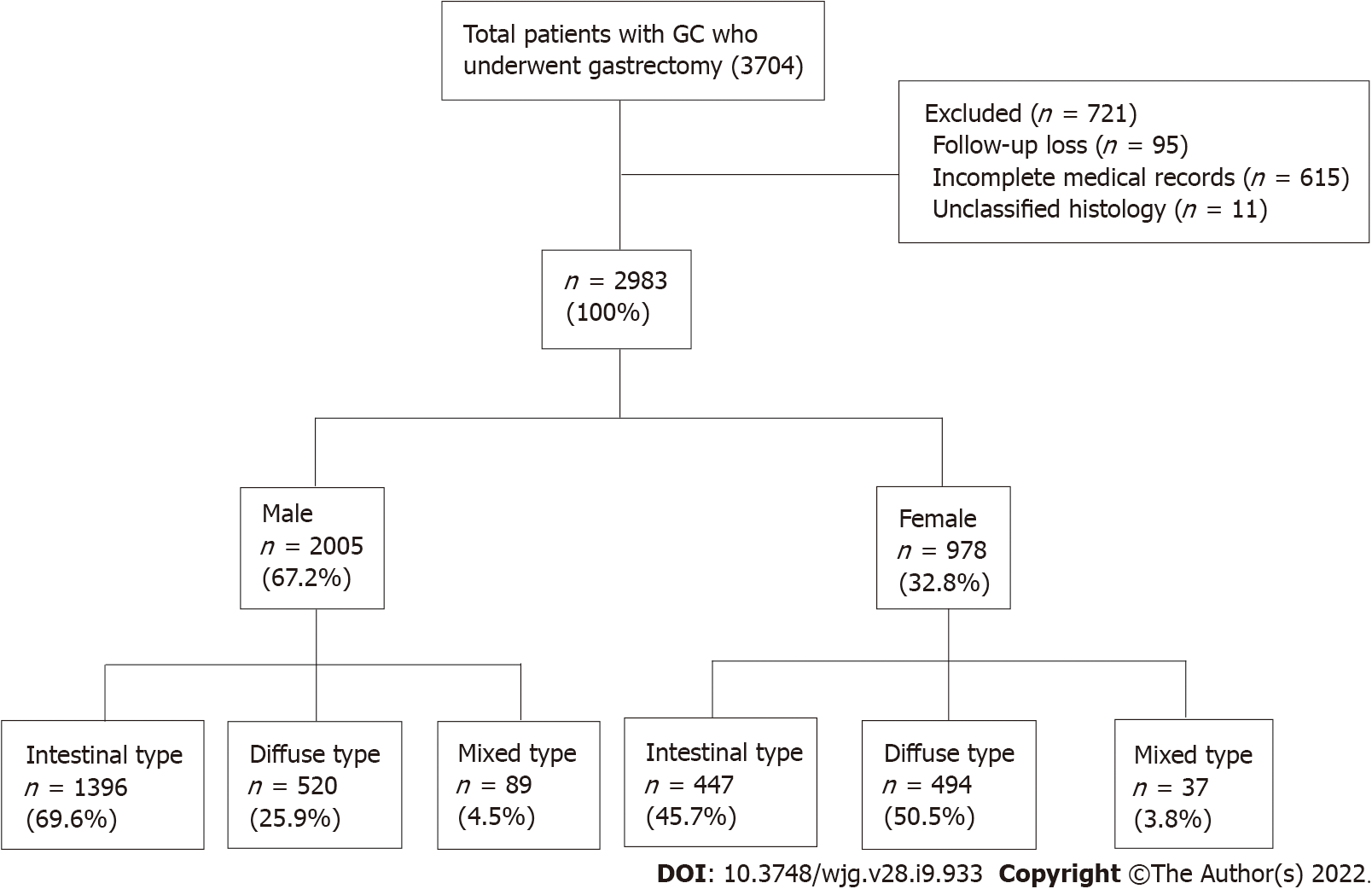
Initially, 3074 patients aged > 18 years were selected from a prospective surgical cohort of patients who were diagnosed with gastric adenocarcinoma and underwent surgical treatment at Seoul National University Bundang Hospital (SNUBH) between 2003 and 2017 (Figure 1). Analyses of the effects of H. pylori eradication treatment, P53 overexpression and the incidence of metachronous GC in this cohort were previously published by our team[17-19]. The following patients were excluded: those with incomplete medical records or unclassified histology, who were lost to follow-up, had a prior history of other cancers at the time of diagnosis, or those who had other diseases with inoperable severity were excluded from the study. Finally, 2983 patients were included in the analysis (Figure 1). The medical records of these patients, including sex, age, death (including cause), histologic type of cancer, and social history such as alcohol consumption, smoking, and family history of GC were collected from surgical and medical cohorts, and reviewed using the Clinical Data Warehouse. The dates and causes of death of the enrolled patients were cross-reviewed with data from the National Statistical Office for verification.
The outcomes were overall survival and GC-specific survival. Univariate and multivariate Cox proportional hazards analyses were used to identify risk factors, and variables with a P value < 0.2 in the univariate analyses, were used as covariates for the multivariate analysis. The Kaplan–Meier estimator method and log-rank tests were used to compare survival. Analyses were performed using IBM SPSS Statistics software (version 25.0; IBM Corp., Armonk, NY, United States). Statistical significance was set at P < 0.05. All data are available upon request from the corresponding author.
The study was reviewed and approved by the Institutional Review Board of SNUBH (IRB No. B-1902–523-107) and registered at clinicaltrials.gov (NCT03978481). All authors have access to the study data and have approved the final manuscript.
The baseline clinicopathological features of the subjects are shown in Table 1. Of the 2983 patients, 2005 were males and 978 were females, indicating a 2:1 sex ratio, with an average age of 61.66 for males and 59.36 for females, indicating a significantly younger onset age in females (P < 0.001). A higher proportion of males had a history of alcohol consumption and smoking (drinking history, P < 0.001; smoking history, P < 0.001). Cancer of the gastric body and diffuse-type cancer were more common in females (tumor location, P < 0.001; histologic type, P < 0.001, respectively). Overexpression of P53 was more common in males than in females (P < 0.001). There were no differences in family history, cancer staging, or H. pylori infection at the time of diagnosis between males and females (family history, P = 0.548; cancer stage, P = 0.189; and H. pylori status, P = 0.062, respectively).
| Characteristics | Total (N = 2983) | Female (n = 978) | Male (n = 2005) | P value |
| Age (yr, mean ± SD) | 60.91 ± 12.31 | 59.36 ± 13.47 | 61.66 ± 11.63 | < 0.001a |
| Drinking history, n (%) | ||||
| No | 1631 (54.7) | 790 (80.8) | 841 (41.9) | < 0.001a |
| Yes | 1352 (45.3) | 188 (19.2) | 1164 (58.1) | |
| Smoking history, n (%) | ||||
| No | 1645 (55.1) | 902 (92.2) | 743 (37.1) | < 0.001a |
| Yes | 1338 (44.9) | 76 (7.8) | 1262 (62.9) | |
| Family history, n (%) | ||||
| No | 2467 (82.7) | 803 (82.1) | 1664 (83.0) | 0.548 |
| Yes | 516 (17.3) | 175 (17.9) | 341 (17.0) | |
| Tumor location, n (%) | ||||
| Upper | 77 (2.6) | 19 (2.0) | 58 (2.9) | < 0.001a |
| Middle | 1332 (44.6) | 497 (50.8) | 835 (41.6) | |
| Lower | 1574 (52.8) | 462 (47.2) | 1112 (55.5) | |
| Atrophic gastritis, n (%) | ||||
| No | 2162 (72.5) | 751 (76.8) | 1411 (70.4) | < 0.001a |
| Yes | 821 (27.5) | 227 (23.2) | 594 (29.6) | |
| Intestinal metaplasia, n (%) | ||||
| No | 1680 (56.3) | 560 (57.3) | 1120 (55.9) | 0.469 |
| Yes | 1303 (43.7) | 418 (42.7) | 885 (44.1) | |
| T stage, n (%) | ||||
| T1 | 2134 (71.5) | 696 (71.2) | 1438 (71.7) | 0.669 |
| T2 | 330 (11.1) | 102 (10.4) | 228 (11.4) | |
| T3 | 420 (14.1) | 144 (14.7) | 276 (13.8) | |
| T4 | 99 (3.3) | 36 (3.7) | 63 (3.1) | |
| N stage, n (%) | ||||
| N0 | 2215 (74.3) | 696 (71.2) | 1519 (75.7) | 0.014a |
| N1 | 423 (14.2) | 163 (16.7) | 260 (13.0) | |
| N2 | 174 (5.8) | 66 (6.7) | 108 (5.4) | |
| N3 | 171 (5.7) | 53 (5.4) | 118 (5.9) | |
| Stage, n (%) | ||||
| I | 2312 (77.5) | 743 (76.0) | 1569 (78.3) | 0.189 |
| II | 405 (13.6) | 151 (15.4) | 254 (12.7) | |
| III | 212 (7.1) | 69 (7.1) | 143 (7.1) | |
| IV | 54 (1.8) | 15 (1.5) | 39 (1.9) | |
| Cancer type, n (%) | ||||
| EGC | 2133 (71.5) | 696 (71.2) | 1437 (71.7) | 0.774 |
| AGC | 850 (28.5) | 282 (28.8) | 568 (28.3) | |
| Histologic type, n (%) (Lauren classification) | ||||
| Intestinal | 1843 (61.8) | 447 (45.7) | 1396 (69.6) | < 0.001a |
| Diffuse | 1014 (34.0) | 494 (50.5) | 520 (25.9) | |
| Mixed | 126 (4.2) | 37 (3.8) | 89 (4.5) | |
| H. pylori status, n (%) | ||||
| Negative | 1267 (42.5) | 379 (38.8) | 888 (44.3) | 0.004a |
| Positive | 1716 (57.5) | 599 (61.2) | 1117 (55.7) | |
| P53, n (%) | ||||
| Negative | 1917 (64.3) | 706 (72.2) | 1211 (60.4) | < 0.001a |
| Positive | 1066 (35.7) | 272 (27.8) | 794 (39.6) |
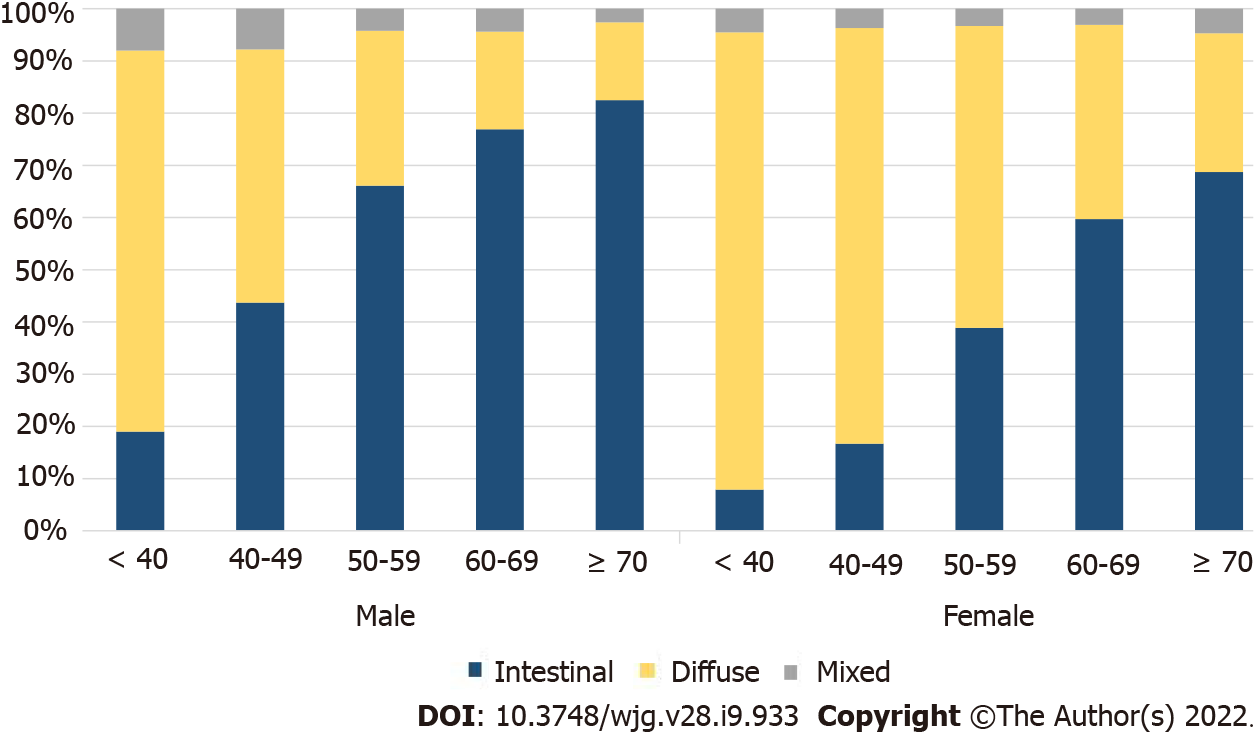
To identify the histological changes in GC by age, the entire group of patients was divided into groups of under 40, 40-49, 50-59, 60-69, and 70+ years, and the trend of an increasing percentage of intestinal-type cancers with age, in both males and females, was noted (Supplementary Table 1). Considering the number of patients and histological ratios, there were more female patients under the age of 40 years and older male patients (Supplementary Figure 1). A higher number of female GC patients were under 40 years of age, while diffuse-type cancer was more common in both males and females than the other histological types (Figure 2). Among the male patients, the proportion of intestinal-type cancer increased steeply from age 50 years, whereas in female patients, the proportion of diffuse-type cancer remained high at 50-59 years of age (Figure 2). The ratio of intestinal- and diffuse-type GC in females approximately 20 years after menopause was similar to that of male patients aged ≥ 70 years (Figure 2).
Meanwhile, a significant correlation was observed between histological type and GC location, with a high ratio of diffuse-type cancer and stomach body cancer in females and a high ratio of intestinal-type cancer and stomach antral cancer in males (Pearson correlation analysis, P < 0.001).
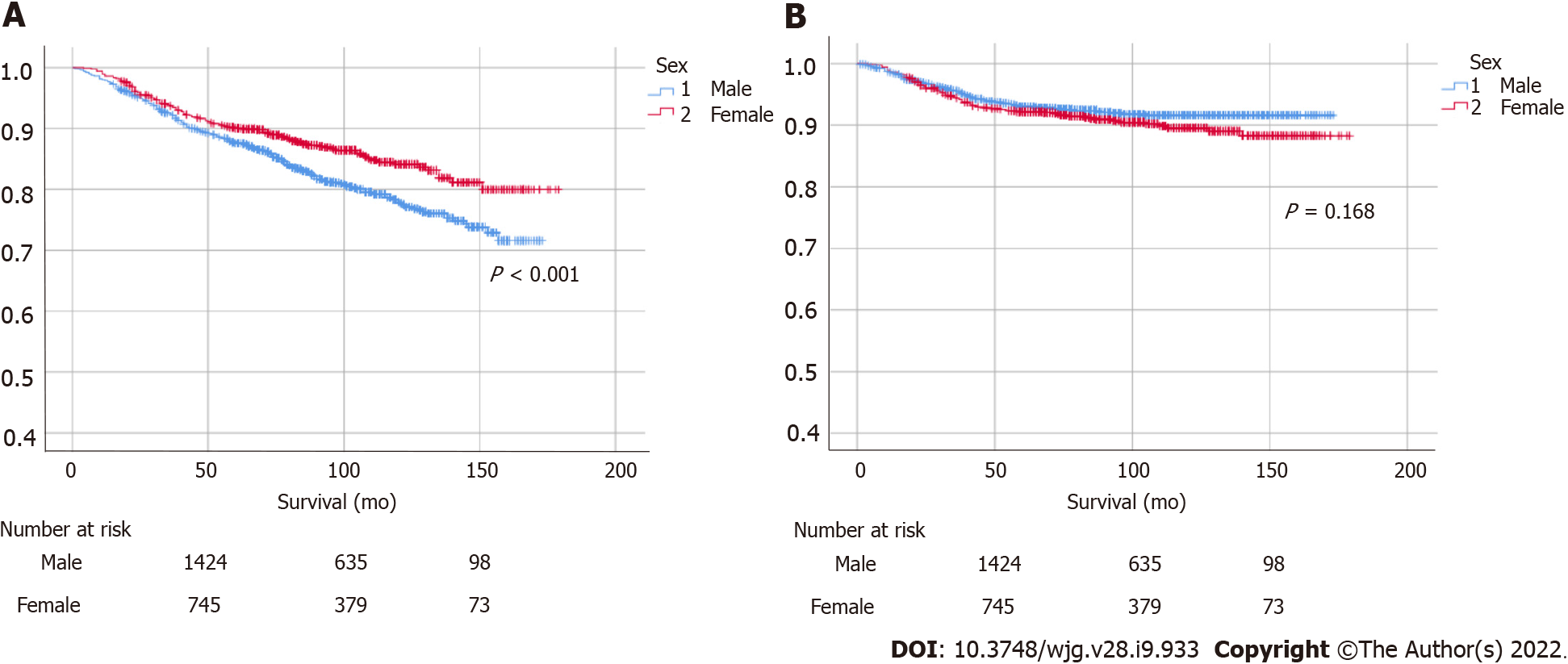
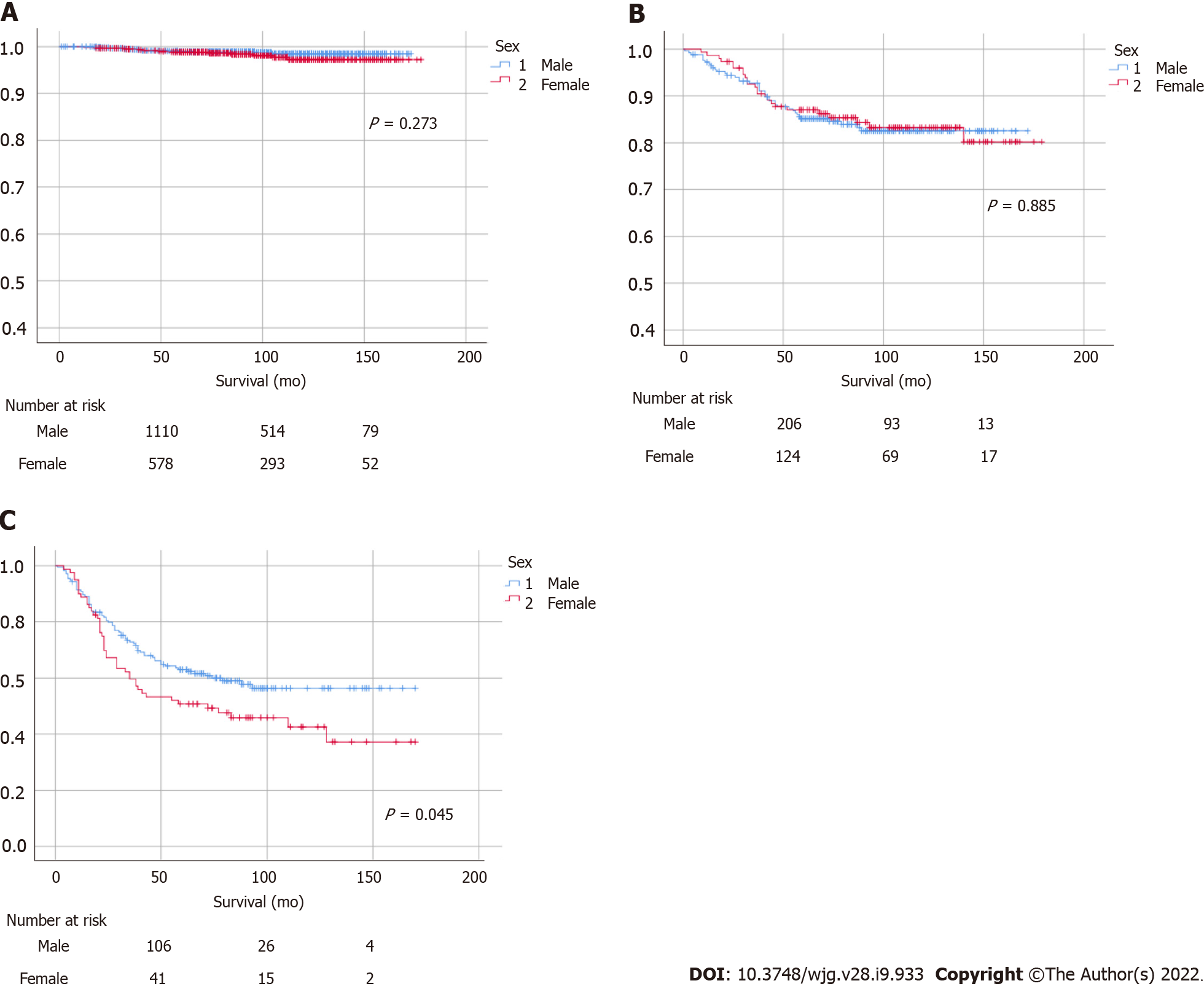
A statistically significant female predominance was identified in overall survival (P < 0.001), while a non-significant male predominance was identified in GC-specific survival (Figure 3). Increasing age, proximal tumor location, and diffuse- or mixed-type histology were identified as risk factors for GC-related morbidity (Table 2). In terms of cancer stage, there were no significant differences in patients with stage I or II GC, whereas a statistically significant male predominance was observed in patients with advanced-stage cancer (stage III or above, P = 0.045; Figure 4). Histologically, patients with intestinal-type GC had a significantly higher survival rate than those with diffuse-type GC, and there were no statistically significant differences between males and females in intestinal- or diffuse-type GC (Supplementary Figure 2).
| Variable | Univariate analysis | P value | Multivariate analysis | P value |
| Sex | ||||
| Male | Ref | 0.169 | Ref | 0.672 |
| Female | 1.22 (0.92-1.61) | 1.06 (0.80-1.42) | ||
| Age | ||||
| < 60 | Ref | 0.001a | Ref | < 0.001a |
| ≥ 60 | 1.64 (1.23-2.18) | 2.02 (1.50-2.73) | ||
| Drinking history | ||||
| No | Ref | 0.996 | ||
| Yes | 1.00 (0.76-1.32) | |||
| Smoking history | ||||
| No | Ref | 0.283 | ||
| Yes | 1.16 (0.88-1.53) | |||
| Family history | ||||
| No | Ref | 0.189 | Ref | 0.165 |
| Yes | 0.77 (0.51-1.14) | 0.75 (0.51-1.12) | ||
| Tumor location | ||||
| Upper | Ref | 0.003a | Ref | < 0.001a |
| Middle | 1.65 (0.41-6.71) | 1.40 (0.34-5.71) | ||
| Lower | 2.61 (0.65-10.54) | 2.63 (0.65-10.64) | ||
| Atrophic gastritis | ||||
| No | Ref | 0.871 | ||
| Yes | 0.97 (0.71-1.34) | |||
| Intestinal metaplasia | ||||
| No | Ref | 0.412 | ||
| Yes | 0.89 (0.67-1.18) | |||
| Histologic type (Lauren classification) | ||||
| Intestinal | Ref | < 0.001a | Ref | < 0.001a |
| Diffuse | 2.16 (1.62-2.89) | 3.07 (2.25-4.19) | ||
| Mixed | 2.25 (1.29-3.92) | 2.50 (1.43-4.35) | ||
| P53 | ||||
| Negative | Ref | 0.651 | ||
| Positive | 1.07 (0.80-1.42) |
In the assessment of comorbidities, we investigated sex-based causes of death. Patients with a prior history of other cancers at the time of diagnosis or having severe diseases with inoperable conditions were excluded from the study, as mentioned above. Among the patients, 453 died including 135 males (6.7%) and 86 females (8.8%) died of GC. Significantly more males died from diseases other than GC (193 males and 39 females). In males, there were more deaths from malignancies such as lung cancer, hepatocellular carcinoma, pancreatic cancer, and respiratory diseases such as interstitial lung disease and chronic obstructive pulmonary disease, while there were relatively more cardiovascular or cerebrovascular deaths in females. Details regarding these are given in Supplementary Tables 2-4.
The results of the subgroup analyses based on sex and histology are presented in Table 3 and Supplementary Table 3. In females, intestinal-type GC was associated with older age and a family history of GC, while diffuse-type GC was associated with younger age and P53 negativity. In males, intestinal-type GC was associated with older age, while diffuse-type GC tended to be associated with younger age and smoking history.
| Characteristics | Intestinal type | Diffuse type | ||||
| Female (n = 447) | Male (n = 1396) | P value | Female (n = 494) | Male (n = 520) | P value | |
| Age (yr, mean ± SD) | 65.72 ± 10.71 | 64.06 ± 10.34 | 0.792 | 53.60 ± 13.06 | 55.82 ± 12.48 | 0.229 |
| Drinking history, n (%) | ||||||
| No | 385 (86.1) | 619 (44.3) | < 0.001a | 374 (75.7) | 183 (35.2) | < 0.001a |
| Yes | 62 (13.9) | 777 (55.7) | 120 (24.3) | 337 (64.8) | ||
| Smoking history, n (%) | ||||||
| No | 420 (94.0) | 530 (38.0) | < 0.001a | 447 (90.5) | 175 (33.7) | < 0.001a |
| Yes | 27 (6.0) | 866 (62.0) | 47 (9.5) | 345 (66.3) | ||
| Family history, n (%) | ||||||
| No | 348 (77.9) | 1138 (81.5) | 0.088 | 419 (84.8) | 447 (86.0) | 0.606 |
| Yes | 99 (22.1) | 258 (18.5) | 75 (15.2) | 73 (14.0) | ||
| Tumor location, n (%) | ||||||
| Upper | 12 (2.7) | 44 (3.2) | 0.053 | 7 (1.4) | 10 (1.9) | < 0.001a |
| Middle | 139 (31.1) | 517 (37.0) | 334 (67.6) | 284 (54.6) | ||
| Lower | 296 (66.2) | 835 (59.8) | 153 (31.0) | 226 (43.5) | ||
| Atrophic gastritis, n (%) | ||||||
| No | 320 (71.6) | 951 (68.1) | 0.168 | 400 (81.0) | 389 (74.8) | 0.018a |
| Yes | 127 (28.4) | 445 (31.9) | 94 (19.0) | 131 (25.2) | ||
| Intestinal metaplasia, n (%) | ||||||
| No | 229 (51.2) | 751 (53.8) | 0.344 | 303 (61.3) | 306 (58.8) | 0.418 |
| Yes | 218 (48.8) | 645 (46.2) | 191 (38.7) | 214 (41.2) | ||
| T stage, n (%) | ||||||
| T1 | 355 (79.4) | 1087 (77.9) | 0.529 | 314 (63.6) | 305 (58.6) | 0.445 |
| T2 | 40 (9.0) | 138 (9.9) | 57 (11.5) | 66 (12.7) | ||
| T3 | 40 (9.0) | 145 (10.4) | 99 (20.0) | 118 (22.7) | ||
| T4 | 12 (2.6) | 26 (1.8) | 24 (4.9) | 31 (6.0) | ||
| N stage, n (%) | ||||||
| N0 | 356 (79.6) | 1140 (81.7) | 0.745 | 319 (64.6) | 335 (64.4) | 0.055 |
| N1 | 56 (12.5) | 149 (10.7) | 97 (19.6) | 85 (16.4) | ||
| N2 | 20 (4.5) | 62 (4.4) | 42 (8.5) | 38 (7.3) | ||
| N3 | 15 (3.4) | 45 (3.2) | 36 (7.3) | 62 (11.9) | ||
| Stage, n (%) | ||||||
| I | 375 (83.9) | 1171 (83.9) | 0.49 | 342 (69.2) | 347 (66.7) | 0.152 |
| II | 47 (10.5) | 142 (10.2) | 96 (19.5) | 89 (17.1) | ||
| III | 23 (5.1) | 65 (4.6) | 43 (8.7) | 66 (12.7) | ||
| IV | 2 (0.5) | 18 (1.3) | 13 (2.6) | 18 (3.5) | ||
| Cancer type, n (%) | ||||||
| EGC | 355 (79.4) | 1086 (77.8) | 0.469 | 314 (63.6) | 305 (58.7) | 0.109 |
| AGC | 92 (20.6) | 310 (22.2) | 180 (36.4) | 215 (41.3) | ||
| P53, n (%) | ||||||
| Negative | 293 (65.5) | 770 (55.2) | < 0.001a | 385 (77.9) | 393 (75.6) | 0.374 |
| Positive | 154 (34.5) | 626 (44.8) | 109 (22.1) | 127 (24.4) | ||
To the best of our knowledge, this study is the first to provide evidence of sex differences in GC with exact histologic diagnosis and long-term follow-up in nearly 3000 patients. In our data, a statistically significant overall survival benefit in females and a non-significant GC-specific survival in males were observed. In both males and females, a high proportion of diffuse-type cancers was observed among younger patients, while intestinal-type cancer became more prominent with increasing age. However, more females of all ages had diffuse-type cancer, while the ratio of diffuse-type to intestinal-type cancer was higher in females until the age of 60 years. In addition, the incidence of higher proportion of diffuse-type and gastric body cancers in females, compared to intestinal-type and antral cancers in males could be the reasons for higher N stage and poor GC-specific survival in females. Furthermore, there were also differences in comorbidities, including causes of death other than GC, between males and females.
There are few studies on the prognosis of GC by sex, and sex-based differences in GC are not clear, as in other cancers such as colorectal cancer[6,7]. A recent study based on the Surveillance, Epidemiology, and End Results (SEER) database in the United States reported survival advantages in females[20]. A female advantage was observed in both overall survival and GC-specific survival, and the prognosis of GC and the risk of developing GC were significantly worse in males than in females in that study, so the authors insisted on the necessity for early intervention in high-risk male patients due to their relatively poor prognosis[20]. However, this difference from our results could be due to a difference in the histologic type of GC, with a higher proportion of adenocarcinoma and a lower proportion of signet ring cell carcinoma (SRC), especially in females. In a large meta-analysis of data obtained from the Korea Central Cancer Registry and National Statistical Office reported by Song et al[21], the prognosis of female GC patients was also better than that of male GC patients, with differential incidence and mortality patterns among age groups. However, females tend to have a worse prognosis when they are diagnosed later than 40 years of age. In that study, the histologic type or anatomic subsites of GC could not be identified. Since the 2000s, many early GC (EGC) patients have been identified and treated through a national endoscopic surveillance project in Korea, possibly showing different results from data prior to the 2000s[22]. Contrary to earlier results, a recent study in Korea reported poor prognosis in females[23], similar to the present study. The authors concluded that female GC patients were significantly younger, had more poorly differentiated adenocarcinomas, and were more likely to have SRC than male GC patients. In addition, females with advanced GC (AGC) and SRC had significantly poorer overall survival rates. In our data, among patients with advanced-stage disease (stage III or above), females had significantly lower GC-specific survival rates than males. In our data, the ratio of diffuse-type (undifferentiated) GC was relatively high, especially among younger females. Diffuse-type GC is known to be related to genetic factors such as E-cadherin mutations, feature a poorer prognosis due to rapid growth and poor treatment response, and is generally more common in younger patients[24,25]. Comparing the characteristics of GC in Korea and the United States, more upper-third and differentiated cancers were observed in the United States, while Korean patients showed fewer upper-third cancers with poorer cancer differentiation, deeper invasion, and poorer prognosis; hence, a difference in GC characteristics between Korean and United States populations is suspected[26].
The same results have been shown in previous studies in relation to histologic type and GC location, as more diffuse-type, gastric body location cases were noted in females versus more intestinal-type, stomach antrum location cases in males[27,28]. Based on previous reports on GC location, the distribution was reported as cardia 4%-8%, body 15%-30%, and antrum 60%-80% in a study of EGC in Korea[29]. In another Korean study of patients who underwent endoscopic resection for EGC, the most common location for EGC was the antrum (57.5%) and lesser curvature (37.8%), and body cancers were associated with younger patient age, larger tumor size, and more frequent poorly differentiated or SRC histology than cancers at other sites[30]. Our data are consistent with those of the aforementioned studies, and this relation to histologic type and GC location is believed to be due to differences in the composition of gastric mucous cells, such as the gastric body with a large distribution of parietal cells and the antrum with a large distribution of G cells[31].
The effects of sex hormones may cause this sex-based difference in GC. Epidemiological studies have reported that exogenous sex hormone exposure reduces the risk of esophageal adenocarcinoma[32,33] and esophageal squamous cell carcinoma[34], and a decrease in the risk of GC[35] and colorectal cancer[33,36] have been reported in females taking oral contraceptives or hormone replacement therapy. A large cohort study in Japan also reported that females in early menarche had a decreased risk of GC, especially differentiated-type GC, in subgroup analyses of histologic subtypes[37]. In addition, in a Chinese study of approximately 2000 surgically treated GC patients, the proportion of female GC patients showed a decreasing tendency, and the proportion of male GC patients showed an increasing tendency with age, but this trend stopped after 60 years of age[38]. Furthermore, a recent study in Korea reported that no premenopausal females had intestinal-type GC and that the ratio of intestinal-type GC increased in females after menopause and became similar to that of males about 10 years after menopause; this parity was associated with an increased risk of intestinal-type GC in females after menopause[27].
These results suggest that estrogen plays a role in curbing the development of GC in females, especially in intestinal-type GC. However, the specific mechanisms of estrogen in different histologic subtypes have not yet been established. Several studies have attempted to explain this by investigating the role of estrogen receptors (ER) in GC. First, Yi et al[39] showed that ERα expression was associated with diffuse-type GC and shorter disease-free survival. Wang et al[40] reported that well-differentiated gastric adenocarcinoma has a higher expression rate of ERβ and that poorly differentiated gastric adenocarcinoma is associated with a reduction or loss of ERβ. According to previous studies, diffuse-type GC may be initiated by the downregulation of E-cadherin by 17β-estradiol (E2), the most potent isoform of estrogen, through ERα[41-43].
In addition to the action of estrogen, Gan et al[44] reported that the four sex hormone receptors, ERα, ERβ, progesterone receptor, and androgen receptor (AR), were expressed independently and showed a decreased expression pattern in gastric tumors compared to adjacent normal tissues, suggesting that sex hormone receptors may be partly involved in gastric carcinogenesis. Jukic et al[2] reported a significantly higher frequency of cases with AR-positive cells in the stroma of intestinal-type GC in males than in females, which may be the reason for the greater invasiveness of this cancer type in males and presented the possibility of AR-targeted agents in GC treatment. Another study by Hsu et al[45] showed that males were more likely to develop tumor recurrence and liver metastasis than females, especially in cases of stage III GC. The authors suggested that the cause was higher programmed death ligand 1 expression in males and GC patients aged 65 years or older, and supporting data suggest that sex hormones are the basis of these differences[46,47].
The changes in the proportion of intestinal-type and diffuse-type cancers in the present study suggest that estrogen might have a protective effect on intestinal-type GC[27,28]. Thus, intestinal-type GC is much less common in young females than in males, and the prevalence of intestinal-type GC increases in females after menopause, which is likely to be similar to males about 20 years after menopause according to our data (approximately 70 years of age). Additional in-depth studies are needed to confirm the role of sex hormones, including estrogen, in the pathogenesis and progression of GC, depending on the tissue type.
The pattern of P53 overexpression also differed by sex; P53 overexpression was more frequent in males and intestinal-type GC patients. In our previous report on P53 overexpression, the clinical and prognostic significance differed by histological type of GC; P53 overexpression was more common in intestinal-type GC, but was associated with a poor prognosis for diffuse-type GC[18]. Therefore, it is also likely to act as a factor that affects GC prognosis differently in males and females.
Our study has several limitations. First, the enrolled subjects were patients who underwent surgical treatment after receiving a diagnosis of GC; therefore, early cases treated with endoscopic resection and advanced inoperable cases were not included. Hence, in terms of GC-related survival and mortality, the data from our study are likely to be slightly different from those of all patients with GC. To compensate for this limitation, we are conducting a follow-up study of patients diagnosed with and treated for over 14000 GC in SNUBH. The results of our data analyses to this point showed no significant differences between males and females according to the treatment method. Second, there are no data on estrogen exposure such as menopause, childbirth, and breastfeeding in this study, making it difficult to provide additional evidence that estrogen has protective effects against intestinal-type cancer. Further research, including a history of sex hormone use, is required. Third, the eradication of H. pylori was not confirmed in all patients, although postoperative H. pylori eradication treatment may affect prognosis or survival[17]. In the future, additional research is needed that considers both H. pylori infection and sex. In contrast, our research has several strengths over existing studies. Studies involving subjects before the year 2000 reported that the prognosis of GC was relatively good in females; however, these studies did not reflect the situation in East Asia, where the prevalence of GC is high[20], or the exact histologic type of GC was not analyzed[21]. A relatively recent large-scale Korean study reflecting histological types reported that the prognosis of GC was poorer in females than in males, similar to our results[23]. In this study, the changes in the histology of GC according to age was examined, and the change in the ratio according to aging and menopause was confirmed, suggesting that female hormones would affect the development and progression of GC. However, we further analyzed the comorbidities of GC patients with respect to survival.
In conclusion, differences in the epidemiology of GC incidence, including a higher proportion of diffuse-type histology and mortality, and poorer survival in AGC in females, were observed. The proportion of diffuse-type cancer was found to be higher in younger patients, the frequency of intestinal-type histology increased with age, and the ratio of diffuse-type cancer was higher until the age of 60 years in females. Differences in Lauren histologic type and tumor location by sex were also observed, with a high proportion of diffuse-type and gastric body location in females. Comorbidities, including other malignancies and respiratory diseases, are more common in males. These differences may originate from hormonal factors and should be considered in the diagnosis, treatment, and prediction of prognosis of GC in individuals.
Despite the nationwide large-scale screening campaign, the incidence of gastric cancer (GC) in Korea is still high. The incidence is approximately twice as high in males than in females.
However, studies so far have not fully explained the different characteristics of GC between the sexes. These differences might be due to the difference in exposure to the known risk factors for GC, such as frequent Helicobacter pylori infection, smoking, and alcohol consumption in males, but we thought that there is a possibility that sex hormones were based on this difference.
This study aimed to analyze sex-based differences in clinicopathological features, staging, survival, and comorbidities in GC.
A total of 2983 patients diagnosed with gastric adenocarcinoma who received surgical treatment at the Seoul National University Bundang Hospital between 2003 and 2017 were included, and clinicopathological characteristics, histologic type of GC, overall and GC-specific survival rates, and associated risk factors were analyzed.
The male to female ratio was 2:1, and the average age of the female group was lower than that of the male group. Diffuse-type GC was more common in younger patients, especially in females younger than 60 years of age, and the proportion of intestinal-type GC increased with age. The overall survival rate was significantly higher in females, whereas GC-specific survival tended to be higher in males. Comorbidities, including other malignancies and respiratory diseases, are more common in males.
Differences in the epidemiology of GC incidence, including a higher proportion of diffuse-type histology, mortality, including poorer survival in the advanced stage in females, and comorbidities were observed. These differences may be due to hormonal factors.
We believe that a larger study including patients who received non-surgical treatment is needed. Individual sex hormone data, including menopause, childbirth, and breastfeeding, would be analyzed to prove the protective effect of estrogen against intestinal-type GC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu JB, Yu F S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1188] [Cited by in F6Publishing: 1424] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 2. | Jukic Z, Radulovic P, Stojković R, Mijic A, Grah J, Kruslin B, Ferencic Z, Fucic A. Gender Difference in Distribution of Estrogen and Androgen Receptors in Intestinal-type Gastric Cancer. Anticancer Res. 2017;37:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 4. | Massarrat S, Stolte M. Development of gastric cancer and its prevention. Arch Iran Med. 2014;17:514-520. [PubMed] [Cited in This Article: ] |
| 5. | Lee TY, Wang CB, Chen TT, Kuo KN, Wu MS, Lin JT, Wu CY; Taiwan Gastrointestinal Disease and Helicobacter Consortium. A tool to predict risk for gastric cancer in patients with peptic ulcer disease on the basis of a nationwide cohort. Clin Gastroenterol Hepatol. 2015;13:287-293.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | DeCosse JJ, Ngoi SS, Jacobson JS, Cennerazzo WJ. Gender and colorectal cancer. Eur J Cancer Prev. 1993;2:105-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 7. | Yang Y, Wang G, He J, Ren S, Wu F, Zhang J, Wang F. Gender differences in colorectal cancer survival: A meta-analysis. Int J Cancer. 2017;141:1942-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Mungan NA, Aben KK, Schoenberg MP, Visser O, Coebergh JW, Witjes JA, Kiemeney LA. Gender differences in stage-adjusted bladder cancer survival. Urology. 2000;55:876-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Li R, Singh M. Sex differences in cognitive impairment and Alzheimer's disease. Front Neuroendocrinol. 2014;35:385-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 11. | Cerri S, Mus L, Blandini F. Parkinson's Disease in Women and Men: What's the Difference? J Parkinsons Dis. 2019;9:501-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 254] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 12. | Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 13. | Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20:5679-5684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 116] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016;22:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Gong EJ, Lee JY, Bae SE, Park YS, Choi KD, Song HJ, Lee GH, Jung HY, Jeong WJ, Cheon GJ, Yook JH, Kim BS. Characteristics of non-cardia gastric cancer with a high serum anti-Helicobacter pylori IgG titer and its association with diffuse-type histology. PLoS One. 2018;13:e0195264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Mengardo V, Treppiedi E, Bencivenga M, Dal Cero M, Giacopuzzi S. Tailored treatment for signet ring cell gastric cancer. Updates Surg. 2018;70:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Choi Y, Kim N, Yun CY, Choi YJ, Yoon H, Shin CM, Park YS, Ahn SH, Joong Park D, Lee HS, Kim JW, Lee KW, Chang W, Park JH, Lee YJ, Lee KH, Kim YH, Lee DH, Kim HH. Effect of Helicobacter pylori eradication after subtotal gastrectomy on the survival rate of patients with gastric cancer: follow-up for up to 15 years. Gastric Cancer. 2020;23:1051-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Kim KW, Kim N, Choi Y, Kim WS, Yoon H, Shin CM, Park YS, Lee DH, Ahn SH, Park DJ, Kim HH, Lee HS, Kim JW, Lee KW, Chang W, Park JH, Lee YJ, Lee KH, Kim YH. Different effects of p53 protein overexpression on the survival of gastric cancer patients according to Lauren histologic classification: a retrospective study. Gastric Cancer. 2021;24:844-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Choi Y, Kim N, Yoon H, Shin CM, Park YS, Lee DH, Ahn SH, Suh YS, Park DJ, Kim HH. The Incidence and Risk Factors for Metachronous Gastric Cancer in the Remnant Stomach after Gastric Cancer Surgery. Gut Liver. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Li H, Wei Z, Wang C, Chen W, He Y, Zhang C. Gender Differences in Gastric Cancer Survival: 99,922 Cases Based on the SEER Database. J Gastrointest Surg. 2020;24:1747-1757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Song M, Kang D, Yang JJ, Choi JY, Sung H, Lee Y, Yoon HS, Choi Y, Kong SH, Lee HJ, Yang HK, Kim WH. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer. 2015;18:580-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, Jung KW, Lee CW, Choi IJ, Park EC, Lee D. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology. 2017;152:1319-1328.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 23. | Kim HW, Kim JH, Lim BJ, Kim H, Park JJ, Youn YH, Park H, Noh SH, Kim JW, Choi SH. Sex Disparity in Gastric Cancer: Female Sex is a Poor Prognostic Factor for Advanced Gastric Cancer. Ann Surg Oncol. 2016;23:4344-4351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Waldum HL, Fossmark R. Types of Gastric Carcinomas. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Ansari S, Gantuya B, Tuan VP, Yamaoka Y. Diffuse Gastric Cancer: A Summary of Analogous Contributing Factors for Its Molecular Pathogenicity. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Shim JH, Song KY, Jeon HM, Park CH, Jacks LM, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Is gastric cancer different in Korea and the United States? Ann Surg Oncol. 2014;21:2332-2339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Kim SM, Min BH, Lee J, An JY, Lee JH, Sohn TS, Bae JM, Kim JJ, Kang WK, Kim S, Choi MG. Protective Effects of Female Reproductive Factors on Lauren Intestinal-Type Gastric Adenocarcinoma. Yonsei Med J. 2018;59:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Jung YJ, Kim HJ, Park CH, Park SJ, Kim N. Effects of Reproductive Factors on Lauren Intestinal-Type Gastric Cancers in Females: A Multicenter Retrospective Study in South Korea. Gut Liver. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Kim SJ, Choi CW. Common Locations of Gastric Cancer: Review of Research from the Endoscopic Submucosal Dissection Era. J Korean Med Sci. 2019;34:e231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Kim K, Cho Y, Sohn JH, Kim DH, Do IG, Lee HJ, Do SI, Ahn S, Lee HW, Chae SW. Clinicopathologic characteristics of early gastric cancer according to specific intragastric location. BMC Gastroenterol. 2019;19:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Walsh JH, Lam SK. Physiology and pathology of gastrin. Clin Gastroenterol. 1980;9:567-591. [PubMed] [Cited in This Article: ] |
| 32. | Lagergren K, Lagergren J, Brusselaers N. Hormone replacement therapy and oral contraceptives and risk of oesophageal adenocarcinoma: a systematic review and meta-analysis. Int J Cancer. 2014;135:2183-2190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Green J, Czanner G, Reeves G, Watson J, Wise L, Roddam A, Beral V. Menopausal hormone therapy and risk of gastrointestinal cancer: nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer. 2012;130:2387-2396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Freedman ND, Lacey JV Jr, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer. 2010;116:1572-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:20-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Song J, Jin Z, Han H, Li M, Guo Y, Guo H, Guo W, He J. Hormone replacement therapies, oral contraceptives, reproductive factors and colorectal adenoma risk: a systematic review and dose-response meta-analysis of observational studies. Colorectal Dis. 2019;21:748-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Persson C, Inoue M, Sasazuki S, Kurahashi N, Iwasaki M, Ye W, Tsugane S; JPHC Study Group. Female reproductive factors and the risk of gastric cancer in a large-scale population-based cohort study in Japan (JPHC study). Eur J Cancer Prev. 2008;17:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Yu J, He Y, Guo Z. Age trend of the male to female sex ratio in surgical gastric cancer patients at a single institution. World J Surg Oncol. 2014;12:269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Yi JH, Do IG, Jang J, Kim ST, Kim KM, Park SH, Park JO, Park YS, Lim HY, Kang WK, Lee J. Anti-tumor efficacy of fulvestrant in estrogen receptor positive gastric cancer. Sci Rep. 2014;4:7592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008;22:2085-2098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Helguero LA, Lindberg K, Gardmo C, Schwend T, Gustafsson JA, Haldosén LA. Different roles of estrogen receptors alpha and beta in the regulation of E-cadherin protein levels in a mouse mammary epithelial cell line. Cancer Res. 2008;68:8695-8704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Oesterreich S, Deng W, Jiang S, Cui X, Ivanova M, Schiff R, Kang K, Hadsell DL, Behrens J, Lee AV. Estrogen-mediated down-regulation of E-cadherin in breast cancer cells. Cancer Res. 2003;63:5203-5208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Gan L, He J, Zhang X, Zhang YJ, Yu GZ, Chen Y, Pan J, Wang JJ, Wang X. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12:566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Hsu LW, Huang KH, Chen MH, Fang WL, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. Genetic alterations in gastric cancer patients according to sex. Aging (Albany NY). 2020;13:376-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269-24283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 47. | Özdemir BC, Dotto GP. Sex Hormones and Anticancer Immunity. Clin Cancer Res. 2019;25:4603-4610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |