Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6402
Peer-review started: June 18, 2020
First decision: July 28, 2020
Revised: August 16, 2020
Accepted: September 16, 2020
Article in press: September 16, 2020
Published online: November 7, 2020
Clinically significant post-endoscopic retrograde cholangiopancreatography (ERCP) bacteremia (PEB) occurs in up to 5% of cases, while antibiotic prophylaxis is recommended only when an ERCP is unlikely to achieve complete biliary drainage. However, the current recommendations may not cover all potential risk factors for PEB.
To identify novel risk factors for PEB and evaluate appropriateness of antibiotic prophylaxis.
A retrospective study of 1082 ERCP procedures performed between January 2012 - December 2013 in a single tertiary medical center. Data collection included: Demographic and clinical characteristics such as pre and post procedure antibiotic treatment and bacterial blood cultures. Exclusion criteria were: (1) Age < 18 years; (2) Positive bacterial blood culture before ERCP; (3) Scheduled antibiotic treatment prior to ERCP; (4) Hospitalization longer than 14 d before ERCP; and (5) missing critical data. Stepwise Logistic Regression analysis and Decision Tree algorithms were used for prediction modeling of PEB.
A total of 626 ERCPs performed in 434 patients were included. Mean age 66.49 ± 15.4 years and 46.5% were males. PEB prevalence was 3.7%. Antibiotic prophylaxis was administrated in 139/626 (22.2%) cases but was indicated according to the guidelines only in 44/626 (7%) cases. In all the PEB cases, prophylaxis was deemed not indicated. A stepwise logistic regression [receiver operating characteristic (ROC), 0.766], identified 3 variables as independent risk factors for PEB: Age at ERCP ≥ 75 years (OR, 3.780, 95%CI: 1.519-9.408, P = 0.004); Tandem EUS/ERCP with fine needle aspiration (FNA) (OR, 14.528, 95%CI: 3.571-59.095, P < 0.001); ERCP duration longer than 60 min (OR, 5.396, 95%CI: 1.86-15.656, P = 0.002). In a decision tree model (ROC, 0.778) the probability for PEB without any risk factors was 1% regardless of prophylaxis administration.
The prevalence of PEB in our study is similar to previous reports, despite the fact that antibiotic prophylaxis was administrated more readily than recommended. ERCP duration longer than 60 min, tandem EUS-ERCP with FNA and age above 75 years are significant risk factors for PEB. These factors should be further evaluated as indications for prophylactic antibiotic treatment before ERCP.
Core Tip: Clinically significant post-endoscopic retrograde cholangiopancreatography (ERCP) bacteremia (PEB) occurs in up to 5% of cases, while antibiotic prophylaxis is recommended when an ERCP is unlikely to achieve complete biliary drainage. Our aim was to identify novel risk factors for PEB and evaluate appropriateness of antibiotic prophylaxis. This retrospective cohort study included 626 ERCPs while PEB prevalence was 3.7%. Antibiotic prophylaxis was administrated in 22.2% of cases but was indicated in 7%. Independent risk factors for PEB were: Age ≥ 75 years, Tandem-EUS/ERCP with FNA and ERCP duration ≥ 60 min (P < 0.005). These novel risk factors should be further evaluated as indications for prophylactic antibiotic treatment before ERCP.
- Citation: Deutsch L, Matalon S, Phillips A, Leshno M, Shibolet O, Santo E. Older age, longer procedures and tandem endoscopic-ultrasound as risk factors for post-endoscopic retrograde cholangiopancreatography bacteremia. World J Gastroenterol 2020; 26(41): 6402-6413
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6402
Endoscopic retrograde cholangiopancreatography (ERCP) is currently the method of choice for the treatment of biliary and pancreatic duct obstruction. However, serious post-procedural complications can occur. The most common complication is post-ERCP pancreatitis (1.6%-15.7%) followed by infectious complications (i.e., clinically significant bacteremia) such as cholangitis and sepsis (3%-5%)[1,2]. The necessity of pre-ERCP antibiotic prophylaxis is controversial. According to ASGE recommendations in 2015[1], antibiotic prophylaxis was not recommended when an ERCP was likely to achieve complete biliary drainage, based on high quality evidence. Administration of antibiotic prophylaxis before ERCP was recommended in liver transplantation recipients or patients with known or suspected biliary obstruction. It was recommended that antibiotics be continued after the procedure if biliary drainage was incomplete. This recommendation was recognized as moderate quality of evidence. The recommendations do not address specific populations or procedure-related factors that require specific management. In the nationwide population-based cohort study of the Swedish Registry of Gallstone Surgery and ERCP (GallRiks) administration of prophylactic antibiotics led to a 26% relative risk reduction and 2.6% absolute risk reduction of post-ERCP adverse events[3]. The beneficial effect was most prominent among patients with obstructive jaundice (32% relative risk reduction and 3.8% absolute risk reduction in post-ERCP complications).
In a Cochrane systematic review of 9 randomized clinical trials (1573 patients), prophylactic antibiotics reduced post-ERCP bacteremia, septicemia and acute cholangitis, but the effect of antibiotics was less prominent in the subgroup of patients with biliary obstruction relieved during the first ERCP[4].
The primary objective of this study was to evaluate possible risk factors for post-ERCP bacteremia (PEB). Secondary objectives were: Evaluation of PEB prevalence and to assess "real-life" practices of antibiotic administration and their competency to ASGE guidelines.
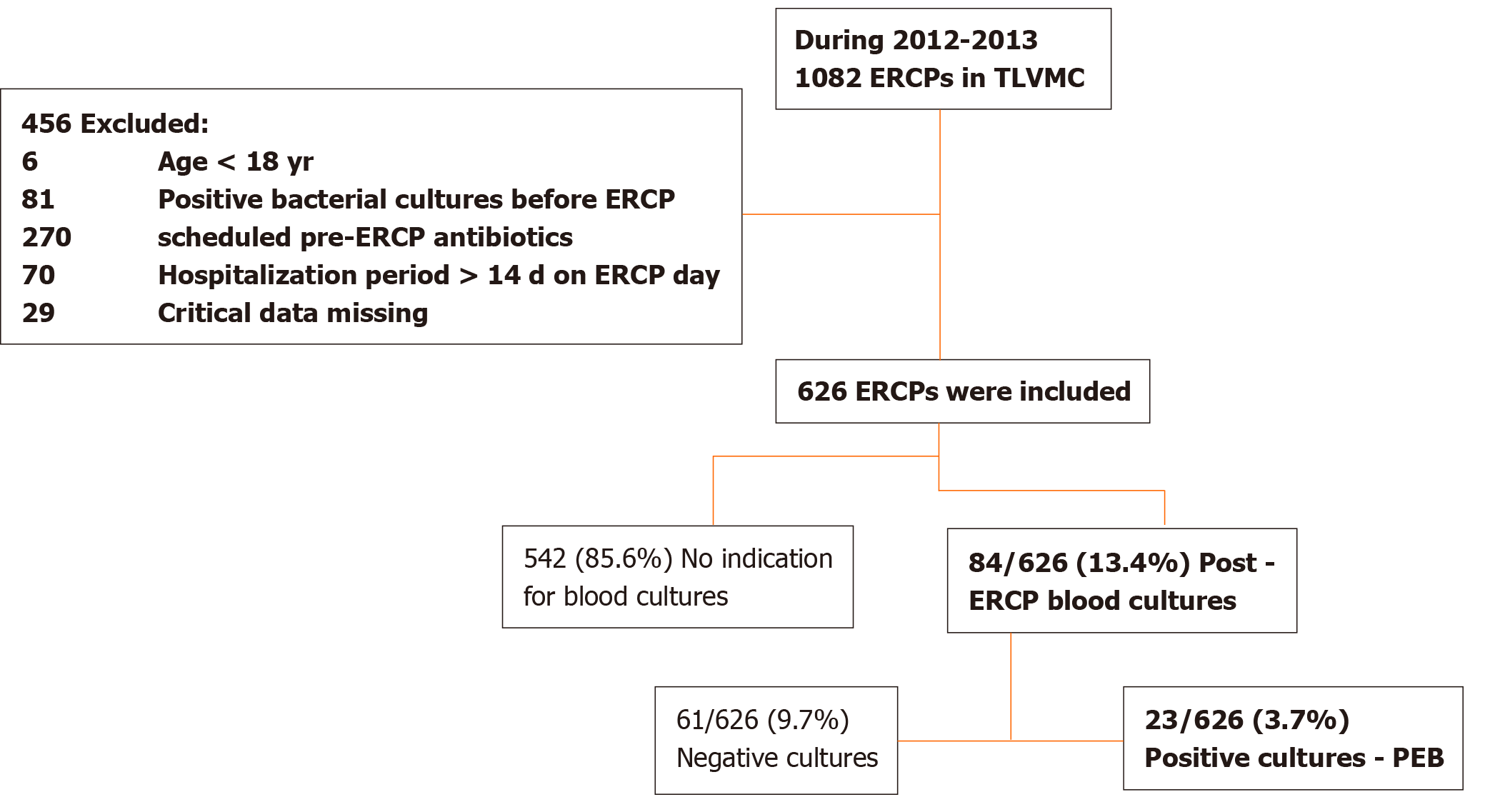
A total of 1082 ERCPs were performed between January 2012 - December 2013 in a single referral center. All ERCPs were performed by one of five certified gastroenterologists with more than 5-years' experience in advanced endoscopy in a single dedicated room. (In only two cases (0.4%) the name of the endoscopist was not documented). Demographic and clinical characteristics including indication, complications, pre and post procedure antibiotic treatment and bacterial blood cultures were collected and documented manually from patient's records. Exclusion criteria were: (1) Age < 18 years; (2) Positive bacterial blood culture before ERCP; (3) Scheduled antibiotic treatment prior to ERCP; (4) Hospitalization longer than 14 days before ERCP; and (5) Missing critical data (mainly medical charts of documented antibiotic treatment). The study was approved by the local Institutional Review Board (IRB No: 0598-13-TLV), data was anonymous and informed consent was waivered.
Native papilla - Patients who previously underwent ERCP with papillotomy or pre-cut were considered "experienced patients" as opposed to patients with "native papilla".
Obstructive malignancy – Bile duct compression by an abdominal tumor or metastases (i.e., pancreatic origin, cholangiocarcinoma, liver metastases etc.). Malignancy without abdominal involvement was not recorded (for example breast cancer).
"Naïve obstructive malignancy" - Patients who had their first ERCP for the indication of obstructive jaundice due to compressive malignancy were labeled as "Naïve obstructive malignancy".
Antibiotic prophylaxis - antibiotic prophylaxis was defined as a single dosage of antibiotic drug given in the window period of 1 h before ERCP and up to the end of the procedure. If the procedure was ambulatory, the decision whether or not to give prophylaxis was made according to the endoscopist discretion. If the procedure was performed during hospitalization the decision was made by the treating physician in the ward.
Tandem procedures – if an endoscopic ultrasound (EUS) was preformed just prior to the ERCP (in the same room, by the same physician and under the same anesthesia).
ERCP duration - the procedure duration was calculated as the time interval between the first documented picture (papilla of Vater) and last picture (final cho-langiography). If tandem procedures were performed, only the ERCP duration was calculated.
Clinically significant PEB - bacterial blood cultures were drawn according to clinical indication (fever, systemic inflammatory response, cholangitis, etc.). PEB was defined as a positive bacterial blood culture within 7 d of ERCP date. Bacterial species and antibiotic resistance were documented.
Appropriateness of antibiotic prophylaxis - a gastroenterology specialist from the advanced endoscopy unit, who did not participate in the ERCPs included and was blinded to the outcome following the ERCPs, reviewed all the cases, and ranked the appropriateness of antibiotic prophylaxis according to the ASGE guidelines. There were 3 categories: (1) Prophylaxis was clearly indicated; (2) Prophylaxis was equivocal but was appropriate in the specific setting; and (3) Prophylaxis was not indicated.
All statistical analyses were performed using MATLAB (Mathwork Inc. version 2015b) and SPSS version 23.0 for Windows (SPSS Inc., Chicago, IL, United State). Continuous variables are presented as means ± SD, while categorical variables are presented in percentage. Univariate analyses were used for the comparison of variable's distribution between the study groups. To test differences in continuous variables between two groups the independent samples t-test (for normally distributed variables) or the Mann-Whitney U test (if non-parametric tests were required) were performed. To test the differences in categorical variables the Pearson χ2-Square test was performed, P < 0.05 was considered statistically significant for all analyses. We used stepwise Logistic Regression analysis with entry probability of 0.2 and Decision Tree algorithms with minimum leaf of 50 cases, for prediction modeling of bacteremia. The statistical methods of the study were reviewed by Leshno M, MD, from the Faculty of Management, Tel-Aviv University, Israel.
A total of 1082 consecutive ERCPs were analyzed and 456 were excluded (Figure 1). Thus, a total of 626 ERCPs performed in 434 patients were included. In 84 cases (13.4%), bacterial blood cultures were drawn based on clinical suspicion. Positive cultures were documented in 23/84 cases (27.4%), thus the rate of clinically significant PEB was 23/626 (3.7%).
Demographics and procedure associated data is shown in Table 1. Mean age at ERCP was 66.49 ± 15.4 years with 46.5% being male. Patient's characteristics were comparable between the PEB and non-PEB groups (Table 1). The most prevalent indication for ERCP in both groups was choledocholithiasis (30.4% and 32.2% for PEB and non-PEB groups respectively, P = NS) followed by elective stent replacement (26.1% and 24.9%, respectively, P = NS). This was a first ERCP intervention (native papilla) in 60.9% of the PEB cases and 44.9% of the non-PEB cases (P = NS). ERCP duration was significantly longer among the PEB group compared to the non-PEB group (40.87 ± 42.7 vs 28.64 ± 24.3 min, respectively, P = 0.02). The prevalence of tandem procedures (EUS followed immediately by ERCP) was significantly higher among the PEB group [5 (21.7%) vs 37 (6.1%), respectively, P = 0.003). In the cases of tandem EUS/ERCP, fine needle aspiration (FNA) from a solid mass was performed in 9/37 cases of the non-PEB group and 4/5 cases of the PEB group (24.3% vs 80%, P = 0.01). Intra ductal ultrasound (IDUS) was used in 2 cases and celiac block was performed in 1 case (all 3 cases were in the non-PEB group). The utilization of sphincterotomy, pre-cut or through the scope (TTS)-dilation was equally prevalent between the groups as well as the use of pancreatic stent. The use of naso-biliary drainage is very rare in our institute and was not documented. There was no difference in the distribution of ERCPs among five operators between the PEB and non-PEB groups (respectively: #1: 26.1% vs 28.4%, #2: 39.1% vs 31.7%, #3: 17.4% vs 15.8%, #4: 13.0% vs 19.6%, 5#: 4.3% vs 4.3%, unknown: 0% vs 0.4%, P = 0.985). Blood tests performed up to 72 h before ERCP were available for a minority of cases and are elaborated in Supplementary table 1.
| All ERCPs (n = 626) | PEB (n = 23) | Non-PEB (n = 603) | P value | |
| Age (yr) | 66.49 ± 15.4 | 71.74 ± 14.2 | 66.29 ± 15.4 | 0.095a |
| Male gender [n (%)] | 291 (46.5%) | 11 (47.8%) | 280 (46.4%) | 0.896b |
| Native papilla [n (%)] | 284 (45.4%) | 14 (60.9%) | 270 (44.9%) | 0.130b |
| Abdominal malignancy at presentation [n (%)] | 193 (30.8%) | 9 (39.1%) | 184 (30.5%) | 0.380b |
| Non | 433 (69.2%) | 14 (60.9%) | 419 (69.5%) | 0.634b |
| Pancreas | 147 (23.5%) | 6 (26.1%) | 141 (23.4%) | |
| Cholangiocarcinoma | 24 (3.8%) | 2 (8.7%) | 22 (3.6%) | |
| Liver metastases | 14 (2.2%) | 1 (4.3%) | 13 (2.2%) | |
| Other abdominal | 8 (1.3%) | 0 (0%) | 8 (1.3%) | |
| s/p liver transplantation | 44 (7.0%) | 0 (0%) | 44 (7.3%) | 0.179b |
| Indications [n (%)] | 0.337b | |||
| Choledocholithiasis | 201 (32.1%) | 7 (30.4%) | 194 (32.2%) | |
| Chronic pancreatitis | 23 (3.7%) | 2 (8.7%) | 21 (3.5%) | |
| Obstructive malignancy | 98 (15.7%) | 6 (26.1%) | 92 (15.3%) | |
| Benign bile duct stricture | 130 (20.8%) | 2 (8.7%) | 128 (21.2%) | |
| Elective stent replacement | 156 (24.9%) | 6 (26.1%) | 150 (24.9%) | |
| Surgical complications | 18 (2.9%) | 0 (0%) | 18 (3.0%) | |
| Procedure characteristics | ||||
| Duration (minutes) | 29.09 ± 25.3 | 40.87 ± 42.7 | 28.64 ± 24.3 | 0.02a |
| Sphincterotomy [n (%)] | 279 (44.6%) | 15 (65.2%) | 264 (43.8%) | 0.136b |
| Pre-cut [n (%)] | 46 (7.3%) | 2 (8.7%) | 44 (7.3%) | 0.772b |
| TTS dilation [n (%)] | 33 (5.3%) | 0 (0%) | 33 (5.5%) | 0.249b |
| Pancreatic stent [n (%)] | 52 (8.3%) | 2 (8.7%) | 50 (8.3%) | 0.945b |
| Tandem EUS/ERCP [n (%)] | 42 (6.7%) | 5 (21.7%) | 37 (6.1%) | 0.003b |
| Tandem EUS/ERCP + FNA [n (%)] | 13 (2.1%) | 4 (17.4%) | 9 (1.5%) | < 0.001b |
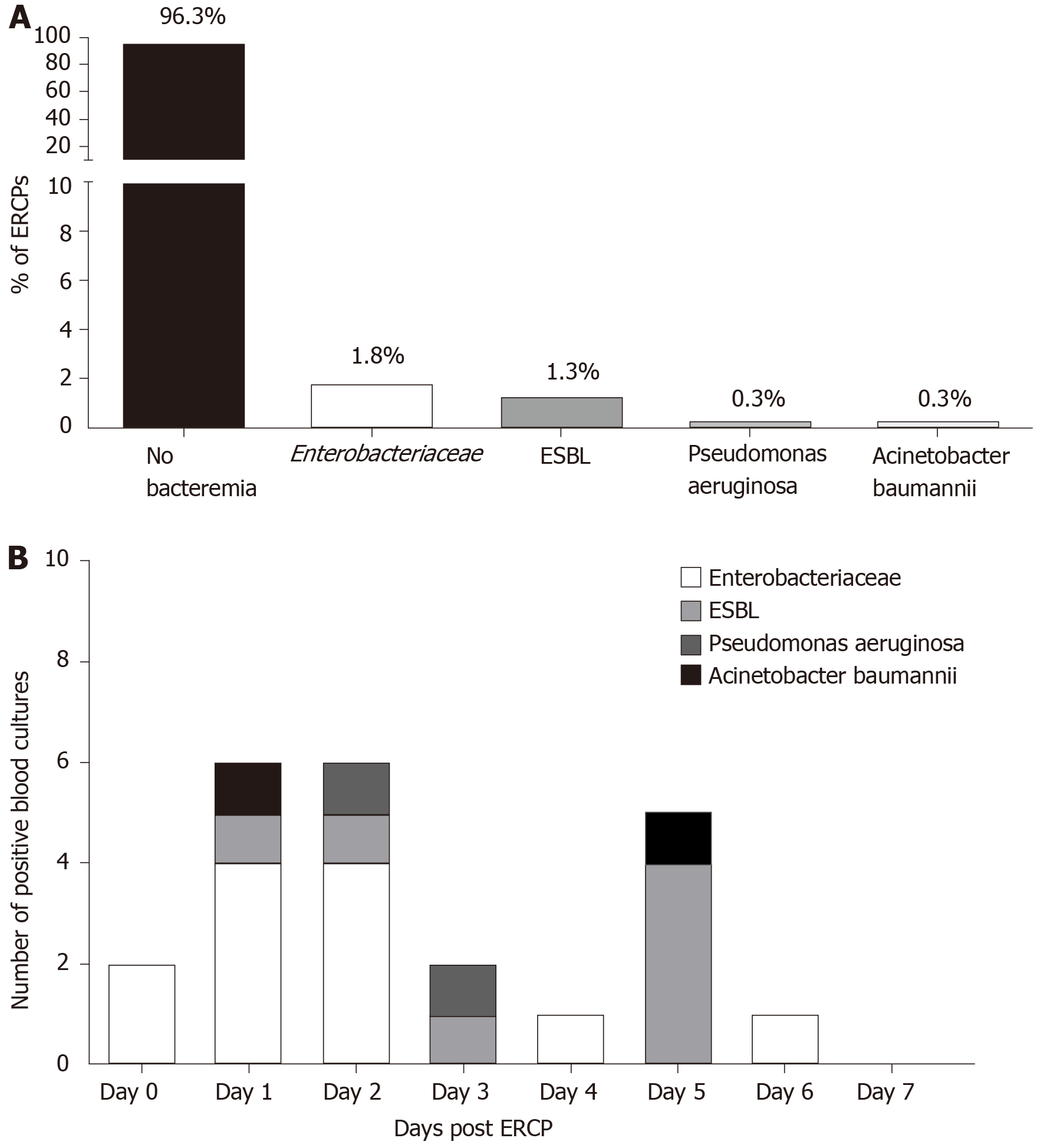
There were 23 cases of Bacteremia: 11 cases (1.8%) were of the Enterobacteriaceae family (E.coli and Klebsiella spp.), 8 cases (1.3%) were extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, 2 cases (0.3%) were Pseudomonas aeruginosa and the last 2 cases (0.3%) were Acinetobacter baumannii (Figure 2A). Seventy percent of the positive cultures were drawn during the three days following the ERCP procedure and 21.7% up to the fifth day, 8.9% were drawn on days 6-7 (Figure 2B).
To rule out other causes for bacteremia, invasive procedures such as percutaneous trans-hepatic drainage (PTD), cholecystostomy or surgery were documented (time interval- 7 days before the procedure and up to the day of bacterial culture collection). Pre-ERCP invasive procedure was not performed in any of the PEB cases. In the non-PEB group, 8 cases (1.3%) had pre-ERCP PTD, cholecystostomy was performed in 1 case (0.2%) and surgery in 12 cases (2%). Invasive procedures post-ERCP were documented in 2 cases (8.7%) from the PEB group, both were PTD insertion, and both were diagnosed with Acinetobacter bacteremia. There were 11 cases of invasive post-ERCP procedures in the non-PEB group (1.9%), with PTD in 4 cases (0.7%), and surgery in 7 cases (1.2%).
In elective ambulatory procedures (520/626 cases, 83.1%), antibiotic prophylaxis with ceftriaxone was administrated in 14.2% of the cases. Administration was in accordance with ASGE recommendations and at the endoscopist discretion. In hospitalized patients (106/626 cases, 16.9%), antibiotic prophylaxis was administrated in 61.3% of cases (61.3% vs 14.2%, in-patients vs out-patients respectively, P < 0.001). Administration was at the treating physician discretion. Prophylaxis in hospitalized patients was administrated as a single drug in 13 cases (20.0%), two drugs in 7 cases (10.8%) and three drugs in 45 cases (69.2%).
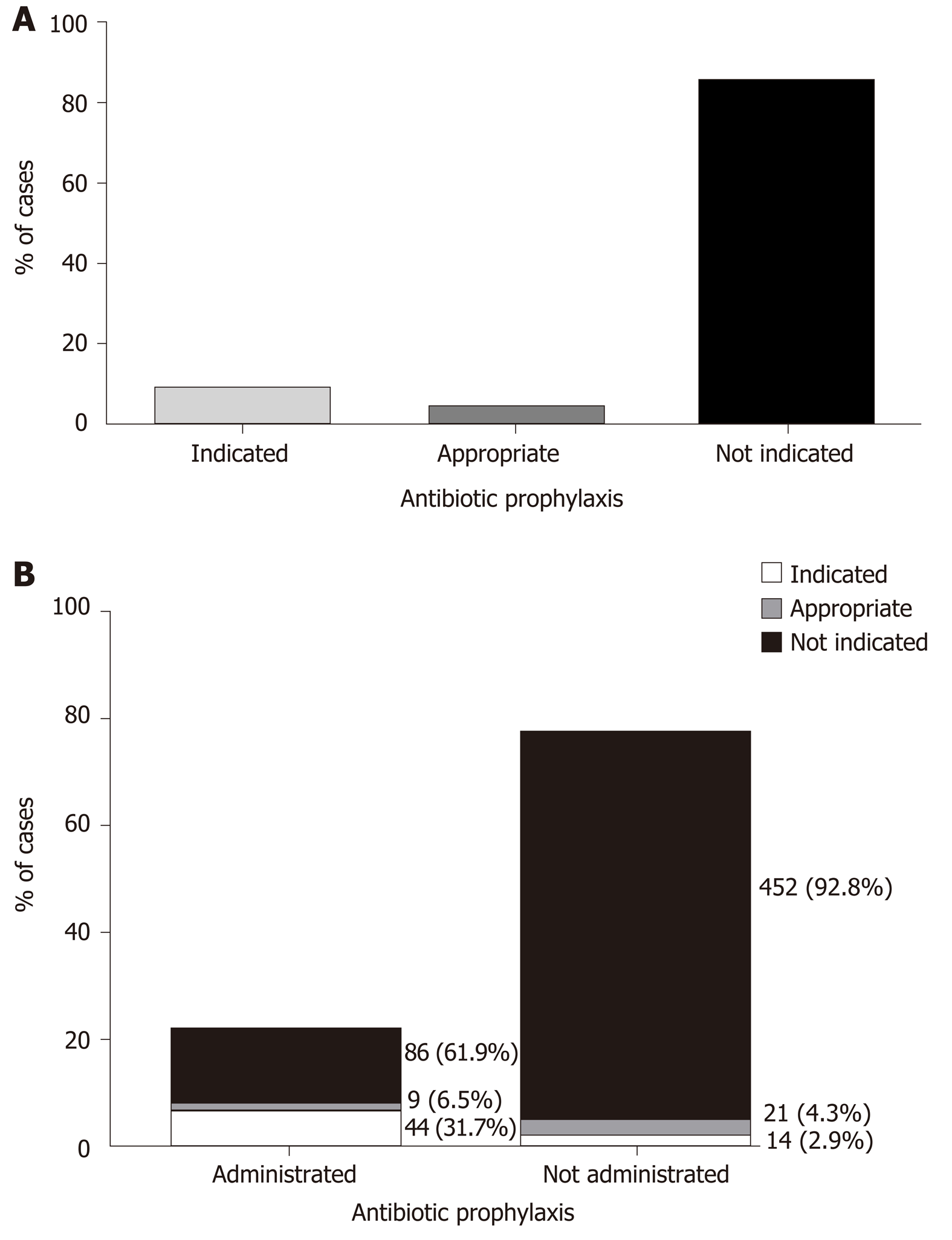
In order to assess the appropriateness of antibiotic administration, a case by case review of the ERCP reports by an advanced endoscopist blinded to drug administration was performed. Antibiotic prophylaxis was clearly indicated in 59 cases (9.3%) and not indicated in 538 cases (85.9%). In 30 cases (4.8%) antibiotic prophylaxis was deemed appropriate in the specific setting, though not clearly indicated by ASGE guidelines (Figure 3A). In line with this classification, the antibiotic prophylaxis was indicated in only 44 cases (31.7%), appropriate in 9 cases (6.5%) and not indicated in 86 (61.9%) out of 139 cases it was given (Figure 3B).
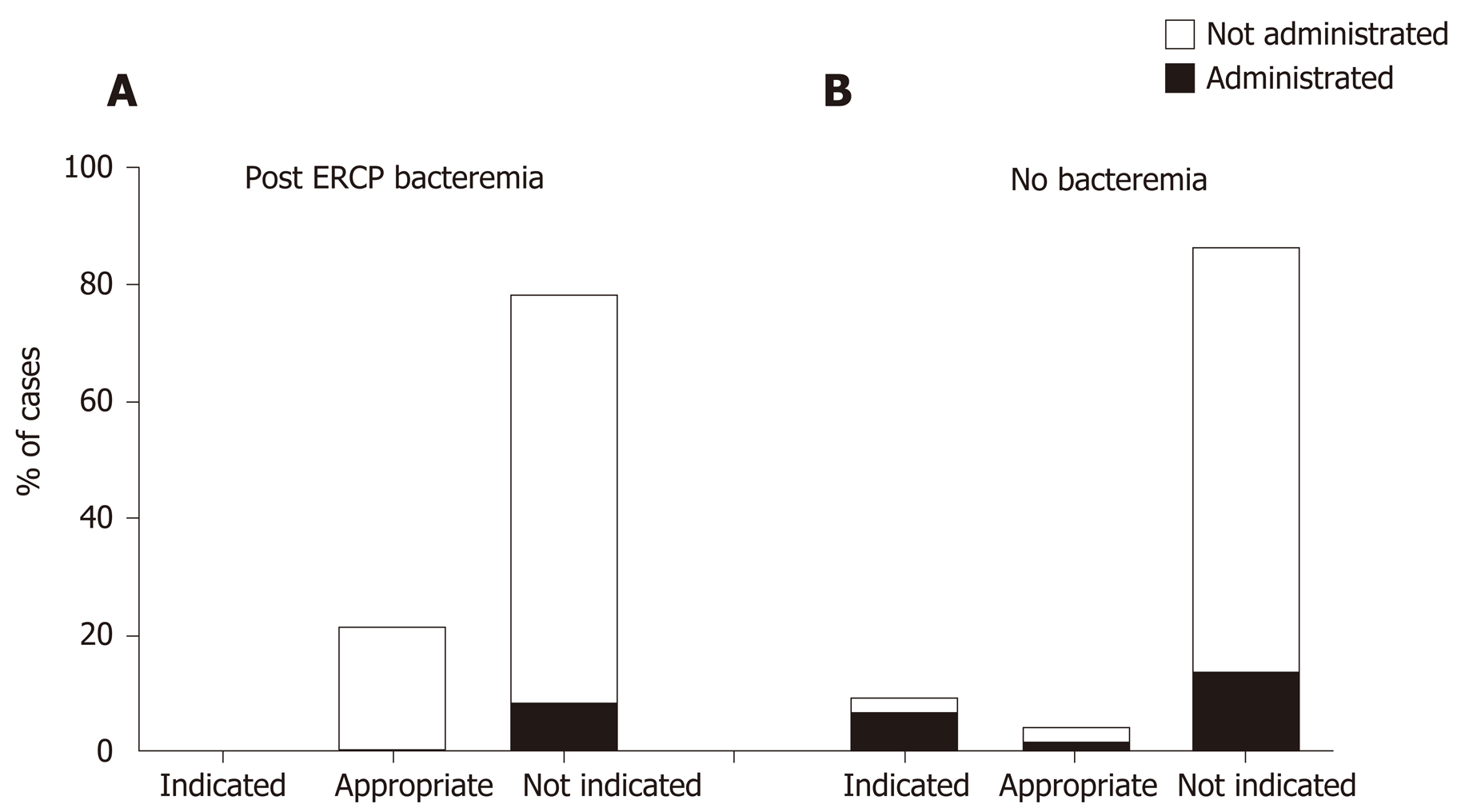
Out of 23 cases of PEB, none (0%) were indicated for antibiotic prophylaxis. Five cases (21.7%) were found appropriate and in 18(78.3%) cases there was no indication for antibiotic prophylaxis. In 2/23 cases of PEB antibiotic prophylaxis was actually administrated (both non-indicated). One case was an ambulatory procedure where Ceftriaxone was administered and Enterobacteriaceae PEB occurred. The other was an in-patient treated prophylactically by three different antibiotics (ceftriaxone, gentamycin and metronidazole) but ended up with ESBL PEB. In both cases the procedure duration was longer than 60 min (Figure 4).
In order to evaluate novel risk factors for PEB, two methods were used: Multivariate logistic regression and decision tree. By univariate logistic regression, PEB (as the dependent variable) and 13 independent variables were evaluated. Four variables were found to be statistically significant: Age at ERCP (years) (OR, 1.027, 95%CI: 0.995-1.060, P = 0.096); Tandem EUS/ERCP (Yes vs No) (OR, 4.130, 95%CI: 1.494-12.084, P = 0.007); Tandem EUS/ERCP with FNA (Yes vs No) (OR, 13.984, 95%CI: 1.552-8.925, P < 0.001); ERCP duration (minutes) (OR, 1.011, 95%CI: 1.001-1.022, P = 0.034) (Table 2). Both appropriateness of prophylaxis administration and actual prophylaxis administration were not shown to increase the risk for PEB (Table 2). We elected and entered three variables (ERCP duration, age at ERCP and tandem EUS/ERCP with FNA) into a decision tree. In this preliminary model the cut points were age ≥ 75 years and ERCP duration ≥ 60 min (not shown). Therefore, we re-analyzed these variables as dichotomous variables in the univariate logistic regression (Table 2). The three mentioned variables were entered to a stepwise multivariate logistic regression along with "antibiotic prophylaxis" as a possible confounder (Table 2). All three factors were significant risk factors: Age at ERCP ≥ 75 years (OR, 3.780, 95%CI: 1.519-9.408, P = 0.004); Tandem EUS/ERCP with FNA (OR, 14.528, 95%CI: 3.571-59.095, P < 0.001); ERCP duration ≥ 60 min (OR, 5.396, 95%CI: 1.86-15.656, P = 0.002). Administration of antibiotic prophylaxis was not a significant beneficial factor.
| Univariate logistic regression | Multivariate logistic regression | |||||
| Variable | Exp (b) | 95%CI | P value | Exp (b) | 95%CI | P value |
| Gender (male vs female) | 1.057 | 0.459-2.434 | 0.444 | |||
| Age at ERCP (yr) | 1.027 | 0.995-1.060 | 0.096 | |||
| Native papilla (Yes vs No) | 1.913 | 0.815-4.487 | 0.136 | |||
| Obstructive abdominal malignancy (Yes vs No) | 1.464 | 0.622-3.443 | 0.382 | |||
| Naïve obstructive malignancy (Yes vs No) | 1.960 | 0.753-5.104 | 0.168 | |||
| Antibiotic prophylaxis (Yes vs No) | 0.324 | 0.075-1.399 | 0.131 | 0.224 | 0.087-1.775 | 0.224 |
| Appropriateness (indicated, appropriate, not indicated) | 1.048 | 0.514-2.135 | 0.898 | |||
| Tandem EUS/ERCP (Yes vs No) | 4.130 | 1.494-12.084 | 0.007 | |||
| Tandem EUS/ERCP with FNA (Yes vs No) | 13.894 | 3.928-49.145 | < 0.001 | 14.528 | 3.571-59.095 | < 0.001 |
| Sphincterotomy (Yes vs No) | 2.408 | 1.006-5.764 | 0.051 | |||
| Precut (Yes vs No) | 1.210 | 0.275-5.329 | 0.801 | |||
| TTS dilation (Yes vs No) | 0.000 | - | 1.000 | |||
| ERCP duration (min) | 1.011 | 1.001-1.022 | 0.034 | |||
| Age at ERCP ≥ 75 years (Yes vs No) | 3.722 | 1.552-8.925 | 0.003 | 3.780 | 1.519-9.408 | 0.004 |
| ERCP duration ≥ 60 minutes (Yes vs No) | 3.933 | 1.438-10.432 | 0.006 | 5.396 | 1.86-15.656 | 0.002 |
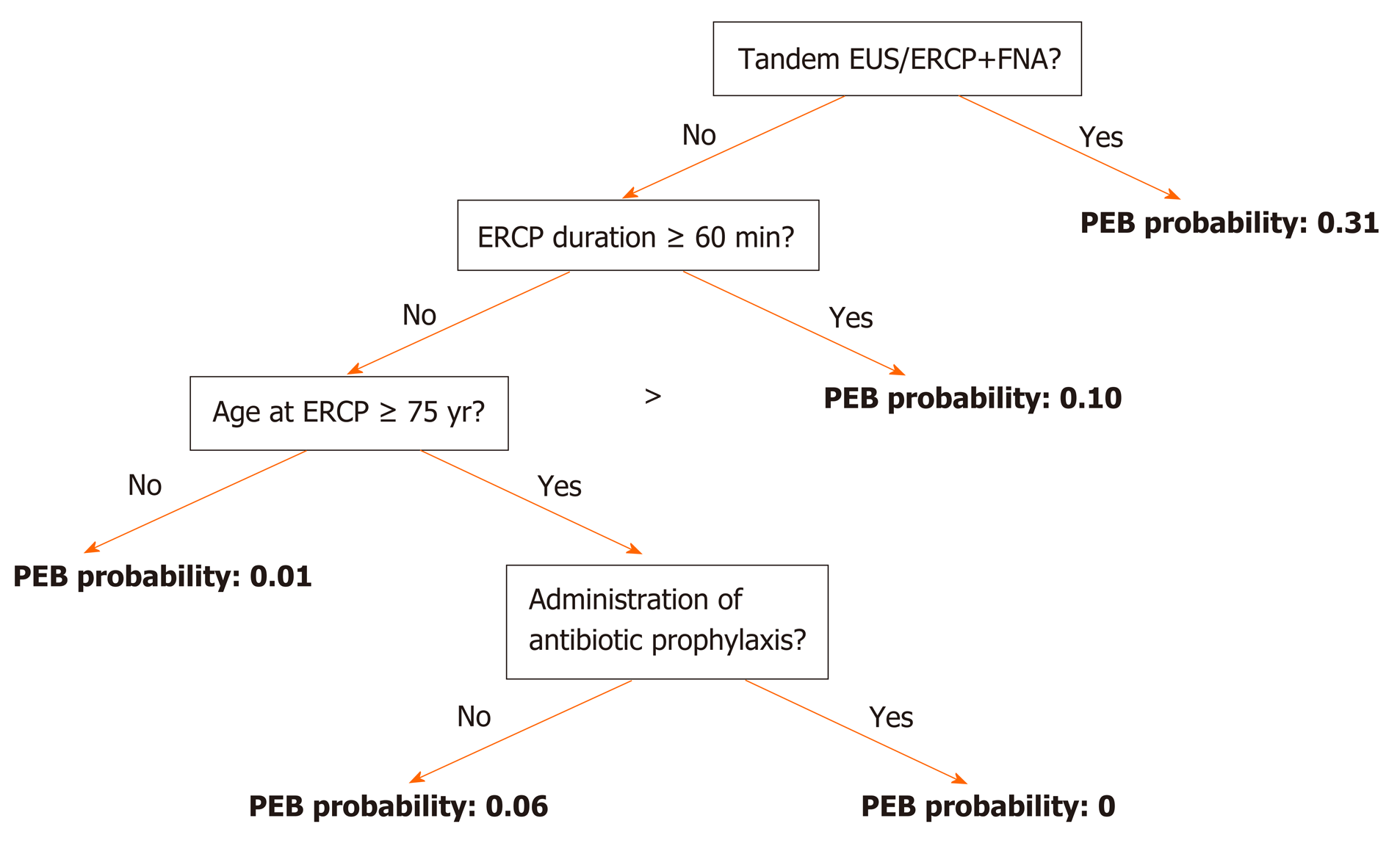
In the second method we entered the selected 3 variables along with "antibiotic prophylaxis" as a possible confounder into a decision tree model (Figure 5). If EUS with FNA preceded ERCP, the probability for PEB was 31%. If not, but the duration of the ERCP was equal to or longer than 60 min, the probability for PEB was 10%. If the duration was less than 60 min and no FNA was preformed but the age of the patient equal or greater than 75 years, the probability for PEB was 6% without prophylaxis and 0% with prophylaxis. If the patient did not have any risk factor, the probability for PEB was 1% regardless of prophylaxis administration.
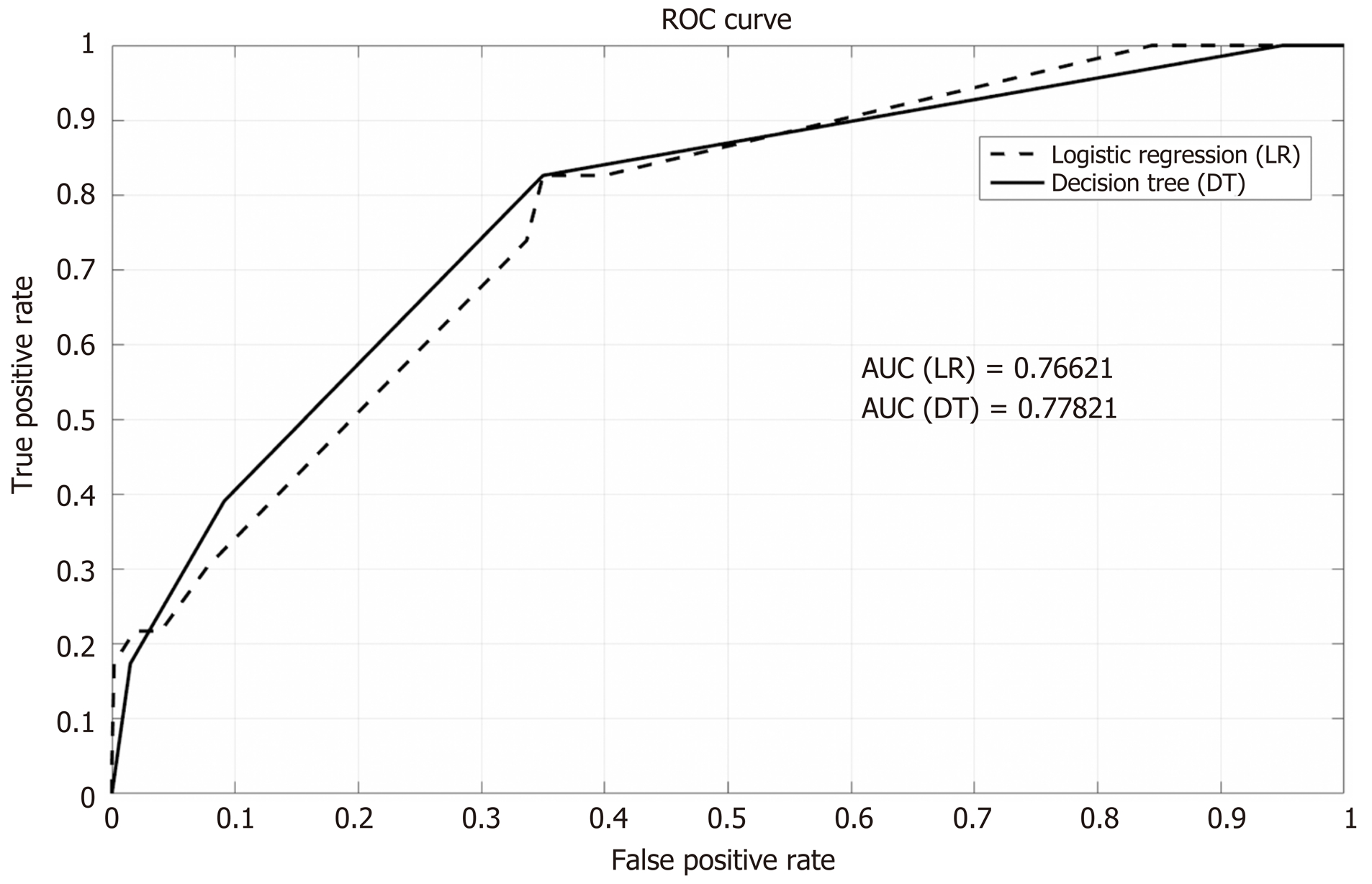
The area under the roc curve of the logistic regression model was 0.766 and the area under the roc curve of the decision tree model was 0.778 (Figure 6).
The rate of bacteremia in our study was 3.7%. Similar rates of 3.56% and 3.1% were described in studies by Du et al[5] and Kwak et al[6] respectively, and can be explained by a uniform definition of PEB occurring up to 7 days from ERCP, and matching inclusion and exclusion criteria that omit patients with suspected pre-ERCP bacteremia or scheduled antibiotic therapy. Much higher rates of bacteremia were described by Thosani et al[7] but in that study blood cultures were actively obtained from all patients regardless of their clinical condition. Moreover, all patients in that study underwent Spyglass choledochoscopy which was proved to be a risk factor for PEB by itself. Supported by Two statistical models (stepwise multivariate logistic regression (ROC, 0.766) and a decision tree model (ROC, 0.778), three independent risk factors for PEB were found in the current study: ERCP duration ≥ 60 min, age at ERCP ≥ 75 years and Tandem EUS/ERCP with FNA. According to the decision tree model, without any risk factors the probability for PEB was 1% regardless of prophylaxis administration.
As in our study, Longer duration of ERCP was also found to be an independent risk factor in the Swedish GallRiks registry[3] where procedures over 30 min carried a higher rate of overall complications (OR, 1.54, 95%CI: 1.43–1.65)[3]. In contrast, Thosani et al[7] found that total ERCP procedure time had no effect on PEB rate. This discrepancy, again, might be explained by the different nature of the studies, where in the last study patients had a more invasive procedure which influenced the rate of overall PEB and, most likely, affected risk factors. As for the patients' age, the Swedish GallRiks registry[3] found age below 70 years to be a significant risk factor for overall complications (OR, 1.26, 95%CI: 1.18–1.35)[3] while in our study, being older than 75 years was an independent risk factor for PEB with odds ratio of 3.780 (1.519-9.408, P = 0.004). This dis-concordance results from different outcome variables. In the Swedish registry the outcome was post-ERCP 30-d overall adverse event rates (including pancreatitis, cholangitis, abscess formation, and perforation). The authors do not describe the prevalence of each complication, but there were 646 patients with septic complications out of 2729 cases with overall complications (23.7% of overall complication events). This can have a major effect on the risk factors. For instance, post-ERCP pancreatitis is well associated with younger age. In accordance to our study, Thosani et al[7] demonstrated that patients with sustained PEB were significantly older than patients who had no documented bacteremia (73 ± 3 vs 61 ± 2, P = 0.0078). Our third independent risk factor for PEB was tandem ERCP and EUS procedures with FNA from a solid lesion. This was the most influential risk factor with a probability of 30% to result in PEB according to the decision tree model and odds ratio of 14.528 (95%CI: 3.571-59.095, P < 0.001) according to the multivariate analysis. Three studies found that the risk of bacteremia after EUS FNA of solid lesions of the upper gastrointestinal (UGI) tract is similar to that for routine endoscopic procedures for which antibiotic prophylaxis is not recommended[8,9]. As a result, the ASGE guidelines[1], recommend against administration of prophylactic antibiotics prior to EUS and FNA from a solid mass. However, very scarce data exist regarding bacteremia after tandem EUS-ERCP procedures. In a study by Gornal et al[10], 3/51 (5.9%) patients had bacteremia after a combined EUS and ERCP procedure. FNA from a suspected malignant tumor was performed in 33 (60%) of all EUS procedures. Study population included both patients with benign disease (choledocholithiasis) and malignant disease among which some had EUS guided biliary drainage (16 procedures). All patients received prophylactic antibiotics. Data regarding bacteremia in each subgroup is not available but overall it seems higher than ERCP alone, as this rate of 5.6% occurred despite a uniform prophylactic antibiotics strategy. It is still questionable if the most influential factor contributing to higher bacteremia rate in that study was the EUS itself, the FNA or the biliary drainage.
Our study showed predominantly Enterobacteriaceae PEB (1.8%). Nevertheless, second in line was ESBL PEB (1.3%). There is a global rise in the prevalence of resistant bacterial strains possibly due to overuse of antibiotics. In a Japanese study[11], biliary drug resistant bacteria was more prevalent in the group receiving antibiotic prophylaxis compared to controls (29.3% vs 5.7%, P = 0.006). Performance of biliary drainage further increased the prevalence of drug-resistant bacteria in both groups, but the difference between them remained statistically significant (36.4% vs 10.0%, P = 0.030). This implies that prophylactic antibiotic treatment should not be given universally and efforts should be made to accurately recognize the patients or the type of procedure in which it deems necessary.
Our study has a few limitations most of them are due to its retrospective nature. However, since the data was documented prospectively and the collection of data was very thorough, missing data was very scarce. Furthermore, the study was not randomized and antibiotic administration was according to the treating physician discretion. Nevertheless, this allowed us to investigate compliance with ASGE guidelines and the association with PEB cases.
In conclusion, PEB is consistently reported in the literature regardless of antibiotic prophylaxis. Moreover, there is upward trend in the emergence of resistant bacteria. Antibiotics administration is a double edge sword, too little will result in PEB, while too much will result in side effects and resistant bacteria. Thus, better classification of risk factors is required. In our study, ERCP duration over 1-hour, Tandem EUS-ERCP with FNA and age above 75 years were found to be significant risk factors for PEB by two independent statistical models. These factors should be further evaluated as valid indications for prophylactic antibiotic treatment before ERCP.
Endoscopic retrograde cholangiopancreatography (ERCP) is currently the method of choice for the treatment of biliary and pancreatic duct obstruction. Clinically significant post-ERCP bacteremia (PEB) occurs in up to 5% of cases, while antibiotic prophylaxis is recommended only when an ERCP is unlikely to achieve complete biliary drainage.
The current recommendations do not address specific populations or procedure-related factors that require specific management in terms of antibiotic prophylaxis. Identification of risk factors for PEB may reduce its occurrence and related complications.
The primary objective of this study was to evaluate possible risk factors for PEB. Secondary objectives were: Evaluation of PEB prevalence and to assess "real-life" practices of antibiotic administration and their competency to ASGE guidelines.
This was a retrospective study of all ERCP procedures performed in a single tertiary medical center. Data collection included: Demographic and clinical characteristics such as pre and post procedure antibiotic treatment and bacterial blood cultures and procedure related characteristics. Strict eligibility criteria were applied and 626 ERCPs were included in the final analysis. Stepwise Logistic Regression analysis and Decision Tree algorithms were used for prediction modeling of PEB.
A total of 626 ERCPs performed in 434 patients were included. PEB prevalence was 3.7%. Antibiotic prophylaxis was administrated in 22.2% cases but was indicated according to the guidelines only in 7% of cases. In all the PEB cases, prophylaxis was deemed not indicated. A stepwise logistic regression (ROC, 0.766), identified 3 variables as independent risk factors for PEB: Age at ERCP ≥ 75 years, Tandem EUS/ERCP with FNA and ERCP duration longer than 60 min. In a decision tree model (ROC, 0.778) the probability for PEB without any risk factors was 1% regardless of prophylaxis administration.
Our study demonstrated that ERCP duration longer than 60 min, tandem EUS-ERCP with FNA and age above 75 years are significant risk factors for PEB. Moreover, the prevalence of PEB in our study was similar to previous reports, despite the fact that antibiotic prophylaxis was administrated more readily than recommended. Both conclusions support a more tailor-made approach regarding antibiotic prophylaxis before ERCP.
Future prospective studies should focus on these risk factors as indications for prophylactic antibiotic treatment before ERCP in order to reduce the prevalence of PEB.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cecinato P, Lu NH S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | ASGE Standards of Practice Committee, Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 240] [Article Influence: 26.7] [Reference Citation Analysis (2)] |
| 2. | ASGE Standards of Practice Committee, Anderson MA, Fisher L, Jain R, Evans JA, Appalaneni V, Ben-Menachem T, Cash BD, Decker GA, Early DS, Fanelli RD, Fisher DA, Fukami N, Hwang JH, Ikenberry SO, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Shergill AK, Dominitz JA. Complications of ERCP. Gastrointest Endosc. 2012;75:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Olsson G, Arnelo U, Lundell L, Persson G, Törnqvist B, Enochsson L. The role of antibiotic prophylaxis in routine endoscopic retrograde cholangiopancreatography investigations as assessed prospectively in a nationwide study cohort. Scand J Gastroenterol. 2015;50:924-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Brand M, Bizos D, O'Farrell P Jr. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database Syst Rev. 2010;CD007345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Du M, Suo J, Liu B, Xing Y, Chen L, Liu Y. Post-ERCP infection and its epidemiological and clinical characteristics in a large Chinese tertiary hospital: a 4-year surveillance study. Antimicrob Resist Infect Control. 2017;6:131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Kwak MS, Jang ES, Ryu JK, Kim YT, Yoon YB, Park JK. Risk factors of post endoscopic retrograde cholangiopancreatography bacteremia. Gut Liver. 2013;7:228-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Thosani N, Zubarik RS, Kochar R, Kothari S, Sardana N, Nguyen T, Banerjee S. Prospective evaluation of bacteremia rates and infectious complications among patients undergoing single-operator choledochoscopy during ERCP. Endoscopy. 2016;48:424-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Barawi M, Gottlieb K, Cunha B, Portis M, Gress F. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Levy MJ, Norton ID, Wiersema MJ, Schwartz DA, Clain JE, Vazquez-Sequeiros E, Wilson WR, Zinsmeister AR, Jondal ML. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57:672-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Gornals JB, Moreno R, Castellote J, Loras C, Barranco R, Catala I, Xiol X, Fabregat J, Corbella X. Single-session endosonography and endoscopic retrograde cholangiopancreatography for biliopancreatic diseases is feasible, effective and cost beneficial. Dig Liver Dis. 2013;45:578-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Minami T, Sasaki T, Serikawa M, Ishigaki T, Murakami Y, Chayama K. Antibiotic prophylaxis for endoscopic retrograde chlangiopancreatography increases the detection rate of drug-resistant bacteria in bile. J Hepatobiliary Pancreat Sci. 2014;21:712-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |