Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4043
Peer-review started: April 28, 2019
First decision: May 24, 2019
Revised: June 11, 2019
Accepted: June 25, 2019
Article in press: June 26, 2019
Published online: August 14, 2019
Liver disease is characterized by breath exhalation of peculiar volatile organic compounds (VOCs). Thanks to the availability of sensitive technologies for breath analysis, this empiric approach has recently gained increasing attention in the context of hepatology, following the good results obtained in other fields of medicine. After the first studies that led to the identification of selected VOCs for pathophysiological purposes, subsequent research has progressively turned towards the comprehensive assessment of exhaled breath for potential clinical application. Specific VOC patterns were found to discriminate subjects with liver cirrhosis, to rate disease severity, and, eventually, to forecast adverse clinical outcomes even beyond existing scores. Preliminary results suggest that breath analysis could be useful also for detecting and staging hepatic encephalopathy and for predicting steatohepatitis in patients with nonalcoholic fatty liver disease. However, clinical translation is still hampered by a number of methodological limitations, including the lack of standardization and the consequent poor comparability between studies and the absence of external validation of obtained results. Given the low-cost and easy execution at bedside of the new technologies (e-nose), larger and well-structured studies are expected in order to provide the adequate level of evidence to support VOC analysis in clinical practice.
Core tip: Since the liver plays a key metabolic role, different volatile organic compounds (VOCs) have been identified in the exhaled breath of hepatopathic patients. VOCs have been already analyzed with promising results concerning disease diagnosis and characterization. To date, translation to the clinic has been limited by the lack of standardization and external validation of the results obtained. Since VOC analysis with new technologies is easy, quick, and cheap, and it was proven to discriminate patients with liver cirrhosis, identify stage disease severity, and predict important adverse outcomes, it should be further explored and hopefully exported to clinical practice.
- Citation: De Vincentis A, Vespasiani-Gentilucci U, Sabatini A, Antonelli-Incalzi R, Picardi A. Exhaled breath analysis in hepatology: State-of-the-art and perspectives. World J Gastroenterol 2019; 25(30): 4043-4050
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4043.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4043
Liver disease is well-known to be accompanied and clinically characterized by the exhalation of peculiar volatile organic compounds (VOCs). In ancient times, the typical musty breath aroma, termed fetor hepaticus, was among the most important clinical signs that physicians considered for diagnosis of liver insufficiency. In more recent years, this empiric approach has been supported by stronger evidence, thanks to the availability of sensitive technologies that have made exhaled breath analysis possible and reproducible on a large scale. VOCs are chemical intermediates generated by cellular metabolism and cleared from tissues and blood through the lungs. Several hepatic metabolic processes may be deranged during the course of chronic liver disease (CLD) and lead to the production and accumulation of various VOCs. Many of them can be odorless or be present in concentrations considerably below human sensorial threshold.
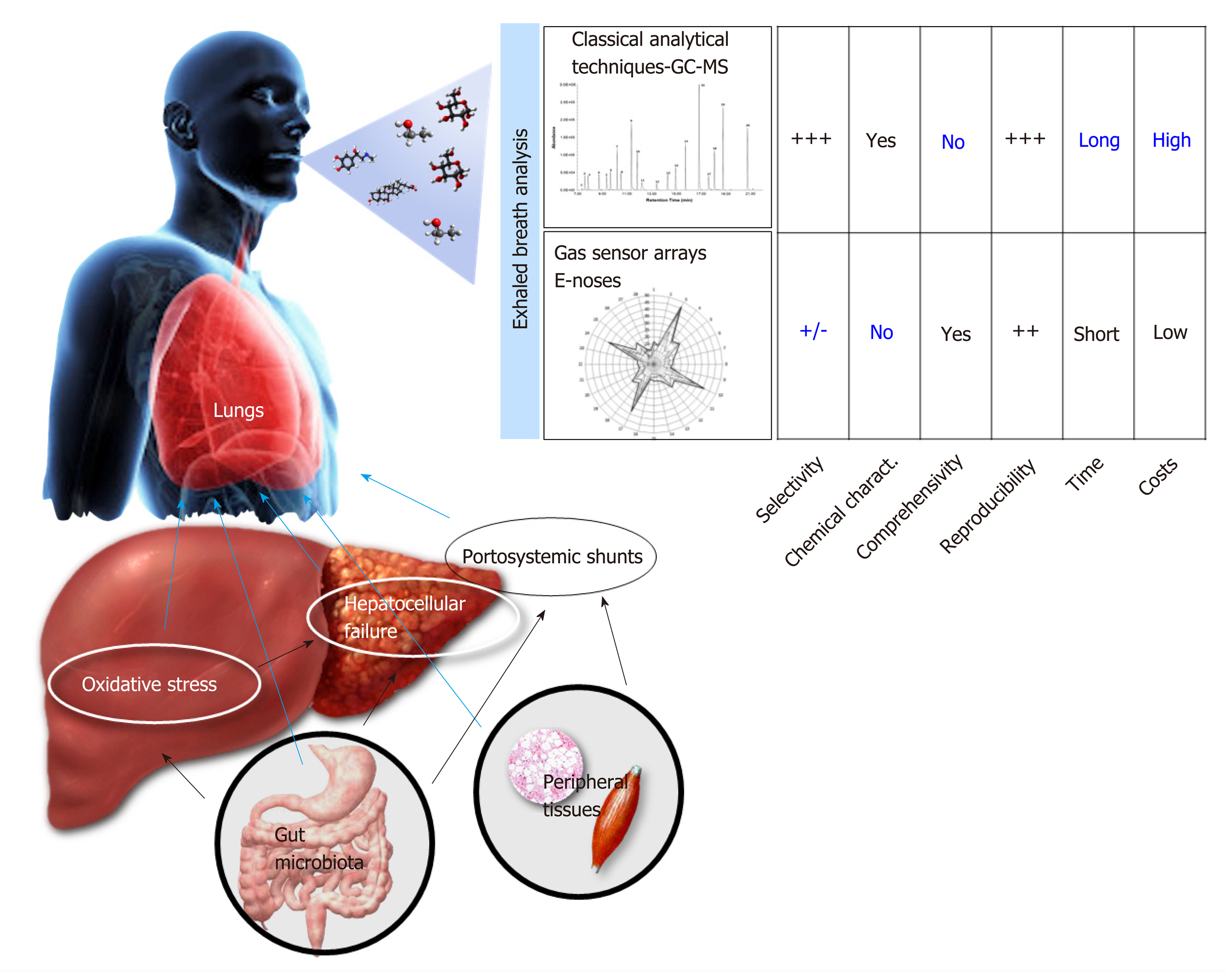
These compounds can be studied using different analytical techniques that are based on gas-chromatography and mass-spectrometry, which allow determining the exact concentration of a large spectrum of VOCs. Thus, these techniques are very useful to clarify biochemical underpinnings of diseases. However, this quantitative breath analysis can hardly provide a set of disease-specific compounds and is limited by a list of technical needs and high costs, making it suboptimal for breath profiling for diagnostic and prognostic purposes in the clinical practice. Conversely, the newer electronic (e-)nose technologies, which are based on the organization of gas sensors into arrays (i.e. gas sensor arrays), have been recently introduced for breath analysis. Although they cannot identify the chemical structure and concentration of each VOC, they provide a qualitative and semi-quantitative profile of the hundreds of VOCs (a sort of fingerprint of exhaled breath, breath-print) that can be associated with selected conditions by pattern recognition. This process mimics the “combinatorial selectivity” that enables natural olfaction to distinguish multiple different odors (Figure 1). A number of existing e-noses are already available[1-4], and this approach has been applied with good results in different clinical contexts[5-7], including the field of hepatology.
The aim of this article is to present the bulk of available studies in which the exhaled breath has been explored in patients with CLD. Starting from work initially focused on the description of VOCs for pathophysiological purposes, more recent research has progressively turned towards the comprehensive assessment of exhaled breath for potential clinical application. The state-of-the-art will be presented, along with a critical discussion of relevant data and perspectives of breath analysis in the field of hepatology.
The pertinent studies were retrieved from MEDLINE using the following search terms: [(exhaled breath analysis OR electronic nose) AND (liver disease OR liver cirrhosis)]. Only English-language studies were considered. To identify additional studies, manual searching of bibliographies from gathered articles and reviews was also performed. Case reports, letters to editor, and commentaries, when adding relevant information, were also considered.
The first works date back to 1970, when Chen et al[8] examined the exhaled breath of two independent groups of 13 and 15 patients with liver cirrhosis (LC), evidencing increased levels of sulfur compounds (such as mercaptans) and volatile aliphatic acids (mainly acetic and propionic acid)[9]. These findings were then confirmed by additional studies[10-16], which further extended this list to nitrogen compounds, various aliphatic acids, alkanes, alkenes, terpenes, ketones, and alcoholic derivatives. Dimethylsulfide was finally identified as the main compound responsible for fetor hepaticus[12]. The exact origin of all these molecules was barely explored, but many pathophysiological speculations linking them with different types of liver disease have been hypothesized involving a wide range of metabolic and inflammatory derangements (presented in Table 1).
| Compounds and molecules | Metabolic disturbances |
| Nitrogen derivatives | |
| Ammonia[14] | Altered urea cycle |
| Trimethylamine[29] | Reduced hepatic catabolism of trimethylamine and/or increased degradation and absorption of dietary phosphatidylcholine and choline mediated by altered intestinal microbiome |
| Ketones[16] | Increased insulin resistance, hepatic glycogen exhaustion, hepatic gluconeogenesis impairment |
| Sulfur derivatives[8] | Incomplete metabolism of sulfur-containing amino acids in the transamination pathway |
| Dimethylsulfide[12] | Main responsible for fetor hepaticus |
| Alkanes, alkenes, terpenes and aliphatic acids[29] | Lipid peroxidation mediated by oxygen radical produced by hepatic CYP activity |
| Ethane and pentane[30] | More typical of alcohol induced liver injury |
| Limonene[13] | Impaired biotransformation by CYP2C (partially dependent on dietary intake of citrus fruits and vegetable) |
| Acetic and propionic acid[9] | Reduced hepatic metabolism of short chain fatty acids produced by gut microbiome |
| Alcohols | |
| Methanol[22] | Pectin degradation; its levels are partially dependent on dietary intake of fruits and on a variable role of intestinal microbiome |
| Ethanol[31] | More typical of NAFLD, even in complete absence of alcohol consumption; possible role of intestinal microbiome in the production of ethanol in obese patients |
Table 2 summarizes the discriminative performances of exhaled breath analysis with respect to the characterization of CLD patients. Van den Velde et al[17] analyzed the breath of 52 patients with established LC. Using gas chromatography mass spectrometry (GC-MS) techniques, they found that dimethylsulfide, acetone, 2-pentanone, and 2-butanone were significantly increased in LC and firstly showed the quite good discriminative capacities of a set of VOCs to distinguish patients with LC from healthy subjects (sensitivity of 100% and specificity of 70%). Subsequently, other studies replicated this finding[18-25], further highlighting the capability of exhaled breath analysis to discriminate LC also from non-cirrhotic CLD[24,25]. Interestingly, a set of 11 VOCs discriminated LC significantly better than five serological markers [alanine aminotransferase (ALT), gamma-glutamyl transferase, bilirubin, albumin and platelets], which are commonly used in clinical practice for this purpose[24].
| Study (year) | Methods of breath analysis | Population | Discriminative capacities |
| LC diagnosis | |||
| Van den Velde et al[17] (2008) | GC-MS | 52 LC vs 50 HC | Sens 100%, spec 70% |
| Netzer et al[18] (2009) | IMR-MS | 37 LC vs 35 HC | AUC 0.84 |
| Millonig et al[19] (2010) | IMR-MS | 37 LC vs 25 HC | AUC 0.88 |
| Dadamio et al[20] (2012) | GC-MS | 35 LC vs 49 HC | Sens 82%-88%, spec 96%-100% |
| Khalid et al[21] (2013) | GC-MS | 34 LC vs 7 HC | Sens 100%, spec 86% |
| Morisco et al[22] (2013) | PTR-MS | 12 LC vs 14 HC | AUC 0.88 |
| Fernández Del Río et al[23] (2015) | PTR-MS | 31 LC vs 30 HC | Sens 97%, spec 70%, AUC 0.95 |
| Pijls et al[24] (2016) | GC-MS | 34 LC vs 87 CLD | Sens 83%, spec 87%, AUC 0.90 |
| De Vincentis et al[25] (2016) | e-nosea | 65 LC vs 39 CLD | Sens 88%, spec 69% |
| Liver function | |||
| Morisco et al[22] (2013) | PTR-MS | 6 CPC B-C vs 6 CPC A | AUC 0.92 |
| De Vincentis et al[25] (2016) | e-nosea | 48 CPC A-B vs 17 CPC C | Sens 88%, spec 64% |
| De Vincentis et al[26] (2017) | e-nosea | 89 LC | aHR 2.8, 95%CI 1.1-7 for mortality and aHR 2.2, 95%CI 1.1-4.2, for hospitalization (analysis adjusted for all potential confounder including CPC and MELD) |
| NAFLD—NASH | |||
| Netzer et al[18] (2009) | IMR-MS | 34 NAFLD vs 35 HC | AUC 0.90 |
| Netzer et al[18] (2009) | IMR-MS | 34 NAFLD vs 20 AFLD | AUC 0.92 |
| Millonig et al[19] (2010) | IMR-MS | 34 NAFLD vs 35 HC | AUC 0.96 |
| Millonig et al[19] (2010) | IMR-MS | 34 NAFLD vs 20 AFLD | AUC 0.95 |
| Verdam et al[28] (2013) | GC-MS | 39 NASH vs 26 HC | AUC 0.77 |
| Hepatic encephalopathy | |||
| Khalid et al[21] (2013) | GC-MS | 11 LC with HE vs 23 LC | Sens 91%, spec 87%, AUC 88% |
| Arasaradnam et al[27] (2016) | e-nose+ | 22 LC with HE vs 20 HC | Sens 88%, spec 73%, AUC 0.84 |
| Arasaradnam et al[27] (2016) | e-nose+ | 13 LC with overt HE vs 9 with covert HC | Sens 79%, spec 50%, AUC 0.71 |
| HCC | |||
| Qin et al[32] (2010) | GC-MS | 30 HCC vs 36 HC | Sens 83%, spec 92%, AUC 0.75 |
| Qin et al[32] (2010) | GC-MS | 30 HCC vs 27 LC | Sens 70%, spec 70%, AUC 0.93 |
Sub-analysis including only patients with LC was carried out in two cases[22,25]. In the first study, exhaled breath analysis correctly classified subjects with de-compensated LC in 92% of the cases[22]. In the second one, a lower accuracy of 70% was found, while end-stage liver disease was predicted with a sensitivity of 88% and a specificity of 64%[25]. These results suggest selected VOC patterns to characterize LC through their patterns changing along with the progressive hepatocellular failure, as represented by Child-Pugh classes (CPC). Although most of these works were cross-sectional, their findings have been recently substantiated by a prospective study in which specific VOCs breath-prints by e-nose were found to predict hospitalization and death of patients with LC even in multiple adjusted models[26]. These associations, independent also of CPC and Model for End-stage of Liver Disease score, suggest that, in the context of LC, the exhaled breath profile may add relevant prognostic information that is not properly captured by the available tools.
On the other hand, limited data are available on the diagnosis and grading of hepatic encephalopathy (HE) by exhaled breath. Khalid et al[21] firstly evidenced 13 VOCS to predict HE with an accuracy of 88% in 33 subjects with alcoholic LC. Later on, Arasaradnam et al[27] confirmed an accuracy of 84% in a wider cohort. Overall, exhaled breath analysis was found to identify patients with HE with respect to healthy controls with sensitivity ranging 88%-91% and specificity ranging 73%-87%[21,27]; however, the only study comparing overt and covert HE in LC patients found a significantly lower diagnostic accuracy (sensitivity of 79%, specificity of 50%, and area under the curve of 0.71)[27]. It is plausible that the broad metabolic de-rangements underlying the great clinical heterogeneity of HE (minimal, episodic, recurrent, and persistent) accounts for the heterogeneous VOC patterns and also for poor discriminatory potential of exhaled breath analysis within HE patients.
Similarly, exhaled breath analysis cannot be used with confidence for dis-crimination of liver diseases from different etiologies. A large study, including patients with infective, alcoholic, and metabolic liver diseases, reported a very poor sensitivity (29%) for the discrimination of infective etiology[25]. Another work, carried out on a much more limited sample, evidenced an accuracy of 65% for the detection of the alcoholic etiology of liver disease[21].
In apparent contrast with these results, nonalcoholic fatty liver disease (NAFLD) seemed to retain a more distinctive breath pattern. In a cohort of 89 subjects, patients with NAFLD were correctly discriminated from healthy subjects in 90% of the cases and from patients with alcoholic liver disease in 92% of the cases[18]. A subsequent similar study reported even higher accuracies (96% and 95%, respectively, for the same comparisons)[19]. Moreover, three VOCs (an alcohol, an alkane, and a nitrogen derivate) were shown to detect nonalcoholic steatohepatitis (NASH)-defined by liver histology- with an accuracy of 77%, which was higher than those observed for other non-invasive biomarkers (e.g., elevated serum ALT or the aspartate aminotransferase to ALT ratio)[28]. The application of breath analysis reduced the proportion of undiagnosed cases from 67%-79% to 10% and that of misdiagnosed cases from 49%-51% to 18%[28].
Overall, despite encouraging results, some limitations seem to arise from the critical appraisal of all the above-mentioned studies. First of all, in the great majority of studies, VOC profiles of patients with CLD were compared to healthy controls rather than to the more appropriate control population. Indeed, it would be more sensible to compare patients with LC to those with non-cirrhotic CLD and patients with HE to patients with LC without HE. Moreover, study cohorts were often limited in numerosity, and subjects were generally free from significant comorbidities, thereby being not particularly representative of the real-life setting. Indeed, when larger populations with a greater burden of coexisting diseases were analyzed[24,25], slightly worse results were obtained.
Except for one study[28], the stage of hepatic fibrosis was not determined through the gold standard method (i.e. liver biopsy). Therefore, we lack data on the relation between VOCs and hepatic inflammatory activity as well as on the VOCs pattern in the different stages of liver fibrosis that precedes LC. Worthy of comment is also the fact that classical analytical GC-MS techniques have been applied in the majority of these works, while the e-nose technologies have been recently tested in two studies[25,26] following the good results obtained in respiratory diseases[6]. Both methods have their own limitations (Figure 1). GC-MS is expensive, is difficult to execute at bedside, and can hardly detect a set of disease specific VOCs; conversely, e-nose is poorly selective and cannot identify the chemical structure of selected VOCs. Hence, this great methodological heterogeneity, as well as the lack of external validation of findings obtained by each study, and the differences by which data was statistically analyzed and reported, weakens the overall relevance of these studies and make them barely comparable.
Potential applications of exhaled breath analysis in patients with liver disease seem to emerge from the bulk of currently available studies and deserve particular consideration. Staging CLD and diagnosing LC are actually achieved either through the physician’s judgment based on different clinical, biochemical, or ultrasound data or by means of liver biopsy, which still represents the gold standard. The former is susceptible to poor accuracy, while the latter is invasive and expensive, hence, not feasible on large scale. Newer non-invasive methods based on clinical scores or elastographic techniques have been recently introduced to overcome these limitations, but their diagnostic performance seems unsatisfactory in selected disease scenarios (e.g., in obese and NAFLD subjects). In this context, exhaled breath analysis could serve as an adjunctive tool to refine diagnosis. In addition, VOCs could qualify as indicators of disease severity and prognosis in patients with LC. In fact, different degrees of liver failure are known to be accompanied by several inflammatory and metabolic derangements, which could be mirrored by different VOC patterns and interpreted for risk stratification. This could be particularly relevant, because, although retaining the strongest prognostic information, available scores (i.e. CPC and Model for End-stage of Liver Disease score) can only moderately capture the great phenotypical variability, which is typical of advanced liver disease. A major contribution to this variability is conferred by the onset of HE, which is among the most invalidating complications of LC with a major impact on health status and quality of life, possibly presenting with a various blend of cognitive, behavioral, and motor function alterations. To date, its diagnosis and classification is based on clinical criteria (West-Haven criteria) and subject to high interobserver variability, and its predictors have not been fully clarified. VOCs might complement the evaluation of patients with HE in order to allow a more effective approach in the diagnostic and prognostic phase. Similarly, VOCs could help in the context of NAFLD for the detection of NASH, which is currently achievable only by performing a liver biopsy. Hence, non-invasive alternatives would be eagerly welcomed.
Despite these perspectives, the clinical significance of exhaled breath analysis in hepatology is still hindered by the absence of a sufficient strength of evidence allowing definitive conclusions. Indeed, larger studies providing a better cha-racterization of patients in terms of liver fibrosis and of comorbidities (e.g., diabetes mellitus, obesity, dyslipidemia, chronic kidney disease) and more representative of real life populations are now needed. These studies are expected to validate the observed discriminative capacities in external cohorts, clarifying the impact of coexistent diseases, which might per se influence VOCs, on disease-specific breath patterns. Aside from better investigating their discriminative capabilities, longitudinal studies should determine whether VOCs could also reliably predict LC patients at increased risk of developing overt episodes of HE within a short period. This property would be clearly invaluable, since HE severely affects the quality of life of patients and their caregivers. Individuating those patients at higher risk of HE could allow for tailoring of individualized prophylactic strategies aimed at improving patients’ safety and social and working related activities.
An associated issue concerns the standardization of exhaled breath analysis techniques. If the intention is to promote an application in clinical practice, cheaper and quicker devices (such as e-noses) should be preferred. In addition, their easy execution at bedside or also at patients’ home makes them particularly suitable for elderly, disabled, and more comorbid subjects (see Figure 1). Conversely, “classical” analytical techniques, based on GC-MS, should be limited only to studies aiming at raising our pathophysiological understanding of the underlying mechanisms in hepatopathies.
In conclusion, exhaled breath analysis could be successfully applied for detecting and monitoring the course of liver diseases only after its diagnostic, classificatory, and predictive properties are comprehensively defined. Efforts should be directed towards the enrollment of better characterized cohorts of patients in larger coo-perative and prospective studies, providing adequate outcome specific follow-up periods. An increasing awareness of the potentialities of this technique is eagerly awaited in order to stimulate future research in this field and involve other research groups.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baddour N, Cheungpasitporn W, Surani S, Teodor Swierczynski J S-Editor: Yan JP L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Baietto M, Wilson AD, Bassi D, Ferrini F. Evaluation of three electronic noses for detecting incipient wood decay. Sensors (Basel). 2010;10:1062-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Electronic Sensor Technology [cited 2019 Apr 22]. In: Electronic Sniffer [Internet]. Available from: https://www.estcal.com. [Cited in This Article: ] |
| 3. | Sensigent [cited 2019 Apr 22]. Available from: https://www.sensigent.com/products/cyranose.html. [Cited in This Article: ] |
| 4. | Santonico M, Pennazza G, Grasso S, D'Amico A, Bizzarri M. Design and test of a biosensor-based multisensorial system: A proof of concept study. Sensors (Basel). 2013;13:16625-16640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, Tutino M, Dragonieri S, Memeo V, de Gennaro G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg. 2013;100:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Scarlata S, Pennazza G, Santonico M, Pedone C, Antonelli Incalzi R. Exhaled breath analysis by electronic nose in respiratory diseases. Expert Rev Mol Diagn. 2015;15:933-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Finamore P, Pedone C, Lelli D, Costanzo L, Bartoli IR, De Vincentis A, Grasso S, Parente FR, Pennazza G, Santonico M, Incalzi RA. Analysis of volatile organic compounds: An innovative approach to heart failure characterization in older patients. J Breath Res. 2018;12:026007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Chen S, Zieve L, Mahadevan V. Mercaptans and dimethyl sulfide in the breath of patients with cirrhosis of the liver. Effect of feeding methionine. J Lab Clin Med. 1970;75:628-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Chen S, Mahadevan V, Zieve L. Volatile fatty acids in the breath of patients with cirrhosis of the liver. J Lab Clin Med. 1970;75:622-627. [PubMed] [Cited in This Article: ] |
| 10. | Kaji H, Hisamura M, Saito N, Murao M. Evaluation of volatile sulfur compounds in the expired alveolar gas in patients with liver cirrhosis. Clin Chim Acta. 1978;85:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Tangerman A, Meuwese-Arends MT, van Tongeren JH. A new sensitive assay for measuring volatile sulphur compounds in human breath by Tenax trapping and gas chromatography and its application in liver cirrhosis. Clin Chim Acta. 1983;130:103-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Tangerman A, Meuwese-Arends MT, Jansen JB. Cause and composition of foetor hepaticus. Lancet. 1994;343:483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Friedman MI, Preti G, Deems RO, Friedman LS, Munoz SJ, Maddrey WC. Limonene in expired lung air of patients with liver disease. Dig Dis Sci. 1994;39:1672-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sehnert SS, Jiang L, Burdick JF, Risby TH. Breath biomarkers for detection of human liver diseases: Preliminary study. Biomarkers. 2002;7:174-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Solga SF, Alkhuraishe A, Cope K, Tabesh A, Clark JM, Torbenson M, Schwartz P, Magnuson T, Diehl AM, Risby TH. Breath biomarkers and non-alcoholic fatty liver disease: Preliminary observations. Biomarkers. 2006;11:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Van den Velde S, Nevens F, Van Hee P, van Steenberghe D, Quirynen M. GC-MS analysis of breath odor compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:344-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Netzer M, Millonig G, Osl M, Pfeifer B, Praun S, Villinger J, Vogel W, Baumgartner C. A new ensemble-based algorithm for identifying breath gas marker candidates in liver disease using ion molecule reaction mass spectrometry. Bioinformatics. 2009;25:941-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Millonig G, Praun S, Netzer M, Baumgartner C, Dornauer A, Mueller S, Villinger J, Vogel W. Non-invasive diagnosis of liver diseases by breath analysis using an optimized ion-molecule reaction-mass spectrometry approach: A pilot study. Biomarkers. 2010;15:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Dadamio J, Van den Velde S, Laleman W, Van Hee P, Coucke W, Nevens F, Quirynen M. Breath biomarkers of liver cirrhosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;905:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Khalid TY, Costello BDL, Ewen R, White P, Stevens S, Gordon F, Collins P, McCune A, Shenoy A, Shetty S, Ratcliffe NM, Probert CS. Breath volatile analysis from patients diagnosed with harmful drinking, cirrhosis and hepatic encephalopathy: a pilot study. Metabolomics. 2013;9:938-948. [DOI] [Cited in This Article: ] |
| 22. | Morisco F, Aprea E, Lembo V, Fogliano V, Vitaglione P, Mazzone G, Cappellin L, Gasperi F, Masone S, De Palma GD, Marmo R, Caporaso N, Biasioli F. Rapid "breath-print" of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS One. 2013;8:e59658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Fernández Del Río R, O'Hara ME, Holt A, Pemberton P, Shah T, Whitehouse T, Mayhew CA. Volatile Biomarkers in Breath Associated With Liver Cirrhosis - Comparisons of Pre- and Post-liver Transplant Breath Samples. EBioMedicine. 2015;2:1243-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Pijls KE, Smolinska A, Jonkers DM, Dallinga JW, Masclee AA, Koek GH, van Schooten FJ. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci Rep. 2016;6:19903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | De Vincentis A, Pennazza G, Santonico M, Vespasiani-Gentilucci U, Galati G, Gallo P, Vernile C, Pedone C, Antonelli Incalzi R, Picardi A. Breath-print analysis by e-nose for classifying and monitoring chronic liver disease: A proof-of-concept study. Sci Rep. 2016;6:25337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | De Vincentis A, Pennazza G, Santonico M, Vespasiani-Gentilucci U, Galati G, Gallo P, Zompanti A, Pedone C, Antonelli Incalzi R, Picardi A. Breath-print analysis by e-nose may refine risk stratification for adverse outcomes in cirrhotic patients. Liver Int. 2017;37:242-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Arasaradnam RP, McFarlane M, Ling K, Wurie S, O'Connell N, Nwokolo CU, Bardhan KD, Skinner J, Savage RS, Covington JA. Breathomics--exhaled volatile organic compound analysis to detect hepatic encephalopathy: A pilot study. J Breath Res. 2016;10:016012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Verdam FJ, Dallinga JW, Driessen A, de Jonge C, Moonen EJ, van Berkel JB, Luijk J, Bouvy ND, Buurman WA, Rensen SS, Greve JW, van Schooten FJ. Non-alcoholic steatohepatitis: A non-invasive diagnosis by analysis of exhaled breath. J Hepatol. 2013;58:543-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Hanouneh IA, Zein NN, Cikach F, Dababneh L, Grove D, Alkhouri N, Lopez R, Dweik RA. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol. 2014;12:516-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Probert CS, Ahmed I, Khalid T, Johnson E, Smith S, Ratcliffe N. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J Gastrointestin Liver Dis. 2009;18:337-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Nair S, Cope K, Risby TH, Diehl AM. Obesity and female gender increase breath ethanol concentration: Potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:1200-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Qin T, Liu H, Song Q, Song G, Wang HZ, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2247-2253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |