Published online Oct 21, 2021. doi: 10.3748/wjg.v27.i39.6572
Peer-review started: January 28, 2021
First decision: May 2, 2021
Revised: May 9, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: October 21, 2021
Pancreatic carcinoma (PC) is one of the leading causes of cancer-related deaths worldwide. Despite early detection and advances in therapeutics, the prognosis remains dismal. The outcome and therapeutic approach are dependent on the stage of PC at the time of diagnosis. The standard of care is surgery, followed by adjuvant chemotherapy. The advent of newer drugs has changed the landscape of adjuvant therapy. Moreover, recent trials have highlighted the role of neoadjuvant therapy and chemoradiotherapy for resectable and borderline resectable PC. As we progress towards a better understanding of tumor biology, genetics, and microenvironment, novel therapeutic strategies and targeted agents are now on the horizon. We have described the current and emerging therapeutic strategies in PC.
Core Tip: An improved understanding of the natural history of pancreatic carcinoma, genetics, and tumor biology has highlighted the role of novel therapeutic strategies. However, despite recent advances in the management of pancreatic carcinoma, the prognosis remains poor. We have attempted to conceptualize the current therapeutic strategies in light of recent advances.
- Citation: Manrai M, Tilak TVSVGK, Dawra S, Srivastava S, Singh A. Current and emerging therapeutic strategies in pancreatic cancer: Challenges and opportunities. World J Gastroenterol 2021; 27(39): 6572-6589
- URL: https://www.wjgnet.com/1007-9327/full/v27/i39/6572.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i39.6572
World over, new cases of pancreatic carcinoma (PC) add up close to three lakh each year[1,2]. There hasn’t been a significant increase in the long-term survival rates, with the 5-year survival rates increasing to 5%-6% over the last 30 years, despite early detection and advances in therapeutics for pancreatic cancer[3,4]. The estimates of leading causes of cancer deaths suggest that PC may become the second, next only to lung cancer in the United States over the next decade[1].
Pancreatic ductal adenocarcinoma (PDAC) is exemplified by abundant genetic mutations, germline or acquired. Among the common ones are CDK2NA and KRAS seen in nearly 90%, TP53 in 75%-90%, and SMAD4/DPC4 in about 50%[5,6]. Additio
The outcomes of PC depend on the stage at diagnosis. Nearly half the cases are diagnosed as metastatic, wherein the survival ranges from 7-11 mo, at best[7,8]. In cases where the disease is non-metastatic but unresectable, there is a modest increase of survival, of nearly 6 months over the metastatic disease. The peculiarity of resectable PC lies in the poor overall survival, of approximately 2 years with adjuvant therapy. This is in stark contrast to most of the other resectable cancers.
The standard of care of resectable PC is surgery followed by adjuvant chemotherapy (CT). The benefit for this approach was established by the European Study Group for Pancreatic Cancer 1 (ESPAC-1) and the CONKO-001 trials, using 5-fluorouracil (5-FU)/leucovorin and gemcitabine respectively[7-9]. The phase III randomized PRODIGE 24 trial using 5-FU/leucovorin with irinotecan and oxaliplatin (FOLFIRINOX) and APACT trial with nab-paclitaxel with gemcitabine changed the landscape of adjuvant therapy following their success noticed in the metastatic setting[10,11]. The role of chemoradiotherapy (CRT) in the adjuvant setting is yet to see the final statement based on the existing literature. Following the lack of survival benefit with CRT in the ESPAC-1 and European Organization for Research and Treatment of Cancer (EORTC) trials and contrasting results with two registry data showing survival benefit of CRT compared to CT, one large series compared the three modalities of systemic CT, CRT or CRT followed by CT. There was a significant survival benefit with CT and CRT followed by CT than in the CRT in patients with stage III disease. This benefit was however not seen in patients with stage I/II disease[12-16].
The management of borderline resectable pancreatic adenocarcinomas (BRPCs) has seen the emergence of adjuvant regimes in the ‘neoadjuvant’ or ‘induction therapy’ role with FOLFIRINOX and nab-paclitaxel with gemcitabine[17,18]. There is no robust data to suggest the survival benefit of these protocols so far; however, there has been demonstrable tolerability and increased resection rates. A clinical challenge has been to offer adjuvant therapy to patients receiving induction therapy. The results of phase II ESPAC-5F trial, presented at the 2020 virtual ASCO meeting, comparing four arms- frontline surgery, induction therapy with gemcitabine and capecitabine, or modified FOLFIRINOX and CRT. The study revealed similar outcomes between the frontline and induction treatment[19].
The metastatic setting is seeing numerous trials with conventional CT as well as targeted agents as the biology and tumor microenvironment, genetics, and molecular concepts are being better understood. This has led to a search for novel therapeutic strategies for managing PC. This review attempts to address the challenge faced by the practicing clinician in optimal sequencing of the available modalities in the various stages of the illness.
A resectable adenocarcinoma does not have metastases to a distant organ or distant lymph nodes; there is no vascular involvement [characterized by absence of superior mesenteric vein (SMV) or portal vein (PV) involvement], tumor thrombosis, or venous encasement > 180°. Also, the fat planes around the celiac axis (CA), hepatic artery (HA), and superior mesenteric artery (SMA) ought to be clear[20].
Surgery is the only treatment option that offers a cure for PDAC. Surgery aims to completely resect the tumor and achieve a microscopically negative tumor margin (R0). R0 dissection is defined as clearance of > 1 mm i.e., the margin of healthy tissue around the removed tumor should be > 1 mm. The various surgical options include pancreaticoduodenectomy (Whipple’s procedure) and distal pancreatectomy. Pancreaticoduodenectomy with SMA first approach is the standard of care for adenocarcinoma localized to the head of the pancreas (HOP). The surgery should involve dissection of greater than 15 lymph nodes and skeletonization of SMA down to adventitia of anterior, left lateral and posterior borders[21,22]. A sampling of para-aortic lymph nodes with an examination of the frozen section is an additional option. For PDAC involving the body and tail of the pancreas, distal pancreatectomy along with splenectomy is the treatment option. This involves dissection of greater than 15 lymph nodes[23,24].
Minimally invasive techniques for pancreatic resection beginning with laparoscopic distal pancreatectomy have been attempted. They offer advantages in the form of reduced blood loss and decreased hospital stay. However, the rate of achieving positive resection margin, morbidity, and mortality of the procedure remains the same as that of an open procedure. The use of robotic techniques in Whipple’s procedure has shown reduced rates of post-procedure complications[25]. Traditionally pre-operative biliary drainage has been advised for patients who present with obstructive jaundice. Recent evidence, however, points towards a higher rate of perioperative complications among those undergoing pre-operative drainage vs those undergoing upfront surgery[26].
The risk of developing tumor recurrence among those patients who undergo curative resection for PC varies from 69%-75% at 2 years to 80%-90% at 5 years post-surgery[27]. Tumor recurrence occurs secondary to locoregional occurrence in a majority of cases. This led to the hypothesis that the use of adjuvant therapy may reduce locoregional tumor recurrence.
Post-operative complications may reduce a patients’ access to adjuvant CT and overall survival. Hence, it becomes imperative to screen patients who are at high risk of post-operative complications, like elderly patients, patients with poor performance status, or higher comorbidity profiles. Preoperative pancreatic resection score (PREPARE) and Surgical results analysis and search (SOAR) are validated and useful scoring systems for assessing the risk of developing complications post-operatively[28,29].
The gold standard treatment for resectable PC is surgery followed by adjuvant CT. The era of adjuvant CT gained prominence when the results of the European Group for Pancreatic Cancer (ESPAC-1) trial showed significant improved median survival and 5-year survival in patients who received adjuvant CT of fluorouracil and folinic acid vs those who underwent surgery alone (20.1 mo vs 15.5 mo, respectively; P = 0.009). This was followed by the CONKO-001 (Charité Onkologietrial) trial, using adjuvant gemcitabine, which showed a median disease-free survival of 13.4 mo and 5-year survival of 20.7% vs 10.4% vs 6.9 mo respectively in the surgery alone group. The efficacy of these two treatment regimens was compared in the ESPAC-3 trial. Results of this study showed no survival benefit of one treatment regimen over the other, however, the treatment-related adverse effects were higher in the fluorouracil and folinic acid group.
To further improve the therapeutic outcome with adjuvant CT, a concept of combination systemic therapy has evolved. Several agents have been studied in various trials (Table 1). Among those of note are the ESPAC-4, PRODIGE and APACT studies. The ESPAC-4 trial carried out a comparison of gemcitabine vs a combination of gemcitabine plus capecitabine, which showed favorable overall survival benefit while using combination therapy [hazard ratio (HR): 0.82, 95% confidence interval (CI): 0.68-0.98; P = 0.032]. However, no significant recurrence-free survival benefit was seen in 2 years of follow-up of these patients (HR: 0.86, 95%CI: 0.73-1.02; P = 0.082). The Partenariat de Recherche en Oncologie Digestive (PRODIGE 24-PA6) trial highlighted the successful use of FOLFIRINOX (oxaliplatin + irinotecan + leucovorin) vs gemcitabine in patients with good performance status (Eastern Cooperative Oncology Group, ECOG: 0-1). The median disease-free survival in patients with combination therapy was 21.6 mo vs 12.8 mo in the gemcitabine group. The latest in series is the Nab-paclitaxel and Gemcitabine vs Gemcitabine Alone as Adjuvant Therapy for Patients with Resected Pancreatic Cancer (APACT) study, which has shown encouraging results of using combination therapy of gemcitabine plus nanoparticle albumin-bound paclitaxel (nab-paclitaxel). Median disease-free survival was not statistically significant in the two arms (19.4 mo in combination arm vs 18.8 mo in gemcitabine only arm). Overall survival favored the combination CT group (HR: 0.82, 95%CI: 0.68-0.996; P = 0.045)[27].
| Study | No. of patients | Treatment arms | Median DFS in mo | Median OS in mo |
| GITSG[33] | 43 | Observation | NR | 20.0 |
| Radiotherapy + 5-FU f/b adjuvant 5-FU | NR | 10.9 | ||
| ESPAC-1[34] | 289 | Observation | NR | 15.5 |
| Chemoradiotherapy | NR | 13.9 | ||
| 5-FU/folinic acid | NR | 20.1 | ||
| Chemoradiotherapy + 5-FU/folinic acid | NR | 19.9 | ||
| CONKO-001[9] | 354 | Observation | 6.7 | 20.2 |
| Gemcitabine | 13.4 | 22.8 | ||
| ESPAC-3[35] | 1088 | 5-FU/folinic acid | 14.1 | 23.0 |
| Gemcitabine | 14.3 | 23.6 | ||
| ESPAC-4[36] | 730 | Gemcitabine | 13.1 | 25.5 |
| Gemcitabine + Capecitabine | 13.9 | 28.0 | ||
| CONKO-005[30] | 436 | Gemcitabine | 11.4 | 26.5 |
| Gemcitabine + Erlotinib | 11.4 | 24.6 | ||
| PRODIGE 24-PA6[37] | 493 | Gemcitabine | 12.8 | 35.0 |
| FOLFIRINOX | 21.6 | 54.4 | ||
| APACT[11] | 866 | Gemcitabine | 18.8 | 36.2 |
| Gemcitabine + nab-paclitaxel | 19.4 | 40.5 |
Another concept is the use of targeted agents (erlotinib or sorafenib) or immunotherapy (algenpantucel-L) in combination with gemcitabine. However, to date, none of these agents have shown any favorable survival outcome vs the use of gemcitabine alone[30-32].
Adjuvant CRT aims to prevent locoregional tumor recurrence. Amongst the first trials to assess the efficacy of CRT in PC was the EORTC trial, which showed no significant survival benefit amongst patients of PDAC who received CRT (40 Gy + continuous infusion of fluorouracil)[13]. This was followed by the ESPAC-1 trial, which employed the following three different adjuvant therapy designs: CRT, CT alone, and CRT followed by adjuvant CT. Patients undergoing adjuvant CT were found to have poor survival benefits after a median follow-up of 47 mo (HR: 1.28, 95%CI: 0.99-1.66; P = 0.05)[34]. The RTOG 9704 trial was carried out to assess the benefit of adding gemcitabine to postoperative radiation + fluorouracil vs adjuvant therapy with fluorouracil. The trial showed no survival benefit in either of the two treatment groups[38].
Although these trials failed to prove any survival benefit, they nevertheless provided useful information on the feasibility and tolerability of these treatment options. Analysis of the US National Cancer Database which included patients of pancreatic adenocarcinoma who underwent curative resection followed by adjuvant CT showed survival benefit (median overall survival with CT + radiation: 22.3 mo and adjuvant CT alone: 20.0 mo; P = 0.001)[14].
There are definite gaps in our knowledge regarding the optimal timing of initiating CT following surgery and follow-up of patients undergoing adjuvant therapy for curable PC. Delay in initiating as well as non-initiation of adjuvant treatment is not uncommon. Post-operative morbidity adversely impacts the initiation of adjuvant treatment. It has been estimated that approximately 20% of patients become ineligible for adjuvant CT. The ESPAC-3 trial aimed to analyze the outcome among those who were initiated on CT within 8 wk of surgery and those who were initiated on CT after 8 wk of surgery. There was no survival benefit in either of the two arms, while successful completion of six cycles of CT was found to be an independent predictor of survival[39]. Similar results have been put forward by analyzing multi-institutional retrospective data of patients who had undergone curative resection for PDAC. Thus, the evidence so far suggests that patients who receive adjuvant therapy more than 12 wk following surgery are still good candidates for adjuvant CT[40].
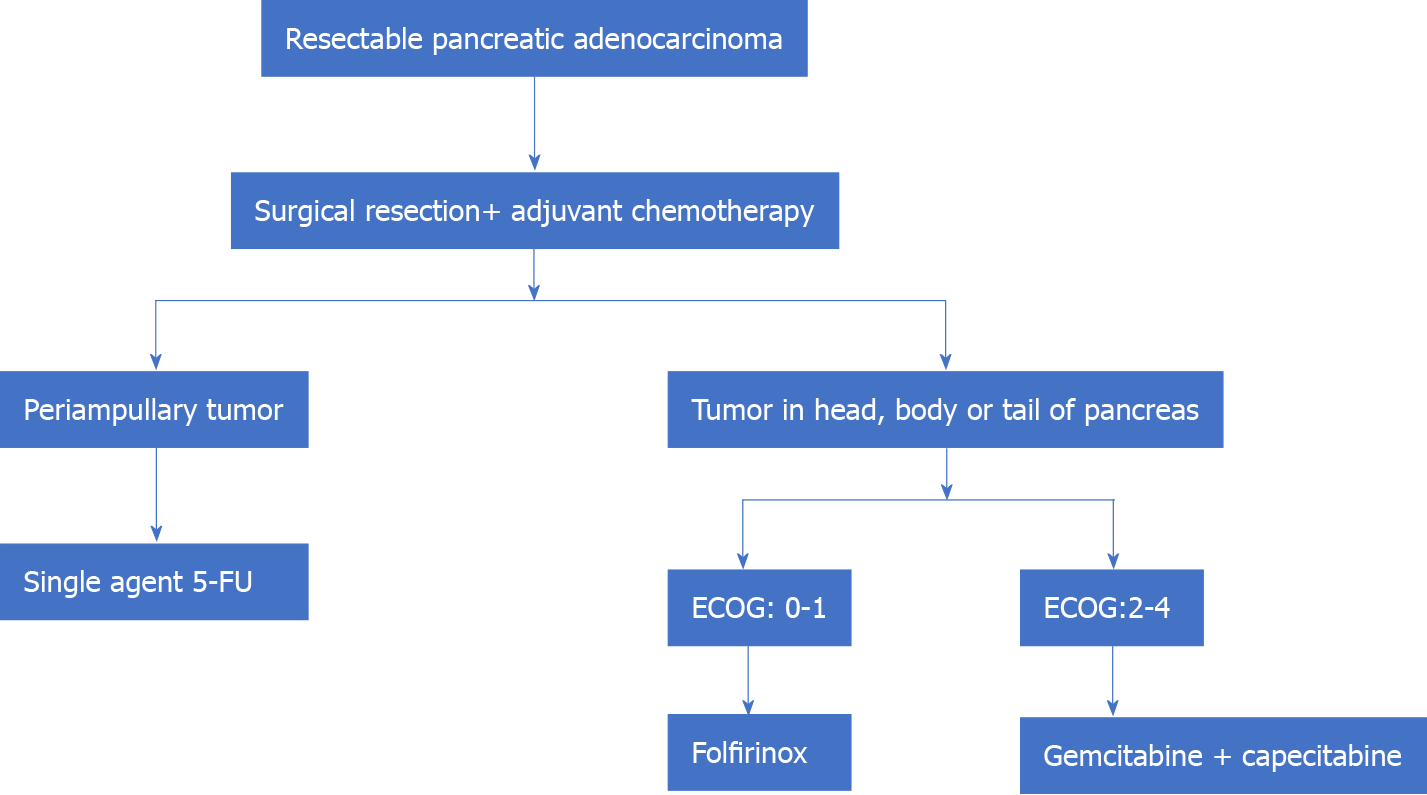
Therefore, in summary, the standard of care in resectable pancreatic adenocarcinoma is surgical resection followed by adjuvant CT. Single-agent (5-FU) is preferred for periampullary tumors of pancreatic origin. Patients with adenocarcinoma in the head, body, or tail of the pancreas may be treated with FOLFIRINOX (patients with good post-operative performance status, ECOG: 0-1) and combination therapy of gemcitabine+ capecitabine in those with poor performance status postoperatively (Figure 1).
Conceptually, the use of neoadjuvant therapy in resectable PC gained prominence when this approach was shown to have improved survival benefit in other gastrointestinal malignancies[41]. It offers the following theoretical advantages: reduction of circulating tumor cells and micrometastasis before surgery, and avoiding surgery in those who experience disease progression while on neoadjuvant therapy, thus, reducing surgical mortality and morbidity. Of particular interest is the ability of neoadjuvant treatment to achieve tumor downsizing or downstaging (lower T and N stages, reducing the rates of vascular, lymphatic, and perineural invasion, and the ability to achieve better R0 resection post-surgery). Moreover, individuals who receive neoadjuvant CT are more likely to be able to access the entire therapeutic sequence[42,43].
The preoperative or postoperative CT for resectable pancreatic adenocarcinoma (PACT-15) trial has shown the efficacy of neoadjuvant CT in patients with resectable PDAC[44]. One of the first published phase III trials assessing the role of neoadjuvant gemcitabine followed by gemcitabine and radiation before surgery is the PREOPANC trial. Although it has not shown statistically significant survival benefit in patients using neoadjuvant CT + CRT (overall survival of 16 mo for neoadjuvant therapy vs 14.3 mo for initial surgery), better R0 resection rates, locoregional disease-free survival, and lower rates of vascular and perineural invasion favor neoadjuvant CT[45]. Analyses of ‘US National Cancer Database data have shown a favorable survival using perioperative CT in patients with early PC as compared with upfront surgery. Another similar cohort study by Mokdad and colleagues[46] demonstrated statistically significant median overall survival in the neoadjuvant group vis a vis upfront surgery group (26 mo vs 23 mo respectively; P = 0.01). Many other phase III trials are being conducted to have a better understanding of this therapeutic aspect[27] (Table 2). Neoadjuvant treatment is well tolerated. The risk of postoperative pancreatic fistula formation (3%-11%), risk of postoperative infections (3%-7%) and mortality (0%-4%) compared to those who have undergone surgery alone[47].
The National Comprehensive Cancer Network has defined BRPC based on the following radiological criteria: Contrast-enhanced computerized tomographic (CECT) scan using the pancreatic protocol, the relationship of tumor with the surrounding vasculature, and the absence/presence of metastasis.
Thus, a BRPC localized to the HOP is one where the tumor is in contact with the common HA but without extension to the CA or artery bifurcation and variant arterial anatomy, contact with the SMA < 180°, contact with the SMV or PV < 180° without venous contour irregularity or thrombosis, which allows for safe and complete arterial and venous resection and reconstruction[20].
Borderline resectable PDAC in the pancreatic body or tail is ‘the solid tumor in contact with the CA of < 180° or contact with the CA > 180° without the involvement of the aorta and gastroduodenal artery to allow a ‘modified Appleby surgery’[20]. Another definition, the Anderson classification for BRPC, classifies it into the following three different groups: Group A includes patients with a tumor that abuts visceral arteries or causes short-segment occlusion of SMV; group B, have findings suggestive of metastasis; and group C patients are those who have marginal performance status[49].
Historically, therapeutic options for BRPC consist of upfront surgery, surgery followed by adjuvant CT/CRT, and neoadjuvant CT. The standard surgical options remain Whipple procedure, total pancreatectomy, or distal pancreatectomy, based on the tumor localization. An approach favoring upfront surgery carries with itself the risk of early failure, which has often been attributed to the poor pre-operative staging of tumor radiologically, inability to carry out a radical surgery, and the aggressive tumor behavior owing to variations in tumor biology. This has brought about an interest in considering neoadjuvant CT for patients with BRPC.
A meta-analysis carried out by the Dutch Pancreatic Cancer Group has shown a median survival of 19.2 mo in patients undergoing neoadjuvant CT vs 12.8 mo in those undergoing upfront surgery[50]. A peculiar problem in comparing different groups arises when different CT/CRT regimens are being compared. A recent patient-level meta-analysis has analyzed the efficacy of neoadjuvant FOLFIRINOX in BRPC, finding a favorable trend using FOLFIRINOX (median overall survival of 22 mo and median disease progression-free survival of 18.0 mo)[18].
A recent randomized control trial has assessed the use of CRT as neoadjuvant therapy for BRPC. The investigators combined 54 Gy in 30 fractions + weekly gemcitabine followed by adjuvant gemcitabine. Results have shown a better resection rate in the neoadjuvant group vs the surgery only group (51.8% vs 26.1% respectively) as well as a statistically significant 2-year survival (40.7% vs 26%; P = 0.028)[51]. The preoperative CRT vs immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1) trial has used intention-to-treat analysis to study the efficacy of neoadjuvant CRT (gemcitabine-based) vs upfront surgery in BRPC. Patients in the neoadjuvant CRT group had a lower resection rate (60%) vs those in the upfront surgery group (72%; P = 0.065). R0 resection rate was statistically higher in the neoadjuvant group vs the upfront surgery group (61% vs 31%; P < 0.001). To add to the benefits, patients receiving neoadjuvant CRT had a longer median time till recurrence vs the surgery only group (9.9 mo vs 7.9 mo; P = 0.023)[48].
Results of these trials have shown the efficacy of both neoadjuvant CT as well as for CRT in BRPC. Several ongoing trials are being carried out to study the efficacy of induction therapy in improving overall survival in BRPC and which of the two therapeutic strategies (CT/CRT) is better suited for the same.
The concept of locally advanced PC (LAPC) has evolved based on the development of radiological criteria of resectability and the availability of neoadjuvant therapy. Practically, the pancreatic tumors which are not metastatic and are unresectable due to ‘irreversible’ vascular invasion (encasement of aorta, invasion of PV or SMV, involvement of the SMA or celiac trunk by > 180°) are considered as LAPC[52,53]. The median survival has been variably reported from 10 mo to 30 mo.
The standard treatment for LAPC is gemcitabine-based CT. For patients with good performance status (ECOG0-1), the FOLFIRINOX-based regimen and for those with ECOG0-2, the nab-paclitaxel-gemcitabine-based regimen may be considered[53]. Some observational studies and pooled analyses of different approaches have advocated induction treatment with either FOLFIRINOX or nab-paclitaxel–gemcitabine followed by CRT. Using this approach median survival of 24.2 mo and disease progression-free survival of 15 mo has been reported[54]. However, the use of either upfront radiation therapy or following induction treatment with gemcitabine is not beneficial for patients with LAPC. Recently published meta-analysis has reported similar overall survival and drug-related side effects of CRT and CT in the setting of LAPC[55]. CRT using capecitabine as a radiosensitizer, however, has been tried for improvement of local control of disease in a subset of patients with LAPC. Since this is not a standard treatment approach, it has been proposed that this may be offered to only a select group of individuals[56].
Ongoing trials in LAPC are trying to assess the efficacy of FOLFIRINOC vs gemcitabine as induction therapy (NEOPAN; NCT02539537), use of nab-paclitaxel-gemcitabine for induction regimen + radiation therapy vs continuous CT, and use of activation of the DPC4 gene (RTOG 1201; NCT01921751).
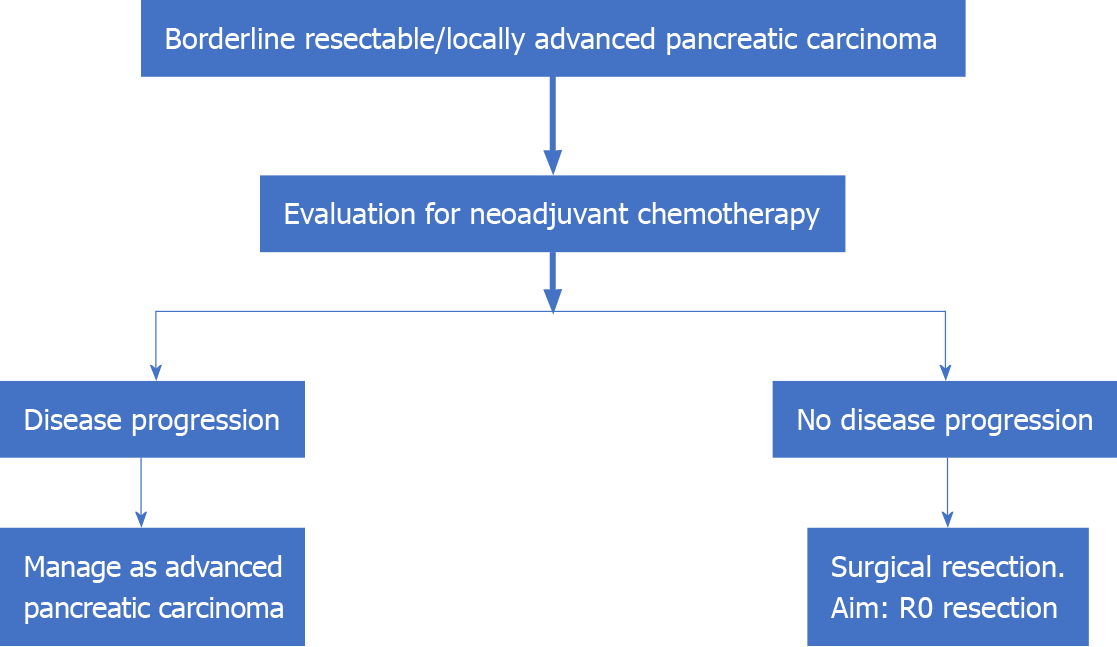
An interesting recent concept that has emerged in the management of LAPC is the role of surgery. This was proposed by researchers from the Medical College of Wisconsin based on the observation that those with an initially unresectable disease as per radiological criteria may convert to the resectable tumor after induction CT ± CRT. They have proposed the classification of patients with LAPC into the following two distinct categories: Type A, tumors that may be considered for resection following induction CT; and type B: Definitively unresectable tumors (> 270° encasement of the SMA, > 180° encasement of the CA, encasement of the aorta, and > 180° encasement of the HA with extension beyond the bifurcation of the proper HA into right and left HA)[52]. Thus, patients with LAPC type A, who have completed induction CT should be considered for surgical exploration. This is especially pertinent as the specificity of a CECT scan to determine tumor staging, operability, and R0 resectability for HOP carcinoma decrease following induction therapy. In such a setting, surgical exploration is the ideal modality to prove/ rule out vascular involvement[57]. Resected patients who have received CT preoperatively can also be considered for an additional course of CT following surgery[58] (Figure 2).
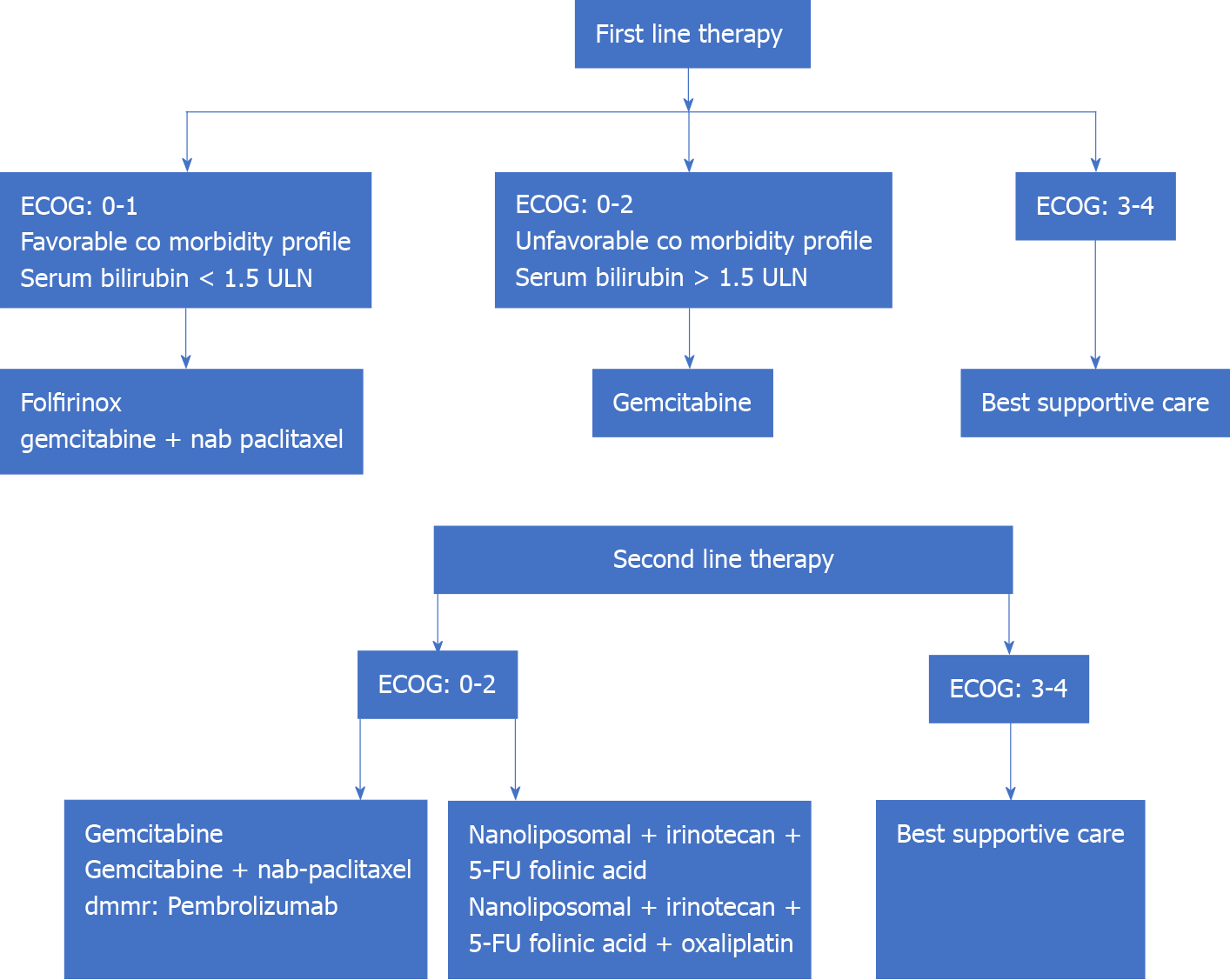
CT forms the backbone of therapeutic regimens for the management of metastatic PC. Till the advent of gemcitabine, fluorouracil was the only approved drug in the management of APC. The use of gemcitabine has brought about benefits of disease progression-free survival, and overall survival (from 4.4 mo to 5.7 mo, P = 0.0025) with similar drug-related side effects as compared to fluorouracil[59]. This was followed by trials that analyzed the survival benefit of adding another cytotoxic drug or targeted therapy to gemcitabine. Use of erlotinib to gemcitabine-based regimen has shown median overall survival benefit (5.91 mo to 6.24 mo, P = 0.038) and 1-year survival benefit (17% to 23%, P = 0.023)[60]. A combination regimen consisting of cisplatin, epirubicin, fluorouracil, and gemcitabine (PEFG) vs gemcitabine has shown ‘four-month progression-free survival’ of 60% vs 28% in gemcitabine alone arm, P = 0.001 with no difference in overall survival[44]. A combination of gemcitabine + nab-paclitaxel in patients with ECOG0-2 has shown an improvement of ‘median overall survival’ vs gemcitabine alone (8.5 mo vs 6.7 mo, P < 0.001). The PRODIGE 4-ACCORD11 trial is a landmark trial that has shown the overall survival benefit of using FOLFIRINOX (median survival: 11.1 mo) in patients with APC vs gemcitabine (6.8 mo, P < 0.001)[10]. FOLFIRINOX has not been compared to nab-paclitaxel + gemcitabine in any prospective trial to date. Thus, the FOLFIRINOX regimen is now the standard of care for patients of APC with ECOG: 0-1, normal serum bilirubin, and no underlying cardiac pathology. The limiting step in the FOLFIRINOX regimen happens to be the performance status of the individual and comorbidity profile with the elderly or low-profile patients likely to have a poorer outcome. Other prognostic factors are the number of metastases, and liver metastases, presence of genetic mutations such as DNA damage response (DDR) gene mutations and BRCA tumor suppressor gene mutations[61-63]. Modified FOLFIRINOX has been tried with similar efficacy and better tolerance profile as compared to the standard FOLFIRINOX regimen. This regimen includes fluorouracil bolus suppression or a dose reduction of irinotecan (or both)[64].
Progress of disease in patients with first-line CT regimens presents a particular challenge with around 50% of patients being eligible for second-line CT[65]. Combination regimens like gemcitabine-platinum and fluoropyrimidine-platinum have shown disease progression-free survival of 2.5 mo vs 1.9 mo for single agents (P = 0.169) but no improvement in overall survival (5.1 mo vs 4.3 mo, P = 0.169)[66]. The various combination regimens that have been tried on the failure of first- and second-line regimens are oxaliplatin, folinic acid, and 5-FU (OFF regimen), and nanoliposomal irinotecan (MM-398), 5-FU, and folinic acid regimen. The CONKO-003 trial has shown overall survival benefit of using the OFF regimen over the 5-FU–folinic acid (FF regimen); the median survival was 5.9 mo vs 3.3 mo respectively (P = 0.01)[67]. The disease progression-free survival with use of nanoliposomal irinotecan (MM-398), 5-FU, and folinic acid regimen after failure of the FOLFIRINOX regimen has been reported to be 5.1 mo with overall survival of 8.8 mo[68]. The results of using targeted agents, namely the Jak1 and Jak2 tyrosine kinase inhibitor ruxolitinib and glufosfamide, have been rather disappointing. However, patients with metastatic solid tumors (including 8 patients with pancreatic tumors), with deficient mismatch repair and failed first-line therapy have shown response to the PD-1 immune checkpoint inhibitor (ICI) pembrolizumab (disease control rate: 77%, objective response: 53% and complete radiological recovery: 21%)[69]. Mutation in the BRCA gene has been reported in around 5% of patients with APC. Targeted therapy in patients with BRCA gene mutation using ‘poly ADP-ribose polymerase (PARP) inhibitors’ is being actively investigated[70] (Figure 3).
Palliative treatment aims to allay patients’ symptoms and improve their quality of life. Pain management, symptomatic relief, and psychological support are the pillars of this strategy.
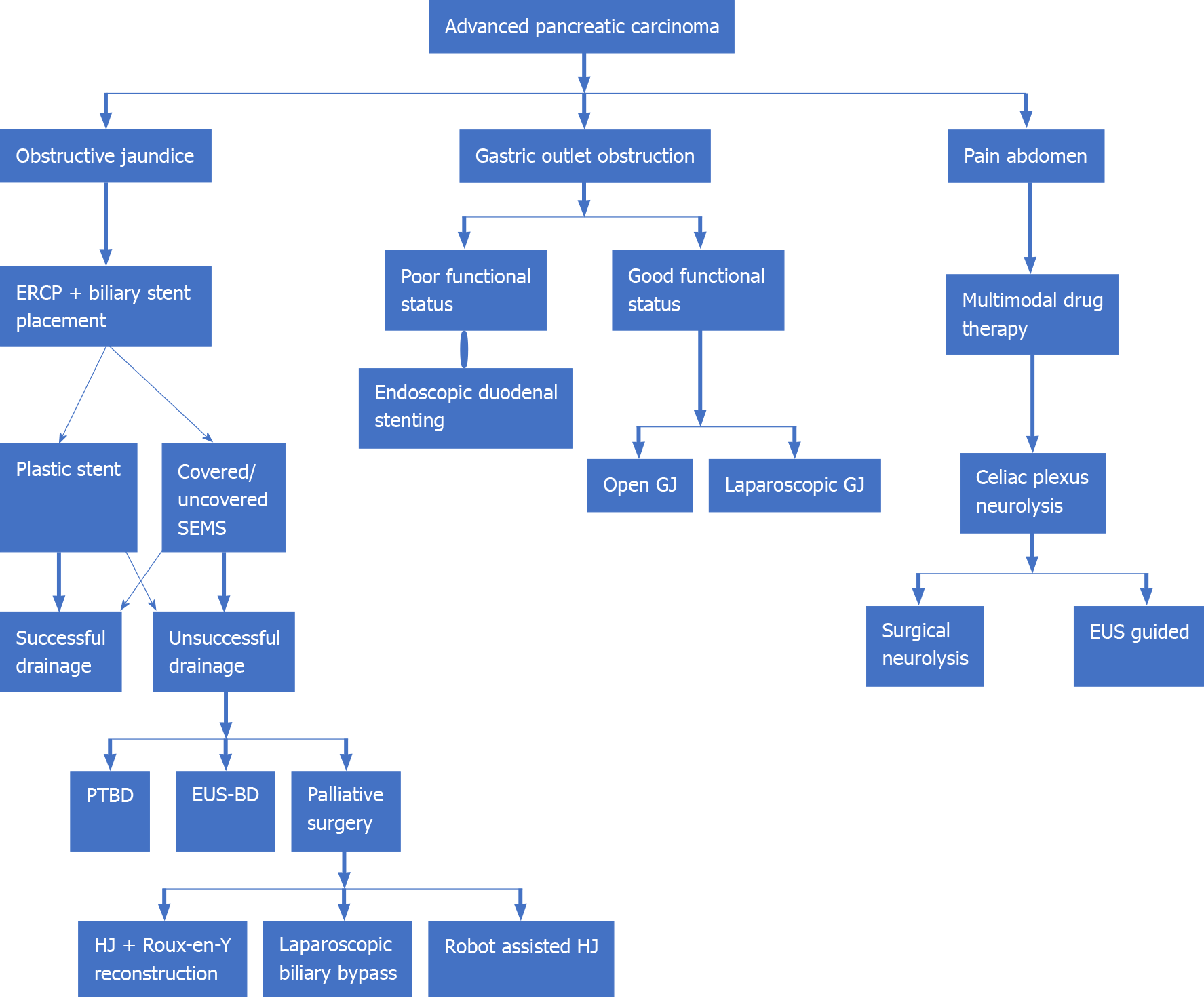
Gastric outlet obstruction (GOO), extrahepatic biliary obstruction (EHBO), and abdominal pain are the three most common disabling symptoms in APC which adversely affect an individual’s quality of life besides being a major source of ‘caregiver fatigue’. GOO, presenting as nausea, vomiting, dehydration, and weight loss, is seen in 10%-25% of all APC cases. Palliative surgery with open gastrojejunostomy (GJ) is the traditional approach to managing a malignant GOO. Placement of endoscopic duodenal stents and laparoscopic GJ has been tried, with varying degrees of success. Surgical procedures offer good functional outcomes at the cost of increased mortality[71]. EHBO can present with obstructive jaundice. Endoscopic retrograde cholangiopancreatography (ERCP)-guided biliary stent placement is the accepted gold standard approach for the management of malignant EHBO. Both plastic and self-expanding metal stents (SEMSs) have been used, with literature favoring the use of covered SEMSs. Failure of ERCP-guided biliary drainage may warrant drainage through the percutaneous route or an endoscopic ultrasonography-guided biliary drainage (EUS BD)[72]. Hepaticojejunostomy with a Roux-en-Y reconstruction and cholecystectomy is the favored palliative surgical procedure for palliation of EHBO secondary to PC. Laparoscopic biliary bypass and robot-assisted laparoscopic hepaticojejunostomy have been technically successful with satisfactory surgical outcomes[73,74]. Thus, patients with good performance status may benefit from a palliative surgical procedure while those with poor performance status warrant endoscopic biliary drainage.
Malignant infiltration of celiac or mesenteric nerve plexus in patients of APC may cause abdominal and back pain. This may adversely affect an individual’s quality of life. Multimodal drug therapy encompassing the use of non-steroidal anti-inflammatory drugs and or opioid analgesics in various combinations form the bedrock of pain management. Intraoperative celiac block using ethanol or a local anesthetic using either laparoscopic or open approach and EUS-guided neurolysis of celiac plexus, have all been tried[71] (Figure 4).
The tumor microenvironment of PC is responsible for aggressiveness as well as chemoresistance. This makes the case for utilizing immunotherapy in the advanced and metastatic settings. However, the approval for ICI is currently for patients with mismatch repair-deficient cases[69]. The updated results of the Keynote-158, using pembrolizumab in patients with advanced PDAC revealed an overall response rate (ORR) of 18.2%, median progression-free survival of 2.1 mo, and median overall survival of 4.0 mo[75]. The results are not very encouraging despite this, due to fewer driver mutations, variable expression of PD-1/PD-L1 and neoantigen burden in the tumor tissue, and absence of DDR, which are hallmarks of this malignancy[76].
The highly actionable mutations detected in molecular profiling of the Know Your Tumor initiative were 27%, of which the common ones involved the KRAS, TP53, MLL3, CDKN2A, SMAD4, TGFBR2, ARID1A, and SF3B1 genes. These mutations, however, do not have any therapeutic modality to target[77,78]. Neurotrophic receptor tyrosine kinase (NTRK) gene fusions have been detected in about 6% of pancreatic adenocarcinomas. Larotrectinib and entrectinib are two newer agents receiving accelerated approval for use in metastatic tumors with NTRK gene biomarker with tissue agnostic indication[79,80]. Three single-arm trials, namely LOXO-TRK-14001, SCOUT and NAVIGATE, had a total of 55 patients with NTRK fusions and had an ORR of 55% with Larotrectinib[81]. The median progression-free survival was not achieved after a median duration of 9.9 mo. Similarly, in three other single-arm trials, including ALKA-372-001, STARTRK-1 and STARTRK-2, with 60 patients in total, the patients with NTRK fusion had an ORR of 100%[82].
Germline BRCA1/2 mutations and homologous recombination deficiency enable the utility of platinum agents as well as poly (adenosine diphosphate-ribose) polymerase (PARP) inhibition as a novel therapeutic modality. Olaparib, a PARP inhibitor was approved by the United States’ Food and Drug Administration in December 2019 for patients with advanced metastatic malignancy having germline BRCA1/2 mutation. The phase III POLO trial utilizing olaparib in PAC, progressing after platinum-based therapy revealed median progression-free survival of 7.4 mo vs 3.8 in the control arm (HR: 0.53, 95%CI: 0.35-0.82; P = 0.004). This did not translate into an overall survival benefit[83].
Macrophages residing in the tumor environment are labeled as tumor-associated macrophages. CD 51 is a marker of macrophages that promotes the stemness of PDAC cells by regulating the TGF-β1/smad2/3 pathway. As a result, CD 51- targeted therapy is evolving as a newer therapeutic modality. Similarly, CD 40 activation, which promotes anti-tumor T-cell responses has been targeted by using anti-CD 40 antibody, CP-870893 along with gemcitabine, providing initial results of response[84].
The ability of cancer vaccines to stimulate dendritic cell responses and activate the adaptive immune responses has been harnessed for many cancers, including PC. The expression of murine enzyme alpha-1,3-galactosyltransferase (alpha-GT) by genetic engineering on PC cell lines HAPa1 and HAPa2 lead to anti-alpha-Gal antibody responses in humans[85]. Algenpanteucel-L was used in a phase II study and phase III (IMPRESS Trial). When algenpanteucel-L was given after gemcitabine and 5-FU based CRT, 81%-86% 1-year disease-free survival and 96% 1-year overall survival were observed[86,87].
GVAX, a line of engineered pancreatic tumor cells secreting GM-CSF has been tested in phase I study along with cyclophosphamide (Cy) with early results of tolerability and survival. In a phase II study, GVAX/Cy was tested against a GVAX/Cy followed by CRS 207 (a live-attenuated Listeria strain that induces tumor-associated antigens) and resulted in better overall survival in the latter arm (6.1 mo vs 3.9 mo, P = 0.02)[88].
There have been numerous trials using targets like vascular endothelial growth factor (VEGF) and VEGF-receptor, RAS-RAF-MEK-ERK pathway, etc. but none have shown robust survival data[89,90]. The rapamycin-insensitive companion of mTOR (RICTOR) expression was found to have a survival benefit in resected PAC patients, with those having a lower expression doubling the survival as compared to the high expressers[91].
We have attempted to provide an inclusive version of the management of PC, with special emphasis on current strategies and the road ahead with emerging modalities of therapy. Of course, there are certain gaps in the understanding of this disease and the evolution of treatment options is always challenging. For times to come, newer modalities appear promising; however, there is no substitute for early diagnosis and management for disease-free survival.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association, No. 1050754; American College of Gastroenterology, No. 51519; American Society for Gastrointestinal Endoscopy, No. 151100; Indian Society of Gastroenterology, No. LM001975.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zeng C S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13300] [Cited by in F6Publishing: 14452] [Article Influence: 2890.4] [Reference Citation Analysis (2)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19774] [Article Influence: 2197.1] [Reference Citation Analysis (17)] |
| 3. | Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol. 2013;24:2657-2671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51:3-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 5. | Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, Kern SE. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731-1734. [PubMed] [Cited in This Article: ] |
| 6. | Moore PS, Orlandini S, Zamboni G, Capelli P, Rigaud G, Falconi M, Bassi C, Lemoine NR, Scarpa A. Pancreatic tumours: molecular pathways implicated in ductal cancer are involved in ampullary but not in exocrine nonductal or endocrine tumorigenesis. Br J Cancer. 2001;84:253-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1779] [Cited by in F6Publishing: 1681] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 8. | Abdel-Rahman O, Elsayed Z, Elhalawani H. Gemcitabine-based chemotherapy for advanced biliary tract carcinomas. Cochrane Database Syst Rev. 2018;4:CD011746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1180] [Cited by in F6Publishing: 1202] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 10. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4838] [Cited by in F6Publishing: 5099] [Article Influence: 392.2] [Reference Citation Analysis (1)] |
| 11. | Tempero MA, Reni M, Riess H, Pelzer U, O'Reilly EM, Winter JM, Oh DY, Li CP, Tortora G, Chang HM, Lopez CD, Tabernero J, Van Cutsem E, Philip PA, Goldstein D, Berlin J, Ferrara S, Li M, Lu BD, Biankin A. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37:15. [Cited in This Article: ] |
| 12. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 1818] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 13. | Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-782; discussion 782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 947] [Cited by in F6Publishing: 1016] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 14. | Rutter CE, Park HS, Corso CD, Lester-Coll NH, Mancini BR, Yeboa DN, Johung KL. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: An analysis of the National Cancer Data Base. Cancer. 2015;121:4141-4149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Hsieh MC, Chang WW, Yu HH, Lu CY, Chang CL, Chow JM, Chen SU, Cheng Y, Wu SY. Adjuvant radiotherapy and chemotherapy improve survival in patients with pancreatic adenocarcinoma receiving surgery: adjuvant chemotherapy alone is insufficient in the era of intensity modulation radiation therapy. Cancer Med. 2018;7:2328-2338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | You MS, Ryu JK, Huh G, Chun JW, Paik WH, Lee SH, Kim YT. Comparison of efficacy between adjuvant chemotherapy and chemoradiation therapy for pancreatic cancer: AJCC stage-based approach. World J Clin Oncol. 2020;11:747-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Cascinu S, Berardi R, Bianco R, Bilancia D, Zaniboni A, Ferrari D, Mosconi S, Spallanzani A, Cavanna L, Leo S, Negri F, Beretta GD, Sobrero A, Banzi M, Morabito A, Bittoni A, Marciano R, Ferrara D, Noventa S, Piccirillo MC. Nab-paclitaxel (Nab) plus gemcitabine (G) is more effective than G alone in locally advanced, unresectable pancreatic cancer (LAUPC): The GAP trial, a GISCAD phase II comparative randomized trial. Ann Oncol. 2019;30:v253-v254. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, Bahary N, Bekaii-Saab T, Bali MA, Besselink MG, Boone BA, Chau I, Clarke S, Dillhoff M, El-Rayes BF, Frakes JM, Grose D, Hosein PJ, Jamieson NB, Javed AA, Khan K, Kim KP, Kim SC, Kim SS, Ko AH, Lacy J, Margonis GA, McCarter MD, McKay CJ, Mellon EA, Moorcraft SY, Okada KI, Paniccia A, Parikh PJ, Peters NA, Rabl H, Samra J, Tinchon C, van Tienhoven G, van Veldhuisen E, Wang-Gillam A, Weiss MJ, Wilmink JW, Yamaue H, Homs MYV, van Eijck CHJ, Katz MHG, Groot Koerkamp B. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J Natl Cancer Inst. 2019;111:782-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 19. | Ghaneh P, Palmer HP, Cicconi S, Halloran C, Psarelli EE, Rawcliffe CL, Sripadam R, Mukherjee S, Wadsley J, Al-Mukhtar A, Jiao LR, Wasan HS, Carter R, Graham JS, Ammad F, Evans J, Tjaden C, Hackert T, Buchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer (ESPAC). ESPAC-5F: Four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J Clin Oncol. 2020;15:4505. [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK, Fountzilas C, Wolpin BM, Al-Hawary M, Asbun H, Behrman SW, Benson AB, Binder E, Cardin DB, Cha C, Chung V, Dillhoff M, Dotan E, Ferrone CR, Fisher G, Hardacre J, Hawkins WG, Ko AH, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Thayer S, Wolff RA, Burns JL, Zuccarino-Catania G. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17:202-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 241] [Article Influence: 60.3] [Reference Citation Analysis (2)] |
| 21. | Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204:244-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Malleo G, Maggino L, Ferrone CR, Marchegiani G, Mino-Kenudson M, Capelli P, Rusev B, Lillemoe KD, Bassi C, Fernàndez-Del Castillo C, Salvia R. Number of Examined Lymph Nodes and Nodal Status Assessment in Distal Pancreatectomy for Body/Tail Ductal Adenocarcinoma. Ann Surg. 2019;270:1138-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Delpero JR, Bachellier P, Regenet N, Le Treut YP, Paye F, Carrere N, Sauvanet A, Autret A, Turrini O, Monges-Ranchin G, Boher JM. Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a French multicentre prospective evaluation of resection margins in 150 evaluable specimens. HPB (Oxford). 2014;16:20-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Negoi I, Hostiuc S, Runcanu A, Negoi RI, Beuran M. Superior mesenteric artery first approach vs standard pancreaticoduodenectomy: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2017;16:127-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 906] [Cited by in F6Publishing: 968] [Article Influence: 161.3] [Reference Citation Analysis (30)] |
| 26. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 717] [Cited by in F6Publishing: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 27. | Lambert A, Schwarz L, Borbath I, Henry A, Van Laethem JL, Malka D, Ducreux M, Conroy T. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol. 2019;11:1758835919875568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 126] [Article Influence: 25.2] [Reference Citation Analysis (1)] |
| 28. | Uzunoglu FG, Reeh M, Vettorazzi E, Ruschke T, Hannah P, Nentwich MF, Vashist YK, Bogoevski D, König A, Janot M, Gavazzi F, Zerbi A, Todaro V, Malleo G, Uhl W, Montorsi M, Bassi C, Izbicki JR, Bockhorn M. Preoperative Pancreatic Resection (PREPARE) score: a prospective multicenter-based morbidity risk score. Ann Surg. 2014;260:857-863; discussion 863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Ragulin-Coyne E, Carroll JE, Smith JK, Witkowski ER, Ng SC, Shah SA, Zhou Z, Tseng JF. Perioperative mortality after pancreatectomy: a risk score to aid decision-making. Surgery. 2012;152:S120-S127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 31. | Sinn M, Liersch T, Gellert K, Riess H, Stübs P, Waldschmidt DT, Pelzer U, Stieler J, Striefler JK, Bahra M, Dörken B, Oettle H. LBA18 - Conko-006: a Randomized Double-Blinded Phase Iib-Study of Adjuvant Therapy with Gemcitabine + Sorafenib/Placebo for Patients with R1-Resection of Pancreatic Cancer. Ann Oncol. 2014;25:v1. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Yoshitomi H, Togawa A, Kimura F, Ito H, Shimizu H, Yoshidome H, Otsuka M, Kato A, Nozawa S, Furukawa K, Miyazaki M; Pancreatic Cancer Chemotherapy Program of the Chiba University Department of General Surgery Affiliated Hospital Group. A randomized phase II trial of adjuvant chemotherapy with uracil/tegafur and gemcitabine vs gemcitabine alone in patients with resected pancreatic cancer. Cancer. 2008;113:2448-2456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987;59:2006-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 34. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 724] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 35. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1008] [Cited by in F6Publishing: 936] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 36. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1327] [Cited by in F6Publishing: 1219] [Article Influence: 174.1] [Reference Citation Analysis (0)] |
| 37. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1427] [Cited by in F6Publishing: 1614] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 38. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, Willett CG, Rich TA. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 527] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 39. | Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P, Rawcliffe CL, Bassi C, Stocken DD, Cunningham D, O'Reilly D, Goldstein D, Robinson BA, Karapetis C, Scarfe A, Lacaine F, Sand J, Izbicki JR, Mayerle J, Dervenis C, Oláh A, Butturini G, Lind PA, Middleton MR, Anthoney A, Sumpter K, Carter R, Büchler MW. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 40. | Xia BT, Ahmad SA, Al Humaidi AH, Hanseman DJ, Ethun CG, Maithel SK, Kooby DA, Salem A, Cho CS, Weber SM, Stocker SJ, Talamonti MS, Bentrem DJ, Abbott DE. Time to Initiation of Adjuvant Chemotherapy in Pancreas Cancer: A Multi-Institutional Experience. Ann Surg Oncol. 2017;24:2770-2776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Newton AD, Datta J, Loaiza-Bonilla A, Karakousis GC, Roses RE. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol. 2015;6:534-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 32] [Reference Citation Analysis (0)] |
| 42. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1085] [Cited by in F6Publishing: 1096] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 43. | Schorn S, Demir IE, Reyes CM, Saricaoglu C, Samm N, Schirren R, Tieftrunk E, Hartmann D, Friess H, Ceyhan GO. The impact of neoadjuvant therapy on the histopathological features of pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Cancer Treat Rev. 2017;55:96-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 44. | Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, Pinelli D, Mosconi S, Doglioni C, Chiaravalli M, Pircher C, Arcidiacono PG, Torri V, Maggiora P, Ceraulo D, Falconi M, Gianni L. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3:413-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 45. | O'Reilly EM, Ferrone C. Neoadjuvant or Adjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer: Which Is Preferred? J Clin Oncol. 2020;38:1757-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 47. | Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am J Clin Oncol. 2016;39:302-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 574] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 49. | Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, Varadhachary GR, Hwang RF. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846; discussion 846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 562] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 50. | Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, van Tienhoven G; Dutch Pancreatic Cancer Group. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 331] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 51. | Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, Oh DY, Chie EK, Lee JM, Heo JS, Park JO, Lim DH, Kim SH, Park SJ, Lee WJ, Koh YH, Park JS, Yoon DS, Lee IJ, Choi SH. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg. 2018;268:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 52. | Evans DB, George B, Tsai S. Non-metastatic Pancreatic Cancer: Resectable, Borderline Resectable, and Locally Advanced-Definitions of Increasing Importance for the Optimal Delivery of Multimodality Therapy. Ann Surg Oncol. 2015;22:3409-3413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Neuzillet C, Gaujoux S, Williet N, Bachet JB, Bauguion L, Colson Durand L, Conroy T, Dahan L, Gilabert M, Huguet F, Marthey L, Meilleroux J, de Mestier L, Napoléon B, Portales F, Sa Cunha A, Schwarz L, Taieb J, Chibaudel B, Bouché O, Hammel P; Thésaurus National de Cancérologie Digestive (TNCD); Société Nationale Française de Gastroentérologie (SNFGE); Fédération Francophone de Cancérologie Digestive (FFCD); Groupe Coopérateur multidisciplinaire en Oncologie (GERCOR); Fédération Nationale des Centres de Lutte Contre le Cancer (UNICANCER); Société Française de Chirurgie Digestive (SFCD); Société Française d’Endoscopie Digestive (SFED); Société Française de Radiothérapie Oncologique (SFRO); Association de Chirurgie Hépato-Bilio-Pancréatique et Transplantation (ACHBT); Association Française de Chirurgie (AFC). Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis. 2018;50:1257-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, El-Rayes BF, Wang-Gillam A, Lacy J, Hosein PJ, Moorcraft SY, Conroy T, Hohla F, Allen P, Taieb J, Hong TS, Shridhar R, Chau I, van Eijck CH, Koerkamp BG. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 613] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 55. | Wang C, Liu X, Wang X, Wang Y, Cha N. Effects of chemoradiotherapy and chemotherapy on survival of patients with locally advanced pancreatic cancer: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e12260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, Crosby T, Jephcott C, Roy R, Radhakrishna G, McDonald A, Ray R, Joseph G, Staffurth J, Abrams RA, Griffiths G, Maughan T. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 57. | Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR; International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155:977-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 585] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 58. | Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, Urba S, Zeh HJ, Katz MHG. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:2324-2328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 59. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4270] [Cited by in F6Publishing: 4042] [Article Influence: 149.7] [Reference Citation Analysis (0)] |
| 60. | Moore K. Endothelin and vascular function in liver disease. Gut. 2004;53:159-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, Scheithauer W, Ramanathan RK, Goldstein D, Penenberg DN, Romano A, Ferrara S, Von Hoff DD. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine vs gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 62. | Sehdev A, Gbolahan O, Hancock BA, Stanley M, Shahda S, Wan J, Wu HH, Radovich M, O'Neil BH. Germline and Somatic DNA Damage Repair Gene Mutations and Overall Survival in Metastatic Pancreatic Adenocarcinoma Patients Treated with FOLFIRINOX. Clin Cancer Res. 2018;24:6204-6211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Fogelman D, Sugar EA, Oliver G, Shah N, Klein A, Alewine C, Wang H, Javle M, Shroff R, Wolff RA, Abbruzzese JL, Laheru D, Diaz LA Jr. Family history as a marker of platinum sensitivity in pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2015;76:489-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Kang H, Jo JH, Lee HS, Chung MJ, Bang S, Park SW, Song SY, Park JY. Comparison of efficacy and safety between standard-dose and modified-dose FOLFIRINOX as a first-line treatment of pancreatic cancer. World J Gastrointest Oncol. 2018;10:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Walker EJ, Ko AH. Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol. 2014;20:2224-2236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Nagrial AM, Chin VT, Sjoquist KM, Pajic M, Horvath LG, Biankin AV, Yip D. Second-line treatment in inoperable pancreatic adenocarcinoma: A systematic review and synthesis of all clinical trials. Crit Rev Oncol Hematol. 2015;96:483-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, Bischoff S, Sinn M, Dörken B, Pelzer U. Second-line oxaliplatin, folinic acid, and fluorouracil vs folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423-2429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 68. | Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R, Bachet JB, Dubreuil O, Marthey L, Dahan L, Tchoundjeu B, Locher C, Lepère C, Bonnetain F, Taieb J. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113:989-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 69. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3799] [Cited by in F6Publishing: 4326] [Article Influence: 618.0] [Reference Citation Analysis (0)] |
| 70. | Domchek SM, Hendifar AE, McWilliams RR, Geva R, Epelbaum R, Biankin A, Vonderheide RH, Wolff RA, Alberts SR, Giordano H, Goble S, Lin KK, Shroff RT. RUCAPANC: An open-label, phase 2 trial of the PARP inhibitor rucaparib in patients (pts) with pancreatic cancer (PC) and a known deleterious germline or somatic BRCA mutation. J Clin Oncol. 2016;15:34. [Cited in This Article: ] |
| 71. | Perinel J, Adham M. Palliative therapy in pancreatic cancer-palliative surgery. Transl Gastroenterol Hepatol. 2019;4:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 73. | Berti S, Ferrarese A, Feleppa C, Francone E, Martino V, Bianchi C, Falco E. Laparoscopic perspectives for distal biliary obstruction. Int J Surg. 2015;21 Suppl 1:S64-S67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Lai EC, Tang CN. Robot-assisted laparoscopic hepaticojejunostomy for advanced malignant biliary obstruction. Asian J Surg. 2015;38:210-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 1525] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 76. | Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ, Narasimhan B, Solheim JC, Batra SK, Jain M. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Pishvaian MJ, Bender RJ, Halverson D, Rahib L, Hendifar AE, Mikhail S, Chung V, Picozzi VJ, Sohal D, Blais EM, Mason K, Lyons EE, Matrisian LM, Brody JR, Madhavan S, Petricoin EF 3rd. Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clin Cancer Res. 2018;24:5018-5027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 78. | Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N; Australian Pancreatic Cancer Genome Initiative, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1442] [Cited by in F6Publishing: 1521] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 79. | National Institutes of Health's National Library of Medicine. DailyMed - VITRAKVI- larotrectinib capsule VITRAKVI- larotrectinib solution, concentrate. In: National Institutes of Health's National Library of Medicine [Internet]. Available from: https://NLM/NIH/HHS/USA.gov. [Cited in This Article: ] |
| 80. | Genentech. Rozlytrek™ (entrectinib) - Information for Healthcare Providers. In: Genentech [Internet]. Available from: www.gene.com. [Cited in This Article: ] |
| 81. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1577] [Cited by in F6Publishing: 1672] [Article Influence: 278.7] [Reference Citation Analysis (0)] |
| 82. | Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, Doebele R, Giannetta L, Cerea G, Marrapese G, Schirru M, Amatu A, Bencardino K, Palmeri L, Sartore-Bianchi A, Vanzulli A, Cresta S, Damian S, Duca M, Ardini E, Li G, Christiansen J, Kowalski K, Johnson AD, Patel R, Luo D, Chow-Maneval E, Hornby Z, Multani PS, Shaw AT, De Braud FG. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7:400-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 550] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 83. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1137] [Cited by in F6Publishing: 1311] [Article Influence: 262.2] [Reference Citation Analysis (0)] |
| 84. | Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O'Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286-6295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 85. | Rossi GR, Mautino MR, Unfer RC, Seregina TM, Vahanian N, Link CJ. Effective treatment of preexisting melanoma with whole cell vaccines expressing alpha(1,3)-galactosyl epitopes. Cancer Res. 2005;65:10555-10561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94-100; discussion p. 100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 87. | Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Algenpantucel-L immunotherapy in pancreatic adenocarcinoma. Immunotherapy. 2016;8:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Sahin IH, Askan G, Hu ZI, O'Reilly EM. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Ann Oncol. 2017;28:2950-2961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 89. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O'Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 645] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 90. | Lemstrova R, Brynychova V, Hughes DJ, Hlavac V, Dvorak P, Doherty JE, Murray HA, Crockard M, Oliverius M, Hlavsa J, Honsova E, Mazanec J, Kala Z, Lovecek M, Havlik R, Ehrmann J, Strouhal O, Soucek P, Melichar B, Mohelnikova-Duchonova B. Dysregulation of KRAS signaling in pancreatic cancer is not associated with KRAS mutations and outcome. Oncol Lett. 2017;14:5980-5988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Schmidt KM, Hellerbrand C, Ruemmele P, Michalski CW, Kong B, Kroemer A, Hackl C, Schlitt HJ, Geissler EK, Lang SA. Inhibition of mTORC2 component RICTOR impairs tumor growth in pancreatic cancer models. Oncotarget. 2017;8:24491-24505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |