Published online Feb 28, 2022. doi: 10.3748/wjg.v28.i8.794
Peer-review started: October 14, 2021
First decision: December 3, 2021
Revised: December 15, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: February 28, 2022
Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs) of the digestive tract are a rare heterogeneous group of tumors that present many challenges in terms of diagnosis and treatment. Over the years, the diagnostic criteria, classification, and clinical behavior of these tumors have been the subjects of ongoing debate, and the various changes in their nomenclature have strengthened the challenges associated with MiNENs. This review is performed to provide an understanding of the key factors involved in the evolution of the designation of these tumors as MiNEN, highlight the current diagnostic criteria, summarize the latest data on pathogenesis and provide information on available treatments. Moreover, this work seeks to increase the awareness about these rare neoplasms by presenting the clinicopathological features and prognostic factors that play important roles in their behavior and discussing their different regions of origin in the gastrointestinal system (GIS). Currently, the MiNEN category also includes tumors in the GIS with a nonneuroendocrine component and epithelial tumors other than adenocarcinoma, depending on the organ of origin. Diagnosis is based on the presence of both morphological components in more than 30% of the tumor. However, this value needs to be reconfirmed with further studies and may be a limiting factor in the diagnosis of MiNEN by biopsy. Furthermore, available clinicopathological data suggest that the inclusion of amphicrine tumors in the definition of MiNEN is not supportive and warrants further investigation. The diagnosis of these tumors is not solely based on immunohistochemical findings. They are not hybrid tumors and both components can act independently; thus, careful grading of each component separately is required. In addition to parameters such as the metastatic state of the tumor at the time of diagnosis and the feasibility of surgical resection, the aggressive potential of both components has paramount importance in the choice of treatment. Regardless of the organ of origin within the GIS, almost MiNENs are tumors with poor prognosis and are frequently encountered in the elderly and men. They are most frequently reported in the colorectum, where data from molecular studies indicate a monoclonal origin; however, further studies are required to provide additional support for this origin.
Core Tip: Mixed neuroendocrine-nonneuroendocrine neoplasms of the gastrointestinal system are a rare heterogeneous group of tumors that present many challenges in terms of diagnosis and treatment. Current data indicate that they are more frequent in the colon and rectum and that most of them consist of aggressive tumors that have poor prognoses in older men. Their correct diagnosis with the proposed criteria and the separate assessment of the grade of each component are crucial in terms of determining the treatment. Although studies have indicated a monoclonal origin, further studies are needed to determine whether these molecular changes could become treatment targets.
- Citation: Elpek GO. Mixed neuroendocrine–nonneuroendocrine neoplasms of the gastrointestinal system: An update. World J Gastroenterol 2022; 28(8): 794-810
- URL: https://www.wjgnet.com/1007-9327/full/v28/i8/794.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i8.794
In epithelial tumors, cells with both neuroendocrine and nonneuroendocrine features coexist in varying amounts, and these tumors occur in almost all organs, including those of the GIS. Although these rarely encountered neoplasms are well known in terms of pathology, debates remain about their diagnosis, classification, pathogenesis, behavior and treatment, and some points are still controversial.
The presence of different proportions of each component in a mixed epithelial tumor (each of which can account for 1% to 99% of the tumor) can result in a wide variety of morphologically heterogeneous tumors as well as different classifications and diagnostic difficulties in pathology[1]. More importantly, these conditions have created problems for oncologists in determining the component that should be targeted primarily in treatment. In addition, the assignment of different definitions to mixed epithelial tumors has led to great inconsistencies in the data obtained from previous studies, especially in determining the prognostic parameters that affect their behavior[2,3]. Therefore, different criteria have recently been introduced for simplifying the diagnosis and classification of these tumors based on the identification of prognostic parameters that may enable effective treatment for mixed epithelial tumors.
The purpose of this review is to highlight the definition of mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs), summarize the current approaches for their histopathological diagnosis and molecular findings related to their pathogenesis and address current approaches in their treatment. Moreover, this work is performed to raise awareness about these rare tumors of the GIS by presenting localization-specific demographic and clinicopathological findings.
Mixed tumors were first described by Cordier[4] at the beginning of the last century as neoplasia in the gastrointestinal tract consisting of adenocarcinoma and neuroendocrine components. However, their classification as a different group and their subcategories under this heading were not recommended until proposed by Levine in 1967[5]. Accordingly, three main subtypes have been proposed based on their nonneuroendocrine and neuroendocrine features: collision tumors, combined tumors, and amphicrine tumors. However, this nomenclature has not been entirely accepted. In addition to the challenges posed by their pathological heterogeneity, many different definitions (some repetitious or overlapping) have been provided for mixed epithelial neoplasms composed of both neuroendocrine and nonneuroendocrine components, which has led to further confusion among clinicians and pathologists by leading to considerable variability in published data[2]. In recent years, many attempts have been made to elucidate the clinical and biological meanings of the different combinations of components and simplify the diagnosis and classification, with the primary goal of developing precise diagnostic criteria that can be used to produce a proper prognostic classification for the management of patients. Standardizing the terminology to provide a prognostic classification of mixed neoplasms of the digestive tract was first suggested by Capella et al[6] in 2000. Accordingly, the term “mixed exocrine– endocrine tumor” was used by the World Health Organization (WHO) to define these neoplasms[7]. This category included previous subcategories suggested by Levine[5], whereas adenocarcinomas or squamous cell carcinomas with scattered neuroendocrine cells were excluded based on previous data about the absence of a relationship between the presence of neuroendocrine cells and prognosis. To emphasize the diagnosis, a subjective cutoff value of 30% was determined for each component to define mixed tumors. Ten years later, the term “mixed exocrine–endocrine tumor” was substituted by “mixed adeno-neuroendocrine tumor” (MANEC)[8]. Since the two components of mixed tumors of the GIS are not always constituted by adenocarcinomas and neuroendocrine adenocarcinoma (NEC), the term MANEC was not sufficient to describe these combinations. This situation encouraged many researchers to find another term that included other associations. In 2016, La Rosa et al[2] proposed the umbrella term "mixed nonneuroendocrine and neuroendocrine neoplasm" (MiNEN). Introduced first for the pancreas by the 2017 WHO classification of tumors of endocrine organs, this term is currently used for all mixed neoplasms of the GIS[9]. According to the WHO, mixed neoplasms consisting of an adenoma and a neuroendocrine tumor (NET) should be classified as MANETs and should not be included in the MiNEN group.
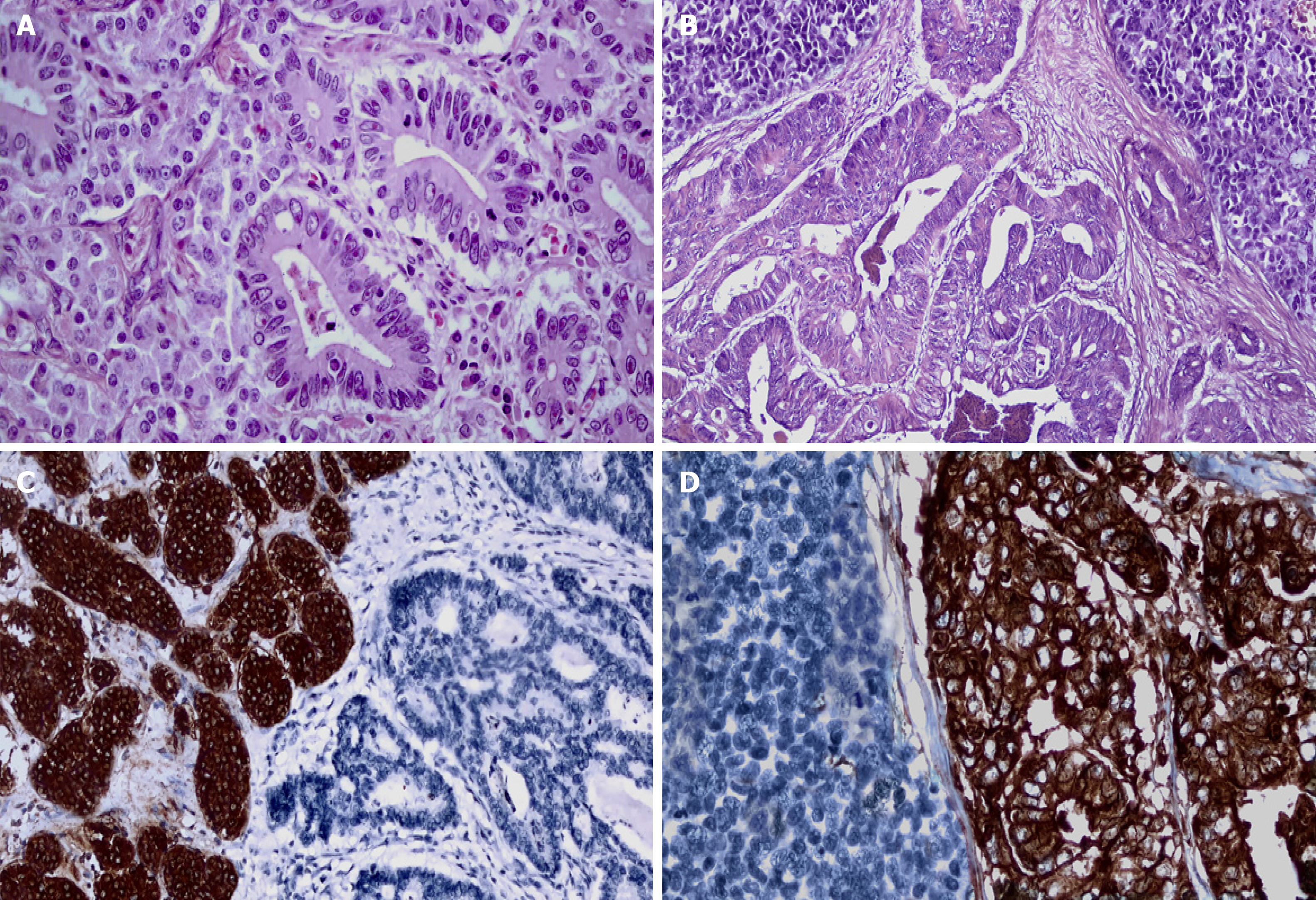
Pathological evaluation of H&E-stained sections is essential for detecting the neuroendocrine and nonneuroendocrine components for the diagnosis of MiNEN. These findings must be confirmed by immunohistochemical (IHC) evaluation. IHC markers used in detecting and grading the endocrine component should be accompanied by markers appropriate for the type of nonneuroendocrine component (Figure 1). The properties and applications of these markers for neuroendocrine neoplasia (NEN) will be briefly mentioned here. However, comprehensive information on this subject can be reviewed in a previous study[10]. Although several biomarkers, including neuron-specific enolase (NSE), CD57, protein gene product 9.5 (PGP 9.5), insulinoma-associated protein 1 (INSM1) and somatostatin receptor subtype 2A (SSTR2A), have been described to date, the most widely used and reliable neuroendocrine markers are chromogranin A, synaptophysin, and CD56[11,12]. In the nonneuroendocrine component, adenocarcinomas express carcinoembryonic antigen, CA 19-9, cytokeratins 7, 19, and AE 1/3. The immunohistochemical features of other tumors that make up this component are presented below according to their localization in different organs of the GIS[10-12].
Both tumor components must account for at least 30% of the whole neoplasm for the diagnosis of MiNEN[8]. This cutoff is arbitrary and was proposed in 1987. The basis for determining this value is the assumption that the prognosis is influenced by the predominant histological component and prevents the management of these cases without consideration of treatment guidelines[1,5]. However, this value has not been reaffirmed in systematic studies. Moreover, the possibility of the negative influence of a small component of high-grade NEN on tumor behavior should not be overlooked[13,14]. This quantitative threshold also poses problems for tissue biopsies. The discrimination of both components could not be performed accurately according to the likelihood of their presence in random biopsies, thus leading to potential underestimations of the frequency of MiNEN diagnosis[15]. Recently, it has been argued that a cutoff value is not mandatory for diagnosing MiNEN because the latest molecular information in the modern classification of these neoplasms has made it possible to demonstrate that both components are clonally related[16].
Another point to be considered is that the determination of NEN on a purely quantitative basis may cause problems in diagnosis, especially with the use of IHC. Indeed, it has been reported that achieving a diagnosis of MiNEN only by quantification with IHC findings has caused inconsistencies and confusion in terminology[17]. Therefore, the WHO has clearly stated that the findings of IHC alone are not sufficient for the diagnosis of MiNEN, and those histopathological findings should be present in each morphological component[9,18].
To date, the definition of MiNEN also excludes nonneuroendocrine neoplasms in which scattered tumor cells express neuroendocrine markers without the presence of neuroendocrine morphology (Table 1). Since neuroendocrine markers can be positive in many nonneuroendocrine tumors, including poorly differentiated adenocarcinomas, performing IHC alone may lead to an overdiagnosis of MiNEN[19-21]. Therefore, it is highly recommended to avoid the application of neuroendocrine markers to tumors that do not have a morphological neuroendocrine component to overcome this difficulty.
| Morphology | Dual differentiation present | Dual differentiation absent | ||||
| Immunohistochemisty | ||||||
| Neuroendocrine markers | (-) | (+) | (+) | (+) | (-) | Few (+) cells1 |
| Nonneuroendocrine markers | (+) | Few (+) cells | (+) | (+) | (+) | (+) |
| Diagnosis | Carcinoma | NEN | MiNEN | Amphicrine tumor | Carcinoma | Carcinoma |
Another issue that should be considered is that the diagnosis of MINEN must be given in cases that have not undergone neoadjuvant treatment. Many studies have observed that the number of neuroendocrine cells may increase after treatment (especially chemotherapy) in GIS adenocarcinomas[22-24]. The effective mechanisms of this phenomenon are not fully known and await clarification, and the latest WHO classification does not include these tumors in the MiNEN group.
As pointed out previously, the behavior of MiNEN does not correspond to the average of the two components,such as hybrid neoplasia, but the sum of the two components because each of component can independently progress and metastasize[17,25]. For this reason, it is crucial to detect and evaluate each component separately during pathological examination to determine the treatment of the tumor. Although the nonneuroendocrine component of many MiNENs is more frequently composed of adenocarcinomas, it may differ according to the location within the GIS (see below), which should be considered during evaluation. This part of the tumor should be graded according to the type of nonneuroendocrine component of MiNEN. Similarly, neuroendocrine components should be evaluated according to the WHO classification and tumors should be graded according to the Ki-67 index percentage and mitotic count.
These tumors are subdivided into three categories. While collision MiNEN is defined as two coexisting cell populations that remain separate without transition, composite MiNEN involves two morphologically distinct components that coexist in an intermingled population[25]. To date, true collision tumors consisting of two independent neoplasms arising in the same organ, even if they abut one another, should not be considered MiNEN unless these components are presumed to be clonally related[18]. The last group includes amphicrine tumors composed of a morphologically one-cell population that displays the phenotypes of neuroendocrine and adenocarcinoma phenotypes. These cells show coexisting morphological, immunohistochemical, and ultrastructural properties that present both neuroendocrine and exocrine differentiation. Although these tumors have been described for many years in many locations, including the GIS, there is no consensus regarding their relationship with MiNENs. These rare neoplasms have been observed in the stomach, pancreas, appendix, and colon[26-28]. In the appendix, where they are observed relatively more frequently, they are now classified as goblet cell adenocarcinomas[29]. Although this nomenclature does not reflect the amphicrine characteristics of the cells, the term goblet cell carcinoid has been used for many years to avoid misclassification. A recent elegant study by Huang et al[30] provided evidence that amphicrine carcinomas arising from the stomach and intestine are distinct tumors with different clinicopathological and pancancer transcriptome features. The latter revealed that although amphicrine neoplasms show similarities to adenocarcinomas, they are not similar to NENs. However, since the nature of NENs was not specified in the study, this finding awaits further investigation. In an elegant study comparing the similarities and differences in genetic alterations between gastric amphicrine carcinomas and MiNENs, Sun et al[31] observed that the copy number (CN) characteristics of gastric amphicrine carcinomas were different from those of MiNENs based on a hierarchical clustering analysis, thus supporting that amphicrine carcinoma is a separate entity from MiNENs. In addition, a higher CN level of C5 (complement C5) was observed in amphicrine carcinomas than in MiNENs, suggesting that these tumors might benefit more from C5 inhibitors than MiNENs.
Currently available data also suggest that the inclusion of amphicrine tumors in the definition of MiNEN is not supported based on clinical and pathological features. Moreover, the fact that amphicrine tumors are a subject of debate does not exclude their consideration in the differential diagnosis of MiNEN.
Although the carcinogenesis of MiNEN has not been clarified, recent studies suggest that they are derived from a single precursor cell that has the capacity for dual differentiation after the initiation of carcinogenesis. Studies on colorectal MiNEN have demonstrated that both components share common driver genetic aberrations in critical oncogenes and/or their protein products, including tumor protein p53 (TP53), retinoblastoma tumor corepressor 1 (RB1), adenomatous polyposis coli (APC), phosphatase and tensin homolog (PTEN), Kirsten rat sarcoma viral oncogene homolog (KRAS), v-myc avian myelocytomatosis viral oncogene homolog (MYC), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PI3KCA), and v-raf murine sarcoma viral oncogene homolog B (BRAF)[32-36]. Recent data have also suggested that microsatellite instability (MSI) and prostaglandin E2 receptor 4 (PTGER4) activation are also involved during the evolution of MiNEN[35]. Many studies have provided essential data about common driver genetic aberrations[32-34,37,38] . Indeed, both components display loss of heterozygosity (LOH) at multiple loci and mutations in key oncogenes with high allele frequencies compared to exclusive alterations of a single component, thus supporting their common clonal origin hypothesis, at least in the earlier steps of carcinogenesis[32-34,37,38].
In a more recent molecular study in gastric tumors with targeted DNA sequencing, a great majority of mutations were shared by both ADC and NEC components, and among them, TP53 was the most commonly mutated gene (69.2%)[39]. A subset of TP53-wild-type tumors had a microsatellite-unstable phenotype or amplifications in various oncogenes, including ERBB2 and NMYC. While differentially altered genes of ADC components were significantly associated with receptor tyrosine kinase signaling pathways, differentially altered genes of NEC components were significantly associated with the NOTCH signaling pathway, thus providing evidence for a possible clonal origin of ADC and NEC components of MiNENs[39].
Due to additional genetic alterations in poorly differentiated NEC (PDNEC), it is suggested that two different components activate separate genetic pathways at some step of carcinogenesis[32,36,38,40]. Another finding supporting a common carcinogenic pathway is that a proportion of either PDNEC or MiNEN of the stomach and colorectum has increased methylation with a mismatch repair-deficient phenotype[41-44]. Since these tumors had less aggressive behavior, similar to sporadic colon adenocarcinoma in the elderly, it is proposed that mismatch repair deficiency could be a pathway between PDNEC/MiNEN and adenocarcinoma[21,25]. In addition, the neuroendocrine component of PDNEC carries mutations specific to the organ from which it originates and is similar to adenocarcinoma in localization; meanwhile, these alterations are different from a common neuroendocrine alteration. When CDK2A and APC mutations were compared in the PDNEC of the colon and pancreas, it was observed that the CDK2A mutation was higher in pancreatic PDNEC compared with APC mutations, which were higher in the colon; this finding was consistent with adenocarcinomas originating from these organs[45]. The comparison of MiNEN with their pure neuroendocrine counterparts in the colon showed that while the former shares a similar copy number aberration profile with adenocarcinoma, the latter displays different structural aberrations; thus, the developmental pathway suggests that MiNEN is related to the nonneuroendocrine component but not to neuroendocrine carcinomas[34,35]. As noted above, exclusive alterations in the neuroendocrine component carry a higher number of aberrations and an imbalance of alleles with a more aggressive phenotype, thus leading some authors to suggest that nonneuroendocrine components give rise to the neuroendocrine component through transdifferentiation, where c-myc and SMARC4 are potentially involved[32,46]. A significant increase in the number of neuroendocrine marker-expressing cells following neoadjuvant therapy in PDNEC supports the development of a neuroendocrine component through the adenoma-adenocarcinoma sequence[22,24]. The presence of cases of MiNEN that combine PDNEC and adenoma without adenocarcinoma is also postulated as evidence of a common carcinogenic pathway[47]. Evidence has also been obtained that the two components demonstrate distinct genetic patterns, suggesting that some MiNENs have polyclonal origins[35,37,38]. Interestingly, well-differentiated NET components of MiNEN do not share similar genetic alterations observed in their adenoma/adenocarcinoma counterparts, such as LOH of APC, KRAS, and TP53, although they do display specific alterations that are usually found in NETs (but not PDNECs), such as LOH of VHL, which may also represent true collision MiNEN with an independent carcinogenic pathway[42,47].
Although there is no complete consensus on the treatment of MiNEN, the presence of tumor metastasis at the time of diagnosis and histopathological MiNEN grading of the tumor play a vital role in the choice of therapeutic options[20]. The classification of MiNEN according to the grade malignancy is presented in Table 2.
| Grade of MiNEN | Nonneuroendocrine component | Endocrine component |
| High-grade | Adenocarcinoma | PDNEC, NET G31 |
| Squamous cell carcinoma | ||
| Cholangiocarcinoma | ||
| Ductal and acinar cell carcinoma | ||
| Ductal adenocarcinoma | ||
| High-grade | Acinar cell carcinoma | NET, G1 or G21 |
| Ductal adenocarcinoma | ||
| Acinar cell carcinoma | ||
| Intermediate grade | Adenocarcinoma | NET, G1 or G21 |
| Low-grade2 | Adenoma | NET, G1 or G2 |
In all localized MiNENs, curative-intent surgery is recommended as the first treatment of choice, if available. Even in high-grade MiNENs, because of their less aggressive behavior than pure NECs, tumors with an acinar component of the pancreas belonging to this category have benefited from such treatment[48-50]. In the same group, although combinations of etoposide (VP16) and platinum salt or a combination of 5-fluorouracil (5FU) with irinotecan (IRI)- or oxaliplatin (OX)-based preoperative and postoperative chemotherapy have been used in tumor management similar to PDNEC therapy, the role of adjuvant therapy is still not completely defined[44,51-53]. Moreover, in the intermediate group, recent studies suggest replacing this therapy with a combination of 5FU and IRI and/or OX or gemcitabine (GEM) and/or OX for treatment parallel to the chemotherapy applied to adenocarcinomas, and in some cases, radiotherapy has been used as a treatment option in addition to chemotherapy[3,54-57].
Unfortunately, extensive surgery does not seem to be an option in this group because the risk outweighs the benefits, especially in high-grade MiNENs. Therefore, systemic chemotherapy is generally performed according to the type present at metastatic sites. In patients with both components diagnosed either at the metastatic site or the primary tumor, therapy is based on the most aggressive component[3,54-57]. Since some intermediate-grade MiNENs with predominant NET components frequently express type 2 somatostatin receptors, such cases may also benefit from long-acting somatostatin analogs and peptide irradiation nucleotide therapy[58-60]. Although MANET is not categorized as MiNEN, its neuroendocrine component can metastasize. Therefore, pure NET-based chemotherapy and peptide irradiation nucleotide therapy are recommended for their treatment. At present, Akt/mTOR mutations have not been investigated in MiNENs. However, some patients who benefit from everolimus have been described[61].
Despite all these findings, the fact that further studies in large series are needed to define the treatment of patients more precisely with MiNEN should not be overlooked. Future studies aiming to determine the molecular vulnerability of both components in MiNEN cases in the GIS diagnosed based on criteria recommended for the diagnosis of these tumors may allow for the development of targeted therapies against both components and improve their treatment.
The treatment approach in MiNENs in both groups is briefly presented in Tables 3 and 4.
| Surgery | Complete resection is possible | Complete resection is not possible | ||||
| MiNEN grade | High | Intermediate | Low | High | Intermediate | Low |
| Chemotherapy | PDNEC-like | ADC-like | NR | PDNEC-like | ADC- like | NET-like |
| Metastatic component | Defined | Not defined | |
| One component | Two components | ||
| Chemotherapy target | The metastatic component | The most aggressive component | The most aggressive component in the primary tumor |
Recently, a comprehensive systematic review and a multicenter study performed by Frizziero et al[3,15] showed that esophageal MiNENs accounted for between 5.9% and 15.9% of all GIS MiNENs. These tumors, which account for approximately one-quarter of the NENs observed in this localization, show an apparent male predominance and are observed at advanced ages (6th decade) in the distal third of the esophagus[2,19,62]. Although the tumor consists of NEC and squamous cell carcinoma (SCC) in most cases, there are rare adenocarcinoma cases, particularly adenocarcinoma, in the background of Barrett's esophagus[40,63,64]. Molecular data indicate their monoclonal origin, as demonstrated by LOH, RB1, TP53, and alterations in TP63, SOX2, DVL3, PTEN, PIK3A, and KRAS[40]. However, the monoclonal origin of esophageal MiNEN with a nonendocrine component composed of adenocarcinoma on the background of Barrett’s metaplasia remains to be elucidated, and some authors postulate that this group reflects a true collision tumor rather than MiNEN[64].
In the esophagus, because the discrimination of basaloid SCC and small-cell NEC (SNEC) has paramount importance and may pose a diagnostic pitfall in routine microscopic evaluation, immunohistochemical staining for high molecular weight cytokeratins, p63, and p40, is highly recommended to discriminate basaloid SCC from SCNEC[17].
Unfortunately, their lower metastatic capacity (25% vs 54%) and longer survival time (28 vs 15 mo) relative to pure PDNEC do not change their poor prognosis[65]. Another important finding is the predictive role of the Ki-67 proliferation index of NEC for prognosis[15,66]. More recently, any statistically significant difference in OS between gastroesophageal GEP MiNEN vs colorectal MiNEN was detected[66].
These tumors constitute 6%–20% of MiNENs located in the GIS, and 7% of NENs are located in the stomach[14,15,67]. Similar to those in the esophagus, stomach tumors are observed in elderly patients (5th-6th decades) and show a male predominance. Based on the macroscopic appearance of these tumors, which are observed equally in the corpus and antrum of the stomach, they are not different from adenocarcinomas, and specific diagnostic findings on endoscopy have not been obtained[19]. Most of these aggressive tumors consist of well-differentiated adenocarcinomas, and the PDNEC component is mainly located deeper in the organ[68,69]. Although cases composed of adenocarcinoma and NETs have been recorded, it is suggested that the term MANEC can be retained for MiNEN at this location[19]. MiNENs composed of gastric NEN and adenocarcinoma have been described in the setting of chronic atrophic gastritis, etiological factors have not yet been entirely identified[70]. Recent molecular studies indicated a monoclonal origin[37,44,71]. Ishida et al[72] compared the molecular pathology of poorly differentiated NEC and MiNEN of the stomach by whole-exome sequencing. The analysis revealed recurrent mutations in 62% of TP53 cases, and they were more frequent in MiNENs than in NECs. Frameshift mutations of APC were observed in two MiNEN cases. In cases of MiNEN, two histological components shared mutations in TP53, APC, and ZNF521, whereas alterations in CTNNB1, KMT2C, PTEN, and SPEN were observed in neuroendocrine components only. They concluded that TP53 is a single, frequently mutated gene in gastric NEC and MiNEN, and alterations in other genes are less common, thus resembling the mutation profiles of gastric adenocarcinomas. Another interesting previous finding is the presence of ATRX gene mutations (primary partial loss) in 37% of cases involving a substantial proportion of gastric MiNEN[73]. However, these findings should be investigated in further studies. Gastric MiNENs are tumors with a poor prognosis that show lymph node and distant metastases at the time of initial diagnosis, and the prognosis is slightly better than that of pure PDNEC[15,74,75]. Similar to these findings, in a recent study including 401 patients, the 5-year disease-free survival was 51.1%, which was significantly better than that of NEC (47,6%) and worse than that of adenocarcinoma (57,8%). Furthermore, in the same series, advanced stages and lymph node metastasis were independent risk factors related to distant recurrence[76].
MiNENs of the small intestines frequently in the duodenum, where they are mainly located in the ampulla[77-81]. MiNENs of the jejunum and ileum are exceedingly rare, similar to PDNECs encountered in these regions[19]. They are equally observed in both sexes and older patients. While adenocarcinoma constitutes the nonneuroendocrine component of tumors in many cases, rare cases of SCC have also been recorded[77,78]. Since the neuroendocrine component is frequently located in the deeper part of the intestinal wall, they are frequently diagnosed as adenocarcinoma from biopsies[81]. The histological subtype of adenocarcinoma forming these tumors was also found to be associated with tumor behavior. MiNENs with intestinal-type adenocarcinoma have a better prognosis than those with the pancreaticobiliary subtype[20,25]. However, ampullary MiNENs are aggressive tumors with a poor prognosis and generally present at advanced stages[15,17]. This finding contrasts with MiNEN in the other part of the duodenum, which combine intestinal-phenotype adenocarcinoma and a well-differentiated somatostatin-secreting NET[20]. They are mostly superficial and not highly aggressive, and distant metastasis is rare.
The last WHO classification of MiNEN indicated that these tumors were composed of two morphologically recognizable components generally represented by adenocarcinoma and NEC, and this classification has provided knowledge about mixed tumors in the appendix[18]. The exclusion of goblet cell carcinoids, which are currently determined to be amphicrine tumors, from the MiNEN group has led to the limited applicability of past clinicopathological data on these tumors located in the appendix, thus necessitating further evaluation of the findings related to this group[29]. A few studies conducted in the recent past indicate that MiNENs constitute 10% of malignancies at this location. On the other hand, a systematic review showed that among the lower gastrointestinal tract organs, these tumors were most frequently (60.3%) localized in the appendix[82]. An interesting finding is that the age-adjusted incidence for MiNENs increased from 0.01/100000 person-years to 0.07/100000 person-years (range 2004-2016), with an annual percentage change (APC) of 13.8%[83]. This finding can be attributed to the increase in clinical recognition and better diagnostic technologies over the years. They are observed in advanced age (between 58-60 years) mostly encountered incidentally and discovered at advanced stages[56,83,84]. Although recent studies indicate that these tumors do not show sex predilection, new findings that APC shows significant differences according to sex (13.81% in females vs 12.24% for males) need to be clarified[83].
Their overall survival rate is 6.5 years, which is better than that of signet ring cell carcinomas (2.1 years) and worse than that of goblet cell adenocarcinomas (13.8 years) and pure NETs (39.4 years)[56,84]. In a more recent study, the prognosis of 315 patients with MiNEN was compared with that of other histological subtypes in the appendix, including NETs, NECs, goblet cell carcinoma, signet ring cell carcinoma, mucinous adenocarcinoma and nonmucinous adenocarcinoma, based on the surveillance, epidemiology and end results program 18 registries. The overall 5-year survival rate was 57.4%, and the level of invasion was the only independent factor influencing tumor behavior. In addition, multivariate analysis demonstrated that the prognosis of MiNENs was worse than that of NETs, NECs, goblet cell carcinoma, and mucinous adenocarcinoma but better than that of nonmucinous adenocarcinoma and signet ring cell carcinoma[83].
Their pathological differential diagnosis should include goblet cell carcinomas, tubular-type carcinoids and pure NETs with glandular configurations.
Tumors in this region constitute more than half of all MiNENs of the GIS[67,85]. In particular, evidence has shown that they are observed more frequently than pure NETs in resections performed in this region[85]. Their distribution among NENs is 14-20% and 1%-3% in the colon and rectum, respectively. They are more common in men than women and observed at an advanced age (6th decade)[25,67,85]. Although they do not have specific clinical findings, they have been defined in the background of inflammatory bowel diseases[86-89]. As a result of their more frequent detection in these regions, the number of molecular studies performed in MiNEN exceeds that of other localizations, and they have provided important data regarding their pathogenesis. The same genetic alterations in both components strongly support their monoclonal origin from a common precursor progenitor cell[33,35,37,38,42]. Parallel to these findings, a recent case showed that in addition to microsatellite instability due to MLH1 promoter methylation, the same mutations affecting the ARID1A, ASXL1, BLM, and RNF43 genes occur in both components, as determined by a multigene next-generation sequencing panel. On the other hand, BRCA2 has been explicitly altered in the neuroendocrine area. Although the latter observation suggested that BRCA2 could be a potential new target for MiNEN, the lack of this alteration in the nonneuroendocrine part of the tumor requires further consideration concerning intratumor heterogeneity[90].
Macroscopically, these tumors form masses (average 5 cm) without distinguishing features from adenocarcinomas. Histologically, most cases are composed of adenocarcinoma and NEC. Rare cases in which the nonneuroendocrine component consisted of squamous cell carcinoma have also been noted[43,91]. If the neuroendocrine component consists of PDNEC, it is consistently observed in metastases. In comparison, adenocarcinomas are observed in one-third of cases[47]. Such tumors show aggressive behavior and have poor prognosis (overall survival: 12.2 mo) according to the Ki67 proliferative index of the NEC component as well as the MSI status and stage[42,43,67]. Recently, a systematic review demonstrated that if the neuroendocrine component consists of NETs, it is unknown which component will be encountered in metastatic sites because of a higher grade, and the predominance of one component does not warrant their presence in metastatic sites[47,92,93]. This finding emphasizes the complexity of MiNENs and the need for an accurate morphological description of all components. A recent systematic review also demonstrated that in MiNENs of the lower gastrointestinal tract, the site of origin in those with metastatic disease at diagnosis appeared to influence prognosis. The median survival time was 12.3 mo for those with primary colonic tumors vs 11.7 mo for those with primary anorectal tumors, with hazard ratios of 1.13 vs 0.80, respectively[82].
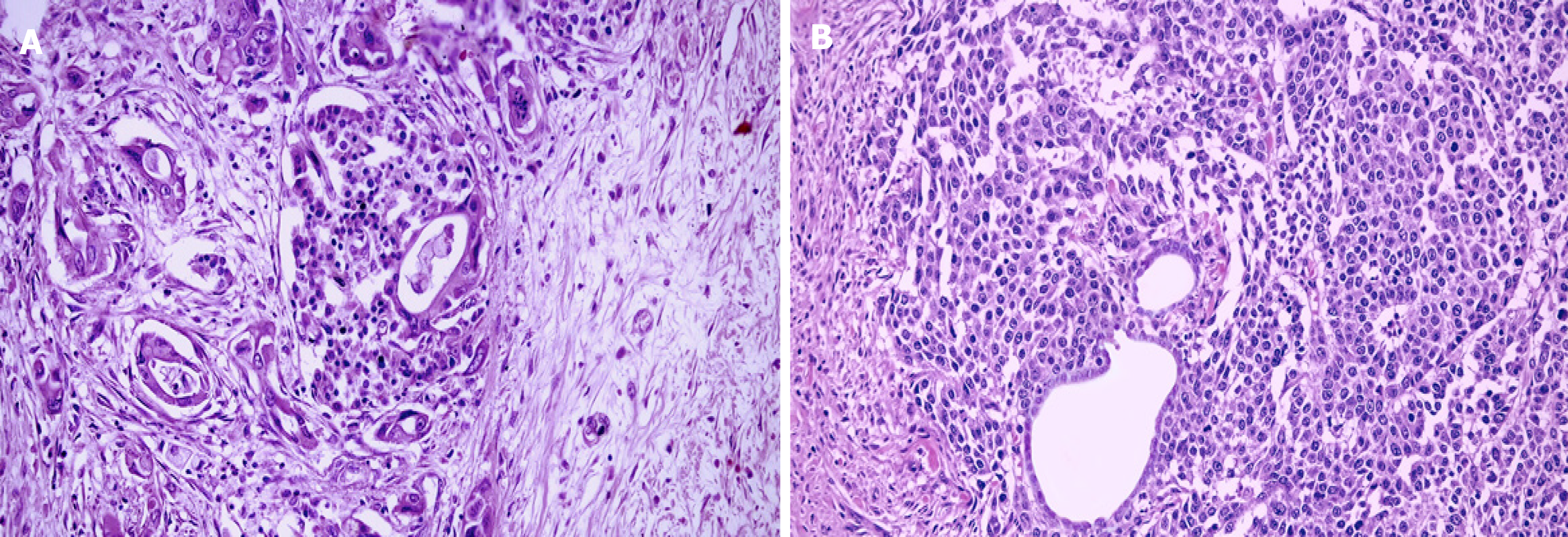
Pancreatic MiNENs are rare tumors in which the nonneuroendocrine component can be formed by ductal or acinar carcinoma[18]. In addition, tumors with a nonneuroendocrine part consisting of ductal and acinar carcinomas are extremely rare and defined as mixed ductal-acinar-neuroendocrine carcinomas[94]. Tumors with mixed ductal–neuroendocrine carcinoma are rare and account for approximately 0.5%–2% of all ductal adenocarcinomas and 5% of all NENs arising from this organ[27]. There is no sex predilection. Although the average age of onset is 68, they are observed in a wide age range, from 21 to 68 years old. They usually consist of NEC accompanying ductal adenocarcinoma[95-97]. They can be located anywhere in the organ and produce clinical symptoms similar to ductal adenocarcinoma without specific clinical findings. Because of the rare nature of these tumors, molecular data are scarce and limited[46,67,98]. Therefore, the diagnosis should be performed using adenocarcinoma and neuroendocrine tumor-specific markers separately and morphologically because they should be clearly distinguished from ductal adenocarcinomas with entrapped islets and NETs with entrapped ductules in the differential diagnosis[17,27]. In the former, the islands present an ovoid shape and have regular contours constituted by endocrine cells without atypia, and express all hormones, which is inconsistent with a predominant cell line of tumor cells; in the latter, however, the absence of atypia of ductal cells , the lack of aberrant P53 staining and the low proliferative index are essential clues in the differential diagnosis (Figure 2). An increase in the number and size of Langerhans islands that accompany chronic obstructive pancreatitis is another diagnostic pitfall in the differential diagnosis. Previous reports indicated that mixed ductal–neuroendocrine carcinomas are aggressive tumors with poor prognoses (5-year survival is 0%)[18,98]. More recently, lymph node metastasis was indicated as an adverse prognostic factor of disease-specific survival in 7 patients with mixed ductal–neuroendocrine carcinomas[99]. Similar findings were also observed by Zhang et al[92] in a larger number of patients. Although data for surgically resected cases are very limited in the literature, a cohort study reported that the median survival was 15.3 mo and all cases died due to disease[100]. However, in a recent study evaluating 8 cases with a median follow-up of 21 mo, the overall survival was 88 mo and the 5-year OS was 58%. In addition, the survival of these tumors was better than that of pancreatic ductal adenocarcinomas; thus, further investigation is warranted[101].
Cases in which the neuroendocrine component consists of NETs have been reported, although their morphology resembles true collision tumors and their monoclonal origin remains to be proven[95,96].
Mixed acinar–neuroendocrine carcinomas are rare and account for nearly one-fifth of all pancreatic ACCs[49,94]. The features of these tumors do not differ from the macroscopic features of ACC, and the tumors are quite large. In the differential diagnosis, morphological and IHC findings should be evaluated together, such as in ductal carcinoma. It is worth noting that 20%-30% of ACCs show a small neuroendocrine cell population, and this morphologically undetected component should not lead to the diagnosis of MiNEN[49]. Immunohistochemical staining with trypsin and bcl-10 [monoclonal antibody directed against the C-terminal portion of bcl-10 (clone 331.3)] is very useful for identifying the acinar component[102] (Figure 3). However, it should be kept in mind that a significant portion of the ACC is stained with synaptophysin.
Moreover, since pure NETs of the pancreas have a better prognosis than these tumors, advanced immunohistochemical evaluations with bcl-10 and trypsin are recommended for all tumors with neuroendocrine-appearing pancreatic neoplasms that show a high mitotic index, abundant necrosis, and evident nucleoli[17]. Although these tumors seem to share the genetic changes observed in pure ACCs, they do not show characteristic mutations that can be found in pancreatic NETs[103,104].
A recent study suggested that c-MYC alterations are involved in mechanisms leading to the neuroendocrine differentiation of ACCs[46]. Surgical resection and tumor stage are the most important prognostic factors, and the reported 5-year survival rate is 30%–50% for patients who undergo surgery[49,105].
The nonneuroendocrine component of MiNENs is mostly hepatocellular carcinoma (HCC) and less frequently cholangiocellular carcinoma, and they are rare liver tumors encountered at advanced ages (43-84 years) predominantly in men[2,106,107]. The neuroendocrine components of these tumors are predominantly NECs, and they have a dismal prognosis, with many cases presenting distant metastasis at the time of diagnosis[2,108,109]. More recently, the 1-year cumulative survival rate of patients was reported to be 53%[107].Although the pathogenesis has not been fully elucidated; neuroendocrine differentiation from existing HCC has been suggested[108,109].
MiNENs of the gallbladder and biliary tract account for 10% of all biliary carcinomas and 2% of all hepatobiliary carcinomas[110,111]. Although considered rare, adenocarcinoma is detected in approximately 30% of NENs, particularly in the gallbladder, and these tumors constitute 35% of NENs in this region, thus indicating that they are more frequent than previously described[79,110]. While the age range is relatively wider than that of many MiNENs, these tumors are observed at an advanced age (mean: 65 years), which is similar to those in other regions of the GIS. However, compared with the male dominance observed in other MiNENs, these tumors are more common in females[18]. The close relationships between MiNENs and inflammatory diseases in these locations suggest that inflammation plays a role in pathogenesis. Recent findings indicate that the NEC component of the tumor is composed of large cell NECs in a great majority of cases (59%), and NECs are incidentally discovered during imaging studies without any specific clinical findings[112]. In parallel, specific findings that differ from the findings for adenocarcinomas on macroscopic examination have not been observed. Although a considerable portion of the tumors are confined to the gallbladder wall at the time of diagnosis, one-fifth of the cases have serosa and one-third have adjacent organ invasion[16]. Therefore, the rarity of distant metastases in these tumors does not exclude the possibility that half of them metastasize to neighboring organs (liver, peritoneum, lymph nodes) at the time of diagnosis. On histopathologic evaluation, the nonneuroendocrine component often consists of adenocarcinoma and rarely consists of squamous cell carcinoma or carcinoma with sarcomatous or osteosarcomatous differentiation[112,113]. Few cases with intracystic papillary neoplasms (IPNs) have also been reported[16]. The NEN component, which is usually more deeply located, often consists of NEC. Molecular studies support that MiNENs in this region also consist of a common precursor[16,114]. Although studies on IPN have indicated that the endocrine component originates from these areas, further studies are needed to support this finding. The one-year survival was four times higher in patients with organ-confined tumors than in those with distant metastases, revealing that distant metastasis is the most effective predictive parameter for the course of the disease, thus emphasizing the importance of staging[79,110]. A recent systemic review based on 53 studies to predict the clinicopathological features and prognosis of biliary MiNENs, including gallbladder MiNENs, showed a median overall survival time of 21 mo. In addition, radical resection and small morphological subtype were independent prognostic factors associated with higher overall survival, and radical resection (R0) and younger age (< 65 years) were associated with higher recurrence-free survival time[115].
In conclusion, MiNENs of the GIS are a rare group of heterogeneous and aggressive tumors that should be diagnosed in patients without neoadjuvant therapy. Morphological findings are indispensable for their histopathological diagnosis. Evaluations based solely on the percentage of cells stained with IHC may lead to overdiagnosis. Since the current therapeutic approach depends on the grade of MiNEN, each component should be evaluated and graded separately. Although many studies support that these tumors are monoclonal, at least in the early stages of carcinogenesis, these data require additional research support.
Similarly, the 30% cutoff value should be reaffirmed by systematic studies because the possibility of the negative influence of a small component of high-grade NEN on tumor behavior should not be ignored. The deep localization of the NEN component in many organs is another potential limitation leading to their underestimation in biopsies. As recent findings suggest that amphicrine tumors may belong to a different tumor category, more studies are needed to reach a complete conclusion regarding these tumors.
In summary, new cases diagnosed as MiNENs in the GIS according to the currently proposed categories will increase awareness of these tumors, provide new data and eliminate diagnostic controversies of the past.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delgado-Gallegos JL, Zhou S S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Brathwaite SA, Smith SM, Wai L, Frankel W, Hays J, Yearsley MM, Abdel-Misih S. Mixed adenoneuroendocrine carcinoma: A review of pathologic characteristics. Hum Pathol. 2018;73:184-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27:284-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 119] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 3. | Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, Khan MS, Morgan M, Christian A, Elshafie M, Shah T, Minicozzi A, Mansoor W, Meyer T, Lamarca A, Hubner RA, Valle JW, McNamara MG. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25:5991-6005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Cordier R. Les cellules argentaffines dans les tumeurs intestinales. Arch In Med Exp. 1924;1:5. [Cited in This Article: ] |
| 5. | Wang J, He A, Feng Q, Hou P, Wu J, Huang Z, Xiao Z, Sun C, Liao W, Wu L. Gastrointestinal mixed adenoneuroendocrine carcinoma: a population level analysis of epidemiological trends. J Transl Med. 2020;18:128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Capella C, La Rosa S, Uccella S, Billo P, Cornaggia M. Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin Diagn Pathol. 2000;17:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Solcia E, Klöppel G, Sobin LH. Histological typing of endocrine tumours. Heidelberg: Springer-Verlag 2000: 7-13. [DOI] [Cited in This Article: ] |
| 8. | Rindi G, Arnold R, Bosman FT. World Health Organization classification of tumours of the digestive system. 4th ed. Lyon: IARC Press, 2010; 13–14. [Cited in This Article: ] |
| 9. | Klöppel G, Couvelard A, Hruban RH. World Health Organization classification of tumours of endocrine organs. 4th ed. Lyon: IARC Press, 2017; 211–214. [Cited in This Article: ] |
| 10. | Uccella S, La Rosa S, Volante M, Papotti M. Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr Pathol. 2018;29:150-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Watanabe H, Ide R, Yamazaki Y, Fujishima F, Kasajima A, Yazdani S, Tachibana T, Motoi F, Unno M, Sasano H. Quantitative digital image analysis of somatostatin receptor 2 immunohistochemistry in pancreatic neuroendocrine tumors. Med Mol Morphol. 2021;54:324-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Volante M, Grillo F, Massa F, Maletta F, Mastracci L, Campora M, Ferro J, Vanoli A, Papotti M. Neuroendocrine neoplasms of the appendix, colon and rectum. Pathologica. 2021;113:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Koppert LB, Wijnhoven BP, Tilanus HW, Stijnen T, Van Dekken H, Dinjens WN. Neuroendocrine in Barrett's mucosa and adenocarcinomas of the gastroesophageal junction. Int J Surg Pathol. 2004;12:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, Yook JH, Kim BS, Kang YK. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014;50:2802-2809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 16. | Sciarra A, Missiaglia E, Trimech M, Melloul E, Brouland JP, Sempoux C, La Rosa S. Gallbladder Mixed Neuroendocrine-Non-neuroendocrine Neoplasm (MiNEN) Arising in Intracholecystic Papillary Neoplasm: Clinicopathologic and Molecular Analysis of a Case and Review of the Literature. Endocr Pathol. 2020;31:84-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Uccella S, La Rosa S. Looking into digestive mixed neuroendocrine - nonneuroendocrine neoplasms: subtypes, prognosis, and predictive factors. Histopathology. 2020;77:700-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Klimstra DS, Klöppel G, La Rosa S. World Health Organization classification of digestive system tumours. 5th ed. Lyon: IARC Press, 2019; 16–19. [Cited in This Article: ] |
| 19. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Hervieu V, Scoazec JY. [Mixed endocrine tumors]. Ann Pathol. 2005;25:511-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Lou L, Lv F, Wu X, Li Y, Zhang X. Clinical implications of mismatch repair deficiency screening in patients with mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN). Eur J Surg Oncol. 2021;47:323-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Kleist B, Poetsch M. Neuroendocrine differentiation: The mysterious fellow of colorectal cancer. World J Gastroenterol. 2015;21:11740-11747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Wang KL, Yang Q, Cleary KR, Swisher SG, Correa AM, Komaki R, Ajani JA, Rashid A, Hamilton SR, Wu TT. The significance of neuroendocrine differentiation in adenocarcinoma of the esophagus and esophagogastric junction after preoperative chemoradiation. Cancer. 2006;107:1467-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Oneda E, Liserre B, Bianchi D, Rota L, Savelli G, Zorzi F, Zaniboni A. Diagnosis of Mixed Adenoneuroendocrine Carcinoma (MANEC) after Neoadjuvant Chemotherapy for Pancreatic and Gastric Adenocarcinoma: Two Case Reports and a Review of the Literature. Case Rep Oncol. 2019;12:434-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | de Mestier L, Cros J. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Ann Endocrinol (Paris). 2019;80:172-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Gravante G, Yahia S, Gopalakrishnan K, Mathew G. Goblet cells carcinoid with mucinous adenocarcinoma of the vermiform appendix: a step towards the unitary intestinal stem cell theory? Eur Rev Med Pharmacol Sci. 2014;18:1591-1594. [PubMed] [Cited in This Article: ] |
| 27. | Reid MD, Akkas G, Basturk O, Adsay V. Pancreatic Neuroendocrine Neoplasms. Cham, Springer, 2015: 155–165. [DOI] [Cited in This Article: ] |
| 28. | Ludmir EB, McCall SJ, Cardona DM, Perkinson KR, Guy CD, Zhang X. Mixed Adenoneuroendocrine Carcinoma, Amphicrine Type, of the Small Bowel. Am J Clin Pathol. 2016;145:703-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Misdraji J, Carr NJ, Pai RK. World Health Organization classification of tumours. Digestive system tumours. 5th ed. Lyon: IARC Press, 2019; 149–151. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1833] [Cited by in F6Publishing: 1677] [Article Influence: 419.3] [Reference Citation Analysis (2)] |
| 30. | Huang D, Ren F, Ni S, Tan C, Weng W, Zhang M, Xu M, Wang L, Xu Q, Sheng W. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int. 2019;19:310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Sun L, Wang C, Zhang J, Shao B, Zhao S, Guo Y, Li X, Sun Y. Genetic alterations in gastric amphicrine carcinomas and comparison with gastric mixed neuroendocrine-non-neuroendocrine neoplasms. Mod Pathol. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Vanacker L, Smeets D, Hoorens A, Teugels E, Algaba R, Dehou MF, De Becker A, Lambrechts D, De Greve J. Mixed adenoneuroendocrine carcinoma of the colon: molecular pathogenesis and treatment. Anticancer Res. 2014;34:5517-5521. [PubMed] [Cited in This Article: ] |
| 33. | Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, Penzel R, Endris V, Arsenic R, Hermann G, Stenzinger A, Weichert W, Pfarr N, Klöppel G. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol. 2017;30:610-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 34. | Farooq F, Zarrabi K, Sweeney K, Kim J, Bandovic J, Patel C, Choi M. Multiregion Comprehensive Genomic Profiling of a Gastric Mixed Neuroendocrine-Nonneuroendocrine Neoplasm with Trilineage Differentiation. J Gastric Cancer. 2018;18:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Sinha N, Gaston D, Manders D, Goudie M, Matsuoka M, Xie T, Huang WY. Characterization of genome-wide copy number aberrations in colonic mixed adenoneuroendocrine carcinoma and neuroendocrine carcinoma reveals recurrent amplification of PTGER4 and MYC genes. Hum Pathol. 2018;73:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Fujita Y, Uesugi N, Sugimoto R, Eizuka M, Matsumoto T, Sugai T. Gastric mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) with pancreatic acinar differentiation: a case report. Diagn Pathol. 2019;14:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, Cingarlini S, Fassan M, Scarpa A. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Woischke C, Schaaf CW, Yang HM, Vieth M, Veits L, Geddert H, Märkl B, Stömmer P, Schaeffer DF, Frölich M, Blum H, Vosberg S, Greif PA, Jung A, Kirchner T, Horst D. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod Pathol. 2017;30:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Koh J, Nam SK, Kwak Y, Kim G, Kim KK, Lee BC, Ahn SH, Park DJ, Kim HH, Park KU, Kim WH, Lee HS. Comprehensive genetic features of gastric mixed adenoneuroendocrine carcinomas and pure neuroendocrine carcinomas. J Pathol. 2021;253:94-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Yuan W, Liu Z, Lei W, Sun L, Yang H, Wang Y, Ramdas S, Dong X, Xu R, Cai H, Li JZ, Ke Y. Mutation landscape and intra-tumor heterogeneity of two MANECs of the esophagus revealed by multi-region sequencing. Oncotarget. 2017;8:69610-69621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 41. | Furlan D, Sahnane N, Mazzoni M, Pastorino R, Carnevali I, Stefanoli M, Ferretti A, Chiaravalli AM, La Rosa S, Capella C. Diagnostic utility of MS-MLPA in DNA methylation profiling of adenocarcinomas and neuroendocrine carcinomas of the colon-rectum. Virchows Arch. 2013;462:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Sahnane N, Furlan D, Monti M, Romualdi C, Vanoli A, Vicari E, Solcia E, Capella C, Sessa F, La Rosa S. Microsatellite unstable gastrointestinal neuroendocrine carcinomas: a new clinicopathologic entity. Endocr Relat Cancer. 2015;22:35-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Silva DJ, Dos Santos J, Vaz AP, Mesquita A. Rectal mixed adenoneuroendocrine carcinoma: Case report. Medicine (Baltimore). 2021;100:e27348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Volante M, Monica V, Birocco N, Brizzi MP, Busso S, Daniele L, La Rosa S, Righi L, Sapino A, Berruti A, Scagliotti GV, Papotti M. Expression analysis of genes involved in DNA repair or synthesis in mixed neuroendocrine/nonneuroendocrine carcinomas. Neuroendocrinology. 2015;101:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | COSMIC database. Available from: http://cancer.sanger.ac.uk/cosmic. [Cited in This Article: ] |
| 46. | La Rosa S, Bernasconi B, Vanoli A, Sciarra A, Notohara K, Albarello L, Casnedi S, Billo P, Zhang L, Tibiletti MG, Sessa F. c-MYC amplification and c-myc protein expression in pancreatic acinar cell carcinomas. New insights into the molecular signature of these rare cancers. Virchows Arch. 2018;473:435-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Chen MH, Kuo YJ, Yeh YC, Lin YC, Tzeng CH, Liu CY, Chang PM, Chen MH, Jeng YM, Chao Y. High neuroendocrine component is a factor for poor prognosis in gastrointestinal high-grade malignant mixed adenoneuroendocrine neoplasms. J Chin Med Assoc. 2015;78:454-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814-2823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 49. | Tang XJ, Fang XF, Zhu LZ, Wei QC, Bai XL, Liang TB, Yuan Y. Metastatic mixed acinar-neuroendocrine carcinoma of the pancreas treated by a multidisciplinary team: A case report and brief review of the literature. J Dig Dis. 2019;20:318-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Kojima M, Chen Y, Ikeda K, Tsukada Y, Takahashi D, Kawano S, Amemiya K, Ito M, Ohki R, Ochiai A. Recommendation of long-term and systemic management according to the risk factors in rectal NETs patients. Sci Rep. 2019;9:2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Yu R, Jih L, Zhai J, Nissen NN, Colquhoun S, Wolin E, Dhall D. Mixed acinar-endocrine carcinoma of the pancreas: new clinical and pathological features in a contemporary series. Pancreas. 2013;42:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Komatsubara T, Koinuma K, Miyakura Y, Horie H, Morimoto M, Ito H, Lefor AK, Sata N, Fukushima N. Endocrine cell carcinomas of the colon and rectum: a clinicopathological evaluation. Clin J Gastroenterol. 2016;9:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Juanmartiñena JF, Fernández-Urién I, Córdoba A, Miranda C, Borda A. Mixed adenoneuroendocrine carcinoma (MANEC) of the gastroesophageal junction: a case report and review of the literature. Rev Esp Enferm Dig. 2017;109:160-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Chen H, Shu M, Chen S, Xue L, Lin Y. Clinicopathological features and lymph node metastatic patterns of gastric mixed adenoneuroendocrine carcinoma. Histol Histopathol. 2019;34:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 55. | Dulskas A, Pilvelis A. Oncologic outcome of mixed adenoneuroendocrine carcinoma (MANEC): A single center case series. Eur J Surg Oncol. 2020;46:105-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Brathwaite S, Yearsley MM, Bekaii-Saab T, Wei L, Schmidt CR, Dillhoff ME, Frankel WL, Hays JL, Wu C, Abdel-Misih S. Appendiceal Mixed Adeno-Neuroendocrine Carcinoma: A Population-Based Study of the Surveillance, Epidemiology, and End Results Registry. Front Oncol. 2016;6:148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Düzköylü Y, Aras O, Bostancı EB, Keklik Temuçin T, Ulaş M. Mixed Adeno-Neuroendocrine Carcinoma; Case Series of Ten Patients with Review of the Literature. Balkan Med J. 2018;35:263-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 59. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1142] [Cited by in F6Publishing: 1122] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 60. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1702] [Cited by in F6Publishing: 1854] [Article Influence: 264.9] [Reference Citation Analysis (0)] |
| 61. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 749] [Cited by in F6Publishing: 781] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 62. | Giannetta E, Guarnotta V, Rota F, de Cicco F, Grillo F, Colao A, Faggiano A; NIKE. A rare rarity: Neuroendocrine tumor of the esophagus. Crit Rev Oncol Hematol. 2019;137:92-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Kawazoe T, Saeki H, Edahiro K, Korehisa S, Taniguchi D, Kudou K, Nakanishi R, Kubo N, Ando K, Nakashima Y, Oki E, Fujiwara M, Oda Y, Maehara Y. A case of mixed adenoneuroendocrine carcinoma (MANEC) arising in Barrett's esophagus: literature and review. Surg Case Rep. 2018;4:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Lim JS, Kurtz J, Borscheid R, Cho E, Osman H, Jeyarajah DR. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs) of the Esophagus. Am Surg. 2020;86:e101-e103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Sohda M, Kuwano H, Saeki H, Miyazaki T, Sakai M, Kakeji Y, Toh Y, Doki Y, Matsubara H. Nationwide survey of neuroendocrine carcinoma of the esophagus: a multicenter study conducted among institutions accredited by the Japan Esophageal Society. J Gastroenterol. 2021;56:350-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Laenkholm IT, Langer SW, Andreassen M, Holmager P, Kjaer A, Klose M, Federspiel BH, Hansen CP, Knigge U. A short report of 50 patients with gastroenteropancreatic mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Acta Oncol. 2021;60:808-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, Vanoli A, Tagliabue G, Pisa E, Messerini L, Centonze G, Inzani F, Scarpa A, Papotti M, Volante M, Sessa F, Fazio N, Pruneri G, Rindi G, Solcia E, La Rosa S, Capella C. Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer. 2018;25:583-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 68. | Rayhan N, Sano T, Qian ZR, Obari AK, Hirokawa M. Histological and immunohistochemical study of composite neuroendocrine-exocrine carcinomas of the stomach. J Med Invest. 2005;52:191-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Ramos MFKP, Pereira MA, Arabi AYM, Mazepa MM, Dias AR, Ribeiro U Jr, Zilberstein B, Nahas SC. Gastric Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Western Center Case Series. Med Sci (Basel). 2021;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Zecchini R, Azzolini F, Cecinato P, Iori V, De Marco L, Zanelli M, Parmeggiani F, Cavina M, Sereni G, Tioli C, Sassatelli, R. A rare case of mixed adeno-neuroendocrine gastric carcinoma(MANEC) associated to autoimmune metaplastic atrophic gastritis (AMAG). Dig Liver Dis. 2016;48:e148. [Cited in This Article: ] |
| 71. | Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, Capella C. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Ishida S, Akita M, Fujikura K, Komatsu M, Sawada R, Matsumoto H, Saegusa J, Itoh T, Kakeji Y, Zen Y. Neuroendocrine carcinoma and mixed neuroendocrine‒non-neuroendocrine neoplasm of the stomach: a clinicopathological and exome sequencing study. Hum Pathol. 2021;110:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Yeo MK, Yoon N, Bae GE. Clinicopathologic and Molecular Characteristics of Gastrointestinal MiNENs. Front Oncol. 2021;11:709097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Choi NY, Kim BS, Oh ST, Yook JH. Comparative Outcomes in Patients With Small- and Large-Cell Neuroendocrine Carcinoma (NEC) and Mixed Neuroendocrine-Non-Neuroendocrine Neoplasm (MiNEN) of the Stomach. Am Surg. 2021;87:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Huang YC, Yang NN, Chen HC, Huang YL, Yan WT, Yang RX, Li N, Zhang S, Yang PP, Feng ZZ. Clinicopathological features and prognostic factors associated with gastroenteropancreatic mixed neuroendocrine non-neuroendocrine neoplasms in Chinese patients. World J Gastroenterol. 2021;27:624-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Lin J, Zhao Y, Zhou Y, Tian Y, He Q, Lin J, Hao H, Zou B, Jiang L, Zhao G, Lin W, Xu Y, Li Z, Xue F, Li S, Fu W, Li Y, Xu Z, Chen J, Zhou X, Zhu Z, Cai L, Li E, Li H, Zheng C, Li P, Huang C, Xie J. Comparison of Survival and Patterns of Recurrence in Gastric Neuroendocrine Carcinoma, Mixed Adenoneuroendocrine Carcinoma, and Adenocarcinoma. JAMA Netw Open. 2021;4:e2114180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Yoshimachi S, Ohtsuka H, Aoki T, Miura T, Ariake K, Masuda K, Ishida M, Mizuma M, Hayashi H, Nakagawa K, Morikawa T, Motoi F, Kanno A, Masamune A, Fujishima F, Sasano H, Kamei T, Naitoh T, Unno M. Mixed adenoneuroendocrine carcinoma of the ampulla of Vater: a case report and literature review. Clin J Gastroenterol. 2020;13:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005;29:588-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 79. | Kim J, Lee WJ, Lee SH, Lee KB, Ryu JK, Kim YT, Kim SW, Yoon YB, Hwang JH, Han HS, Woo SM, Park SJ. Clinical features of 20 patients with curatively resected biliary neuroendocrine tumours. Dig Liver Dis. 2011;43:965-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Adsay V, Ohike N, Tajiri T, Kim GE, Krasinskas A, Balci S, Bagci P, Basturk O, Bandyopadhyay S, Jang KT, Kooby DA, Maithel SK, Sarmiento J, Staley CA, Gonzalez RS, Kong SY, Goodman M. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Zhang L, DeMay RM. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol. 2014;42:1075-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Grossi U, Bonis A, Carrington EV, Mazzobel E, Santoro GA, Cattaneo L, Centonze G, Gallo G, Kazemi Nava A, Romano M, Di Tanna GL, Zanus G. Mixed adenoneuroendocrine carcinoma (MANEC) of the lower gastrointestinal tract: A systematic review with Bayesian hierarchical survival analysis. Eur J Surg Oncol. 2021;47:2893-2899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Zheng M, Li T, Li Y, Zhang T, Zhang L, Ma W, Zhou L. Survival Profile and Prognostic Factors for Appendiceal Mixed Neuroendocrine Non-neuroendocrine Neoplasms: A SEER Population-Based Study. Front Oncol. 2020;10:1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Onyemkpa C, Davis A, McLeod M, Oyasiji T. Typical carcinoids, goblet cell carcinoids, mixed adenoneuroendocrine carcinomas, neuroendocrine carcinomas and adenocarcinomas of the appendix: a comparative analysis of survival profile and predictors. J Gastrointest Oncol. 2019;10:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21:2956-2962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Hock YL, Scott KW, Grace RH. Mixed adenocarcinoma/carcinoid tumour of large bowel in a patient with Crohn's disease. J Clin Pathol. 1993;46:183-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Lyss AP, Thompson JJ, Glick JH. Adenocarcinoid tumor of the colon arising in preexisting ulcerative colitis. Cancer. 1981;48:833-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 88. | Bolzacchini E, Chini C, Cortelezzi CC, Vallini I, Pinotti G, La Rosa S, Uccella S. Poorly Differentiated Neuroendocrine Carcinoma of the Sigmoid Tract in Long-Standing Ulcerative Colitis: Report of a Case and Review of the Literature. Int J Surg Pathol. 2018;26:479-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Kinugasa H, Hiraoka S, Oka S, Okada H. Ulcerative Colitis Associated with a Mixed Neuroendocrine-non-neuroendocrine Neoplasm. Intern Med. 2020;59:2085-2086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Sciammarella C, Bencivenga M, Mafficini A, Piredda ML, Tsvetkova V, Paolino G, Mastrosimini MG, Hetoja S, de Manzoni G, Mattiolo P, Borga C, Fassan M, Scarpa A, Luchini C, Lawlor RT. Molecular Analysis of an Intestinal Neuroendocrine/Non-neuroendocrine Neoplasm (MiNEN) Reveals MLH1 Methylation-driven Microsatellite Instability and a Monoclonal Origin: Diagnostic and Clinical Implications. Appl Immunohistochem Mol Morphol. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Sato H, Shiota M, Urano M, Tsukamoto T, Honda K, Toyama K, Uyama I. Mixed neuroendocrine-non-neuroendocrine neoplasm with squamous cell carcinoma covered by tubulovillous adenoma in the rectum. Clin J Gastroenterol. 2021;14:1136-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 92. | Zhang P, Li Z, Li J, Zhang X, Lu Z, Sun Y, Li Y, Zhou J, Wang X, Peng Z, Shen L, Lu M. Clinicopathological features and lymph node and distant metastasis patterns in patients with gastroenteropancreatic mixed neuroendocrine-non-neuroendocrine neoplasm. Cancer Med. 2021;10:4855-4863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Grillo F, Valle L, Sammito G, Scabini S, Albertelli M, Mastracci L. Rectal mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN): High grade evolution of a MANET? Pathol Res Pract. 2020;216:152869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne). 2015;2:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Wang Y, Gandhi S, Basu A, Ijeli A, Kovarik P, Sekosan M, Demetria M. Pancreatic Collision Tumor of Ductal Adenocarcinoma and Neuroendocrine Tumor. ACG Case Rep J. 2018;5:e39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Merigliano S, Sperti C. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: a case report and review of the literature. World J Surg Oncol. 2017;15:93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Varshney B, Bharti JN, Varshney VK, Yadav T. Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) of pancreas: a rare entity-worth to note. BMJ Case Rep. 2020;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL, Liu X, Zhang L, Giordano TJ, Bellizzi AM, Chen JH, Shi C, Allen P, Reidy DL, Wolfgang CL, Saka B, Rezaee N, Deshpande V, Klimstra DS. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 99. | Tian F, Dai MH, Jia CW, Liu ZW, Li BL. Retrospective analysis of seven cases of pancreatic mixed adenoneuroendocrine carcinoma from a high-volume center and review of the literature. BMC Surg. 2019;19:89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Yang M, Ke NW, Zhang Y, Zeng L, Tan CL, Zhang H, Mai G, Tian BL, Liu XB. Survival Analyses for Patients With Surgically Resected Pancreatic Neuroendocrine Tumors by World Health Organization 2010 Grading Classifications and American Joint Committee on Cancer 2010 Staging Systems. Medicine (Baltimore). 2015;94:e2156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Nießen A, Schimmack S, Weber TF, Mayer P, Bergmann F, Hinz U, Büchler MW, Strobel O. Presentation and outcome of mixed neuroendocrine non-neuroendocrine neoplasms of the pancreas. Pancreatology. 2021;21:224-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Manfrin E, Parisi A, Stefanizzi L, D'Onofrio M, Bernardoni L, Crino SF, Pelosi G, Pancione M, Giordano G, Sina S, Remo A. Bcl-10, trypsin and synaptophysin helps recognize acinar cell and mixed acinar neuroendocrine cell carcinoma of the pancreas on both preoperative cytological samples and needle biopsy specimens. Pathol Res Pract. 2021;226:153593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, Brennan M, Cameron JL, Klimstra DS. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 104. | Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O, Balasubramanian S, Lipson D, Yelensky R, Pao W, Miller VA, Klimstra DS, Stephens PJ. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Kim JY, Brosnan-Cashman JA, Kim J, An S, Lee KB, Kim H, Park DY, Jang KT, Oh YH, Hruban RH, Heaphy CM, Hong SM. Pancreatic acinar cell carcinomas and mixed acinar-neuroendocrine carcinomas are more clinically aggressive than grade 1 pancreatic neuroendocrine tumours. Pathology. 2020;52:336-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Lan J, Guo D, Qin X, Chen B, Liu Q. Mixed Neuroendocrine Carcinoma and Hepatocellular Carcinoma: A Case Report and Literature Review. Front Surg. 2021;8:678853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Nakano A, Hirabayashi K, Yamamuro H, Mashiko T, Masuoka Y, Yamamoto S, Ozawa S, Nakagohri T. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma: case report and literature review. World J Surg Oncol. 2021;19:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Ishida M, Seki K, Tatsuzawa A, Katayama K, Hirose K, Azuma T, Imamura Y, Abraham A, Yamaguchi A. Primary hepatic neuroendocrine carcinoma coexisting with hepatocellular carcinoma in hepatitis C liver cirrhosis: report of a case. Surg Today. 2003;33:214-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 109. | Yang CS, Wen MC, Jan YJ, Wang J, Wu CC. Combined primary neuroendocrine carcinoma and hepatocellular carcinoma of the liver. J Chin Med Assoc. 2009;72:430-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Harada K, Sato Y, Ikeda H, Maylee H, Igarashi S, Okamura A, Masuda S, Nakanuma Y. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch. 2012;460:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 111. | Ishikawa T, Nakano K, Osaka M, Aratani K, Yayoi K, Akioka K, Tsuchiya K, Hosokawa Y. Mixed neuroendocrine-non-neuroendocrine neoplasms of the gallbladder: a case report. Surg Case Rep. 2021;7:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Alawad M, Gupta R, Haseeb MA, Brunicardi FC. Clinicopathologic and Molecular Features of Mixed Neuroendocrine Non-Neuroendocrine Neoplasms of the Gallbladder. Gastroenterology Res. 2020;13:269-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Shintaku M, Kataoka K, Kawabata K. Mixed adenoneuroendocrine carcinoma of the gallbladder with squamous cell carcinomatous and osteosarcomatous differentiation: report of a case. Pathol Int. 2013;63:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 114. | de Bitter TJJ, Kroeze LI, de Reuver PR, van Vliet S, Vink-Börger E, von Rhein D, Jansen EAM, Nagtegaal ID, Ligtenberg MJL, van der Post RS. Unraveling Neuroendocrine Gallbladder Cancer: Comprehensive Clinicopathologic and Molecular Characterization. JCO Precis Oncol. 2021;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 115. | Wen LJ, Chen JH, Xu HJ, Yu Q, Deng Y, Liu K. The clinical profiles, management, and prognostic factors of biliary mixed neuroendocrine nonneuroendocrine neoplasms: A systematic review of the literature. Medicine (Baltimore). 2020;99:e23271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |