Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3524
Peer-review started: December 26, 2021
First decision: April 16, 2022
Revised: April 27, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: July 21, 2022
Sinusoidal obstruction syndrome has been reported after oxaliplatin-based chemotherapy, but liver fibrosis and non-cirrhotic portal hypertension (NCPH) are rarely reported.
Here, we describe the case of a 64-year-old woman who developed isolated gastric variceal bleeding 16 mo after completing eight cycles of oxaliplatin combined with capecitabine chemotherapy after colon cancer resection. Surprisingly, splenomegaly and thrombocytopenia were not accompanied by variceal bleeding, which has been reported to have predictive value for gastric variceal formation. However, a liver biopsy showed fibrosis in the portal area, suggesting NCPH. The patient underwent endoscopic treatment and experienced no further symptoms.
It is necessary to guard against long-term complications after oxaliplatin-based chemotherapy. Sometimes splenic size and platelet level may not always accurately predict the occurrence of portal hypertension.
Core Tip: The occurrence of portal hypertension after oxaliplatin chemotherapy is mostly considered to be related to sinusoidal obstruction syndrome, and few studies clearly support non-cirrhotic portal hypertension (NCPH). We present a case of isolated gastric variceal bleeding after oxaliplatin chemo
- Citation: Zhang X, Gao YY, Song DZ, Qian BX. Isolated gastric variceal bleeding related to non-cirrhotic portal hypertension following oxaliplatin-based chemotherapy: A case report. World J Gastroenterol 2022; 28(27): 3524-3531
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3524.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3524
The third-generation platinum anticancer drug, oxaliplatin, is often used in combination with 5-fluorouracil or capecitabine as one of the main chemotherapy strategies for neoadjuvant/adjuvant treatment of stage III and metastatic colon cancer[1]. Oxaliplatin can induce different degrees of liver injury, from mild to acute liver failure, and sinusoidal obstruction syndrome (SOS) is a characteristic manifestation. In addition, chronic injury from endothelial cell damage and architectural distortion may develop nodular regenerative hyperplasia (NRH) and non-cirrhotic portal hypertension (NCPH) years after chemotherapy[2]. As a result, there are manifestations of portal hypertension, such as splenomegaly, thrombocytopenia, ascites, and esophagogastric varices. Since these complications occur long after chemotherapy completion, clinicians may not attribute these findings to oxaliplatin. Here, we report the case of a 64-year-old woman who was treated with eight cycles of oxaliplatin combined with capecitabine after radical resection of the right colon for ascending colon cancer. Isolated gastric variceal bleeding was diagnosed and treated endoscopically, but there was no obvious accompanying splenomegaly, thrombocytopenia, or broadened portal vein. The liver biopsy was indicative of NCPH.
A 64-year-old woman was transferred to our hospital on account of hematemesis and melena.
The patient complained of hematemesis and melena 2 d prior to presentation. She underwent hemostasis and rehydration at the previous hospital. Colonoscopy and gastroscopy were successively performed, and severe gastric varices were found before administration.
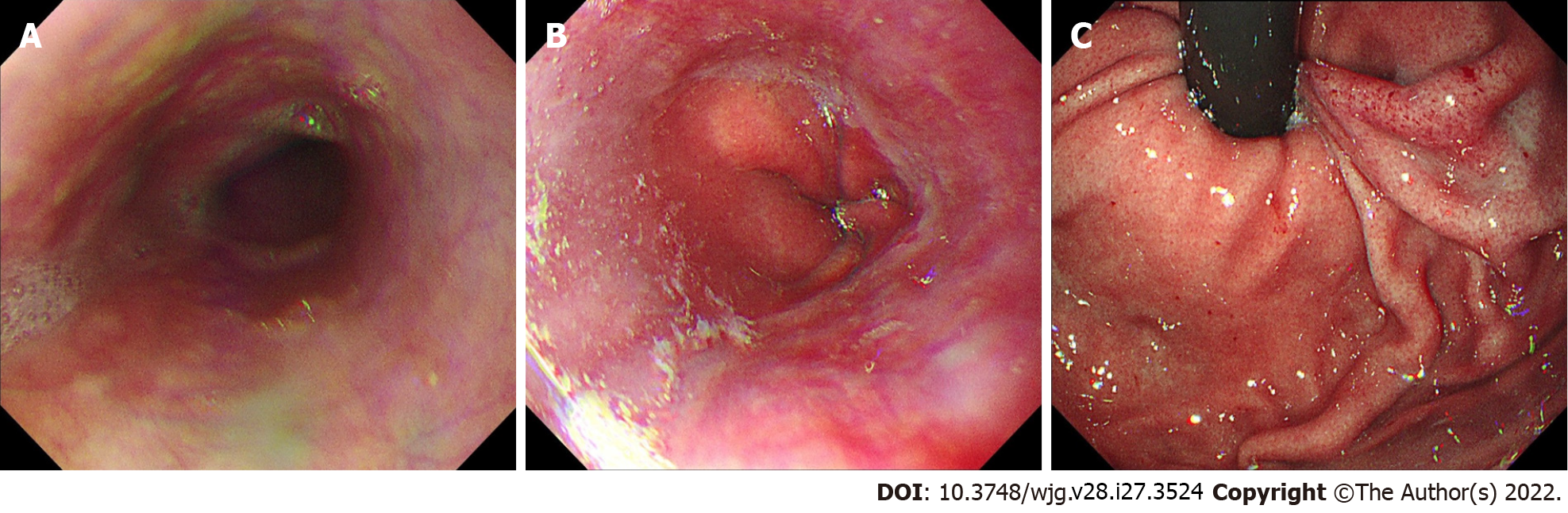
Laparoscopic radical resection of right colon cancer had been performed 16 mo earlier due to ascending colon cancer (pT4N2Mx). Preoperative gastroscopy did not show any abnormalities (Figure 1). She received eight cycles of oxaliplatin combined with capecitabine for 6 mo postoperatively. No tumor recurrence was found after intermittent re-examination.
The patient had no known history of liver disease. She had no history of habitual use of alcohol and no significant history of exposure to liver injury-inducing drugs other than chemotherapy.
The patient’s temperature was 36.6°C, heart rate was 93 bpm, respiratory rate was 16 breaths per minute, blood pressure was 125/65 mmHg, and oxygen saturation in room air was 98%. The patient had an anemic appearance and pale palpebral conjunctiva. No signs of chronic liver disease, such as palmar erythema and spider nevus, were found. No significant enlargement of the liver or spleen was detected on palpation.
Blood examination indicated severe anemia with normal levels of leukocytes and platelets. Prothrombin time was normal with a slight increase in plasma D-dimer level. Liver function test indicated mild hypoproteinemia with normal transaminase and bilirubin levels; renal function was normal. Liver tests were negative for hepatitis viruses; autoimmune liver diseases; and metabolic liver diseases, such as hepatolenticular degeneration and hemochromatosis (Table 1).
| Routine examination | Biochemical examination | Immune examination | |||
| White blood count | 11.95 x 109/L | Alaninetransaminase | 1831 U/L | Hepatitis B surface antigen | (-) |
| Red blood count | 1.9 x 1012/L | Aspartate transaminase | 31 U/L | Hepatitis C antibody | (-) |
| Hemoglobin | 60 g/L | Alkaline phosphatase | 103 U/L | Antinuclear antibody | (-) |
| Platelet | 171 x 109/L | Gamma glutamyl transpeptidase | 29 U/L | Antimitochondrial antibody | (-) |
| Prothrombin time | 14.3 s | Total bilirubin | 12.0 mmol/L | Immunoglobulin G | (-) |
| International standard ratio | 1.12 | Direct bilirubin | 1.2 mmol/L | Immunoglobulin M | (-) |
| D-dimer | 4.38 mg/L | Albumin | 33.7 g/L | Immunoglobulin G4 | (-) |
| Globulose | 29.3 g/L | ||||
| Serum creatinine | 82 mmol/L | ||||
| Potassium | 3.58 mmol/L | ||||
| Ferritin | (-) | ||||
| Ceruloplasmin | (-) |
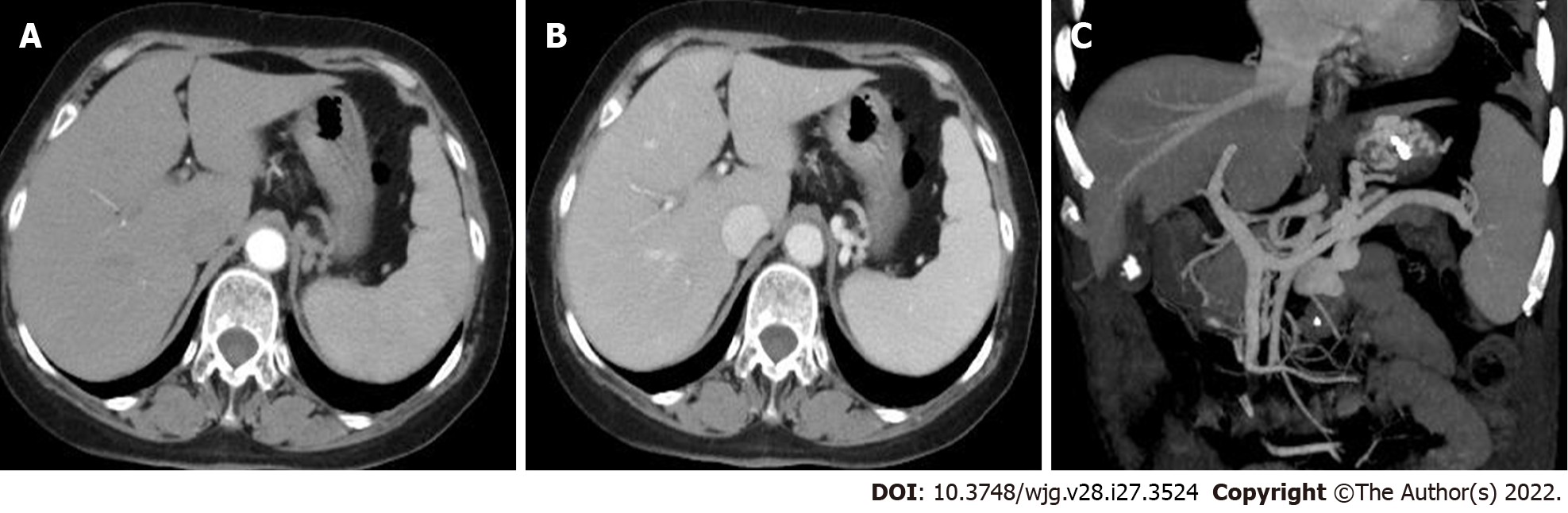
An enhanced computed tomography (CT) scan of the abdomen and pelvis showed postoperative changes of the colon, an irregular liver contour, slightly enlarged spleen, low-grade fatty liver, and varices in the gastric fundus with gastro-renal shunting (Figure 2). Contrast-enhanced ultrasonography showed that the size of the spleen was 4.2 cm × 11.2 cm. The diameter of the retropancreatic splenic vein was 0.67 cm with a mean blood flow velocity of 33.0 cm/s and volume flow of 528.7 mL/min.
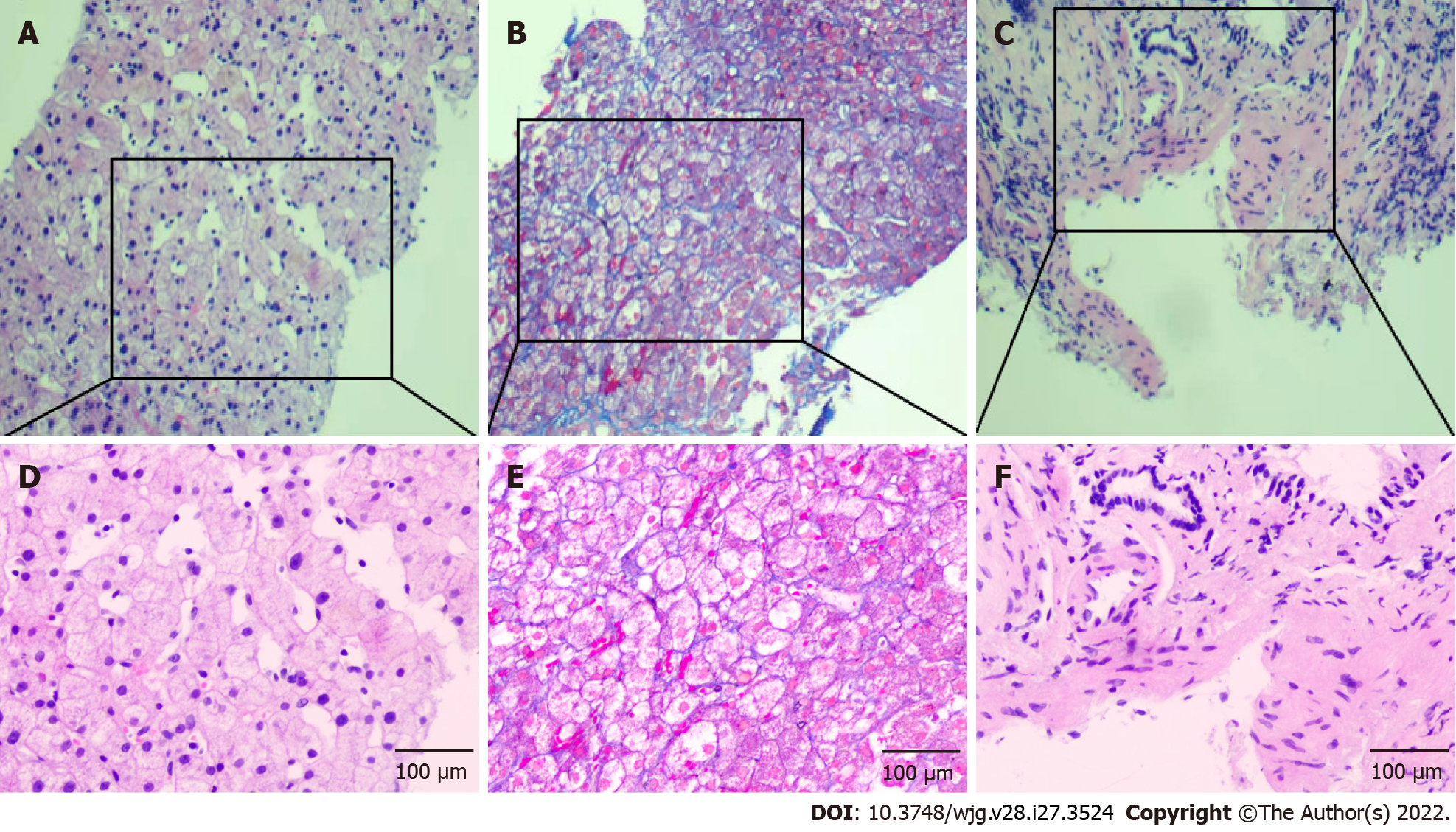
A transjugular liver biopsy was performed, and the hepatic venous pressure gradient (HVPG, wedged hepatic venous pressure – free hepatic venous pressure) was measured as 15 mmHg. Liver biopsy showed that the hepatic lobule was essentially complete with sinusoidal dilatation, and no pseudolobular formation was observed. Hepatocyte nuclear size slightly varied. Focal perisinusoidal fibrosis could be observed in the hepatic central vein area, which showed a diffuse distribution of necrotic hepatocytes engulfed by phagocytes. There were slight inflammatory reactions in hepatic portal areas, with slight fibrosis in the interstitium and around the bile duct. The vascular wall of the portal vein was thickened, indicating portal hypertension, and the final pathological diagnosis considered NCPH (Figure 3).
The final diagnosis of the presented case was NCPH due to oxaliplatin.
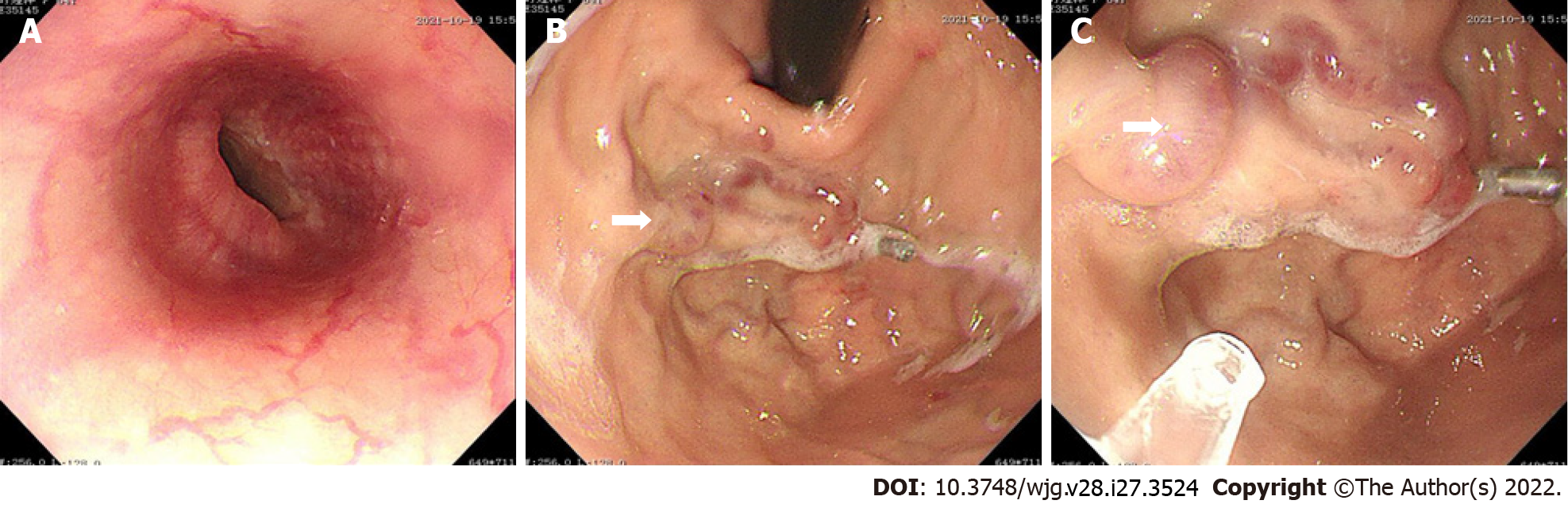
After admission, the patient was immediately administered with octreotide (50 μg/h, iv, for 72 h), proton pump inhibitor, omeprazole (8 mg/h, iv, for 72 h), and hemocoagulase. An erythrocyte suspension of volume, 400 mL, was transfused. Gastroscopy after cessation of active gastrointestinal bleeding revealed severe isolated gastric varices in the fundus of the stomach, Lg-f, F3, Cb, and RC2 (Figure 4). Cyanoacrylate glue was injected.
The patient had an uneventful postoperative clinical course. Therefore, she was discharged 7 d after operation. Beta blockers were not administered because the patient′ s mean heart rate was 58 ± 6 bpm. During a follow-up visit 2 mo after the operation, signs of anemia was absent and a new gastroscopy showed varices in fundus of stomach relieved obviously.
As one of the main chemotherapeutic agents for stage III and metastatic colon cancer, oxaliplatin is widely used in chemotherapy. Common side effects of oxaliplatin are neurotoxicity, gastrointestinal reactions, and hematological toxicity. In recent years, SOS has received more attention as a long-term complication of oxaliplatin use, and its reported incidence rate ranges from 19% to 52%[3,4]. SOS is an obliterative venulitis of the terminal hepatic venules, which is characterized by jaundice, right upper quadrant pain, tender hepatomegaly, ascites, and unexplained weight gain. SOS usually occurs as a result of cytoreductive therapy prior to hematopoietic stem cell transplantation. In China, it is often associated with the oral intake of plants that contain pyrrolidine alkaloids. This patient did not have any significant history of exposure to liver injury-inducing drugs other than chemotherapy. Therefore, the liver damage was considered to be directly related to oxaliplatin-containing chemotherapy.
The mechanism by which oxaliplatin causes hepatic sinusoidal injury is relatively clear. The inflammation caused by oxaliplatin can increase the expression of many cytokines and chemokines[5], leading to atrophy and apoptosis of hepatocytes around the hepatic sinusoids, which decreases the supporting capacity. Oxidative stress[6] and glutathione depletion[7] caused by oxaliplatin metabolism could destroy the integrity and permeability of liver sinusoidal endothelial cells (LSECs). Necrotic LSECs and red blood cells can then form emboli with platelets, which causes obstruction and expansion of the hepatic sinusoids, leading to SOS. LSECs produce cytokines and inflammatory factors, such as interleukin-6, platelet-derived growth factor, tissue inhibitor of metalloproteinase, matrix metalloproteinases, and vascular endothelial growth factor. These activate hepatic stellate cells to increase collagen levels in the extracellular matrix, ultimately leading to hepatic fibrosis[8] that can progress to NRH, NCPH, and portal sclerosis in subsequent years.
In the pathological examination of our patient, there was an obvious expansion of the hepatic sinusoids with fibrosis in the hepatic sinusoids and portal areas. The lack of typical SOS manifestations, such as intrasinusoidal hemorrhage, thrombosis, and obstruction, suggested NCPH. This pathological manifestation was similar to that described by Ryuta et al[9]. In that case, esophageal and gastric varices were found 3.5 years after the termination of oxaliplatin-based chemotherapy, compared to 16 mo in this report. Both patients developed long-term portal complications after chemotherapy completion. Vigano et al[10] analyzed liver injury reversibility after the interruption of chemotherapy and reported that SOS may resolve within 9 mo. Therefore, we suspected that the sinusoidal injury in this patient was not enough to cause typical clinical presentations of SOS, which had gradually recovered after chemotherapy termination. However, fibrosis persisted in the hepatic sinusoids and portal areas, resulting in portal hypertension and severe gastric variceal bleeding.
NCPH etiology can be divided into five groups: infection, immune disorders, thrombophilia, genetic defect, and exposure history of drugs or poisons[11]. Currently, drugs that can reportedly induce NCPH include difenoxin, azathioprine, mercaptopurine, and allopurinol[12,13]. A recent study proposed that oxaliplatin is related to NCPH[2]. The main clinical manifestations of NCPH are esophagogastric varices, splenomegaly, thrombocytopenia, and ascites, with the first being the most common[14]. Park et al[15] investigated the predictors of portal hypertension formation related to oxaliplatin use and found that noninvasive fibrosis prediction models including, the age-platelet index, aspartate aminotransferase-to-platelet ratio index, platelet-to-spleen ratio, and fibrosis-4 score, have good predictive values. Satta et al[16] reported that platelet count and spleen index under CT correlated with esophagogastric variceal formation. These indicators are often used to screen whether patients need gastroscopy because they are easily obtained. However, in this case, the patient had severe isolated gastric varices accompanied by gastro-renal shunting. No obvious increase in splenic volume or thrombocytopenia during or after chemotherapy was observed, and no widening of the portal vein was observed on enhanced CT, which was different from common esophagogastric varices related to oxaliplatin. These findings exemplify why diagnoses may be missed. Apparently, gastroscopy was not performed during follow-up after surgery and chemotherapy, which is why the varices were not discovered until bleeding occurred.
Notably, NCPH is considered as pre-sinusoidal portal hypertension. Therefore, HVPG in patients with NCPH may not exactly reflect portal hypertension with measurements lower than those in patients with liver cirrhosis. A retrospective study reported that the average HVPG of patients with NCPH was 8.3 ± 4.5 mmHg, 60% of patients had normal or slightly elevated HVPG (≤ 10 mmHg), and 40% of patients had HVPG > 11 mmHg[14]. The HVPG level in our patient was 15 mmHg, which was significantly higher than normal, possibly due to hepatic sinusoidal injury and peri-sinusoidal fibrosis.
Ligation and cyanoacrylate glue injection under gastroscopy are the first-line treatment options for esophageal and gastric variceal bleeding in liver cirrhosis[17], but whether they are appropriate for esophageal and gastric variceal bleeding related to NCPH remains controversial. A previous study reported that the effect of endoscopic therapy in preventing variceal rebleeding in patients with NCPH is not ideal, compared to its effect in preventing hepatitis B cirrhosis-related portal hypertension[18]. Due to good functional liver reserve in patients with NCPH, transjugular intrahepatic portosystemic shunt (TIPS) treatment has a lower rebleeding rate, and the incidence and mortality of postoperative hepatic encephalopathy are lower[19]. For these reasons, TIPS may be used as the first-line treatment for esophageal and gastric variceal bleeding due to NCPH.
However, this report describes a patient with NCPH who underwent colon cancer resection. Therefore, it is necessary to guard against the possibility of malignant tumor recurrence and maintain good liver function, in case reoperation and additional chemotherapy rounds are required. Liver compensation ability may decrease after TIPS treatment. Instead, gastroscopic therapy was performed with successful cyanoacrylate glue injection, and the patient was discharged.
Oxaliplatin can cause hepatic sinusoidal injury and SOS, which could lead to portal hypertension years later. Splenomegaly and thrombocytopenia have a certain predictive value for SOS occurrence in patients who previously received chemotherapy, but few unique cases remain in the clinic. The formation of esophageal and gastric varices is the only manifestation of portal hypertension; therefore, these patients are likely to be misdiagnosed. Therefore, gastroscopy should be considered as part of follow-up after oxaliplatin-based chemotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Lithuania; Fiorentini G, Italy; Yeoh SW, Australia; Yoshida H, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2653] [Cited by in F6Publishing: 2584] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 2. | Tavernier E, Chalayer E, Cornillon J, Pouvaret A, Martignoles JA, Casteillo F, Terreaux J, Daguenet E, Guyotat D. Fulminant hepatitis due to very severe sinusoidal obstruction syndrome (SOS/VOD) after autologous peripheral stem cell transplantation: a case report. BMC Res Notes. 2018;11:436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 731] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey JN, Mentha G, Terris B. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 5. | Robinson SM, Mann J, Vasilaki A, Mathers J, Burt AD, Oakley F, White SA, Mann DA. Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model. J Hepatol. 2013;59:318-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Takada S, Miyashita T, Yamamoto Y, Kanou S, Munesue S, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita J, Makino I, Nakamura K, Tajima H, Takamura H, Ninomiya I, Fushida S, Ohta T. Soluble Thrombomodulin Attenuates Endothelial Cell Damage in Hepatic Sinusoidal Obstruction Syndrome. In Vivo. 2018;32:1409-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Vreuls CP, Olde Damink SW, Koek GH, Winstanley A, Wisse E, Cloots RH, van den Broek MA, Dejong CH, Bosman FT, Driessen A. Glutathione S-transferase M1-null genotype as risk factor for SOS in oxaliplatin-treated patients with metastatic colorectal cancer. Br J Cancer. 2013;108:676-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Rubbia-Brandt L, Tauzin S, Brezault C, Delucinge-Vivier C, Descombes P, Dousset B, Majno PE, Mentha G, Terris B. Gene expression profiling provides insights into pathways of oxaliplatin-related sinusoidal obstruction syndrome in humans. Mol Cancer Ther. 2011;10:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Shigefuku R, Watanabe T, Mizukami T, Matsunaga K, Hattori N, Ehira T, Suzuki T, Nakano H, Sato Y, Matsuo Y, Nakahara K, Ikeda H, Matsumoto N, Tsuda T, Katayama M, Koizumi S, Okuse C, Suzuki M, Otsubo T, Nakajima TE, Yasuda H, Itoh F. Esophagogastric varices were diagnosed in a non-cirrhotic liver case during long-term follow-up after oxaliplatin-based chemotherapy. Clin J Gastroenterol. 2018;11:487-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Vigano L, De Rosa G, Toso C, Andres A, Ferrero A, Roth A, Sperti E, Majno P, Rubbia-Brandt L. Reversibility of chemotherapy-related liver injury. J Hepatol. 2017;67:84-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Schouten JN, Garcia-Pagan JC, Valla DC, Janssen HL. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54:1071-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 12. | Cachay ER, Peterson MR, Goicoechea M, Mathews WC. Didanosine Exposure and Noncirrhotic Portal Hypertension in a HIV Clinic in North America: a Follow-up Study. Br J Med Med Res. 2011;1:346-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Seinen ML, van Asseldonk DP, de Boer NK, Bouma G, van Nieuwkerk CM, Mulder CJ, Bloemena E, van Bodegraven AA. Nodular Regenerative Hyperplasia of the Liver in Patients with IBD Treated with Allopurinol-Thiopurine Combination Therapy. Inflamm Bowel Dis. 2017;23:448-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, Turon F, Hernandez-Gea V, Bosch J, Garcia-Pagán JC. Idiopathic portal hypertension: natural history and long-term outcome. Hepatology. 2014;59:2276-2285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Park S, Kim HY, Kim H, Park JH, Kim JH, Kim KH, Kim W, Choi IS, Jung YJ, Kim JS. Changes in Noninvasive Liver Fibrosis Indices and Spleen Size During Chemotherapy: Potential Markers for Oxaliplatin-Induced Sinusoidal Obstruction Syndrome. Medicine (Baltimore). 2016;95:e2454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Satta Y, Shigefuku R, Watanabe T, Mizukami T, Tsuda T, Suzuki T, Ehira T, Hattori N, Kiyokawa H, Nakahara K, Ikeda H, Matsunaga K, Takahashi H, Matsumoto N, Okuse C, Suzuki M, Sunakawa Y, Yasuda H, Itoh F. Prediction of esophagogastric varices associated with oxaliplatin administration. JGH Open. 2021;5:1289-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | He FL, Qi RZ, Zhang YN, Zhang K, Zhu-Ge YZ, Wang M, Wang Y, Jia JD, Liu FQ. Transjugular intrahepatic portosystemic shunt and splenectomy are more effective than endoscopic therapy for recurrent variceal bleeding in patients with idiopathic noncirrhotic portal hypertension. World J Clin Cases. 2020;8:1871-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Huang X, Li F, Wang L, Xiao M, Ni L, Jiang S, Ji Y, Zhang C, Zhang W, Wang J, Chen S. Endoscopic treatment of gastroesophageal variceal bleeding after oxaliplatin-based chemotherapy in patients with colorectal cancer. Endoscopy. 2020;52:727-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Lv Y, Li K, He C, Luo B, Zhang B, Liu H, Wang Z, Guo W, Wang Q, Chen H, Bai W, Yuan X, Yu T, Li X, Yuan J, Han N, Zhu Y, Niu J, Xie H, Wang J, Chen L, Yin Z, Fan D, Li Z, Han G. TIPSS for variceal bleeding in patients with idiopathic non-cirrhotic portal hypertension: comparison with patients who have cirrhosis. Aliment Pharmacol Ther. 2019;49:926-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |