Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1450
Peer-review started: October 5, 2016
First decision: October 20, 2016
Revised: November 20, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 28, 2017
To detect the expression of Arpin, and determine its correlation with clinicopathological characteristics and the prognosis of gastric cancer (GC) patients.
A total of 176 GC patients were enrolled as study subjects and classified into groups according to different clinicopathological variables. GC mucosal tissues were obtained via surgery. Another 43 paraffin-embedded tissue blocks of normal gastric epithelium (> 5 cm away from the edge of the tumor) were included in the control group. Immunohistochemistry (IHC) for the Arpin and Arp3 proteins was performed on the formalin-fixed, paraffin-embedded GC tissues. Additionally, expression of the Arpin protein in 43 normal gastric tissues was also determined using IHC.
Expression of the Arpin protein in GC was lower than that in normal gastric mucosa (30.68% vs 60.47%, P < 0.001). A χ2 test of the 176 GC samples used for IHC showed that decreased Arpin expression was associated with advanced TNM stage (P < 0.01) and the presence or absence of lymph node metastasis (80.92% vs 35.56%, P < 0.001). Additionally, a significant correlation was observed between the expression of Arpin and the presence of the Arp2/3 complex in GC tissues (χ2 = 30.535, P < 0.001). Moreover, a multivariate Cox regression analysis revealed that Arpin expression [hazard ratio (HR) = 0.551, P = 0.029] and TNM stage (HR = 5.344, P = 0.001) were independent prognostic markers for overall survival of GC patients. Regarding the 3-year disease-free survival (DFS), the recurrence rate of GC patients with low Arpin expression levels (median DFS 19 mo) was higher than that in the high-Arpin-expression group (median DFS 34 mo, P = 0.022).
Low Arpin levels are associated with clinicopathological variables and a poor prognosis in GC patients. Arpin may be regarded as a potential prognostic indicator in GC.
Core tip: Arpin, a newly found Arp2/3 complex inhibitor reported in Nature, in 2013, was shown to restrict the rate of actin polymerization and control cell migration. However, little is known about whether the expression of Arpin is altered in gastric cancer (GC) tissues, and the detailed mechanisms for invasion and metastasis of GC remain unknown. Our research shows that expression level of Arpin is low in GC, and decreased Arpin expression is associated with the characteristics of clinical pathology and poor prognosis of GC patients. It may be regarded as a potential prognosis indicator for clinical outcomes in GC.
- Citation: Li T, Zheng HM, Deng NM, Jiang YJ, Wang J, Zhang DL. Clinicopathological and prognostic significance of aberrant Arpin expression in gastric cancer. World J Gastroenterol 2017; 23(8): 1450-1457
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1450
Gastric cancer (GC) remains the fourth most common malignancy and the third leading cause of cancer-related death worldwide, despite its steadily decreasing incidence and mortality since 1930s[1,2]. Although the early detection rate of GC has increased, many patients still suffer from distant metastasis, resulting in a median survival of only 3-5 mo[3-6]. At present, understanding of the multidisciplinary treatment of cancer is a concern of doctors and researchers. However, surgery is still the treatment of choice for most early solid tumors and even some advanced malignant tumors. With the progress made in molecular biology research, accumulating evidence has shown that the carcinogenesis of the gastric mucosa is a multi-factor, multi-step, and multi-stage development process that involves a variety of related genes. Moreover, stages of GC development are affected by different genes. Therefore, it is necessary to identify novel markers that can accurately reflect the biological characteristics of GC tumors, provide new therapeutic strategies, and predict clinical outcomes.
Invasion and metastasis are the two main characteristics of malignant tumors. In general, invasion and metastasis accompany the movement of cancer cells from the primary focus in cancer tissues to other normal tissues or organs, and actin polymerization is necessary for this movement. Actin, a structural protein composed of actin filaments, exists in two forms, monomers and polymers. The actin polymerization process can be divided into two distinct steps: actin monomer polymerization in the nucleus, followed by the addition of actin monomers to the formed nuclei or fibers. Cell movement results from the mutual cooperation of polymerization and depolymerization of the cytoskeleton itself and adhesion and desorption between different parts of the cell and extracellular matrix. Moreover, the formation of lamellipodia is closely associated with actin polymerization. Lamellipodia play an important role in the process of exploration of the external environment by cancer cells and the formation of new adhesion contacts with the extracellular matrix, which allow motility and spreading. The process of lamellipodiaformation is often driven by spatially and temporally regulated actin polymerization at the leading edge[7]. The molecular mechanisms of cancer cell motility and migration are more complicated than that expected, the movement and migration of cancer cells is a result of multi-step process initiated by the formation of membrane protrusions in response to migratory and chemotactic stimuli. It is generally believed that lamellipodia in driving cancer cell migration plays a main role, and it is caused by adhesion force to pull the cell body forward in the basement membrane. However, many cytokines can form new actin fibers, and each factor can form a specific network. The actin-related protein 2/3 (Arp2/3) complex, the most investigated molecule by far, is the sole machine that generates branched actin networks, and is also considered to be a key regulator of cell motility[8]. It has been reported to be involved in the development and migration of some cancers, such as pancreatic, gastric, colorectal, and breast cancer[9-12]. By binding actin filaments to the side of an existing filament and initiating branch formation, the Arp2/3 complex accomplishes its role of nucleating actin filaments[13]. Therefore, the Arp2/3 complex is thought to be involved in cancer cell invasion and metastasis and is controlled by the tumor-stromal interaction, but the specific guiding mechanism of cell invasion in tumors has not been extensively explored. Relevant research results show that the Arp2/3 complex and its activators, such as the WAVE complex, are deregulated in diverse cancers. The Arp2/3 complex plays an important role in the invasion and metastasis of tumor cells. However, it is worth noting that the Arp2/3 complex is also vital for the maintenance of normal cell function.
In 2013, Dang et al[14] reported a new protein, Arpin, which contains 220 amino acid residues that are localized to the cell membrane. It is encoded by the C15ORF38gene, which is present in multicellular animals and unicellular amoebas. Interestingly, this protein contains a carboxyl structure region but no C-terminal end, helix, or single actin binding domain. However, it does inhibit the Arp2/3 complex, resulting in the inhibition of actin polymerization. Arpin contains the putative binding site of the Arp2/3 complex[14], which has been reported to be closely related to the development and migration of cancer cells due to its key role in filopodia initiation[15,16]. Arpin can guide the direction of cell movement, and it is also known as “the steering factor”. However, this process is not dependent on the formation of lamellipodia and is not based on actin dynamics. Arpin acts in a dose-dependent manner and induces the cell to move. Arpin and its acidic motif can compete with nucleation promoting factors for Arp2/3 binding, thereby inhibiting the activation of the Arp2/3 complex[14,17], resulting in the inhibition of actin filament polymerization. Related research has shown the significant relationship between decreased Arpin, the clinical features of breast cancer, and the poor prognosis of breast cancer patients with low Arpin expression relative to the 5-year replase-free survival[18]. However, little is known about the expression of Arpin in GC tissue and its relationship with the clinicopathological characteristics and prognosis of GC patients. In this study, we investigated the issues mentioned above, preliminarily determined the correlation between Arpin and GC as well as between Arpin and the Arp2/3 complex, and provided a theoretical and experimental basis for further research of the specific signaling transduction mechanism of Arpin and the Arp2/3 complex in advanced gastric carcinoma.
The study cohort was composed of samples from 176 patients with gastric adenocarcinoma, including 110 men and 66 women (mean age 56.3 years) who had undergone a gastrectomy at Qingdao Municipal Hospital and the Affiliated Hospital of Qingdao University between March 2013 and August 2013. Following surgery, routine chemotherapy was administered to patients with advanced disease, but no radiation treatment was administered to any of the patients included in the present study. The eligibility criteria for this study included the following: (1) histologically proven adenocarcinoma; (2) no history of gastrectomy or other malignancy; (3) no other gastric tumors such as gastric stromal tumors; (4) availability of complete clinicopathological and survival data; (5) patients had not received neoadjuvant chemotherapy; and (6) no distant metastasis. This study was approved by the Human Subjects Institutional Committee of the Affiliated Hospital of Qingdao University. All study participants or their legal guardian provided informed written consent prior to study enrollment.
The clinicopathological data were recorded prospectively for the retrospective analysis. The clinicopathological data of the 176 GC patients included age, gender, size of the primary tumor, depth of invasion, lymph node metastasis, TNM stage, degree of differentiation, and location of the primary tumor. The TNM stages of the specimens subjected to immunohistochemistry (IHC) assays were as follows: 70 (39.78%) in TNM stage I, 50 (28.41%) in stage II, and 56 (31.81%) in stage III. Forty-five patients did not have lymph node metastasis, while 131 patients exhibited metastasis. Another 43 paraffin-embedded tissue blocks of normal gastric epithelium (> 5 cm away from the edge of the tumor) were included in the control group. The clinicopathological factors of GC patients are shown in Table 1.
| Clinicopathological features | Number of patients (n = 176) | Arpin expression | χ2 | P value | |
| High (n = 54) | Low (n = 122) | ||||
| Age (yr) | |||||
| > 50 | 97 (55.11) | 29 (29.90) | 68 (70.10) | 0.063 | 0.802 |
| ≤ 50 | 79 (44.89) | 25 (31.65) | 54 (68.35) | ||
| Gender | |||||
| Male | 110 (62.50) | 31 (28.18) | 79 (71.82) | 0.865 | 0.353 |
| Female | 66 (37.50) | 23 (34.85) | 43 (65.15) | ||
| Tumer size (cm) | |||||
| > 5 | 120 (68.18) | 37 (30.83) | 83 (69.17) | 0.004 | 0.949 |
| ≤ 5 | 56 (31.82) | 17 (30.36) | 39 (69.64) | ||
| Depth of invasion | |||||
| T1 + T2 | 44 (25.00) | 24 (54.55) | 20 (45.45) | 15.79 | 0.000 |
| T3 + T4 | 132 (75.00) | 30 (22.73) | 102 (77.27) | ||
| Lymph node metastasis | |||||
| Yes | 131 (74.43) | 25 (19.08) | 106 (80.92) | 32.404 | 0.000 |
| No | 45 (25.57) | 29 (64.44) | 16 (35.56) | ||
| TNM stage | |||||
| I | 70 (39.77) | 30 (42.86) | 40 (57.14) | 9.379 | 0.009 |
| II | 50 (28.41) | 14 (28.00) | 36 (72.00) | ||
| III | 56 (31.82) | 10 (17.86) | 16 (82.14) | ||
| Histology | |||||
| Well + moderate | 80 (45.45) | 21 (26.25) | 59 (73.75) | 1.354 | 0.245 |
| Poor | 96 (54.55) | 33 (34.38) | 63 (65.62) | ||
| Tumor site | |||||
| Upper | 66 (37.50) | 24 (36.36) | 42 (63.64) | 1.648 | 0.439 |
| Middle | 16 (9.09) | 4 (25.00) | 12 (75.00) | ||
| Low | 94 (53.41) | 26 (27.66) | 68 (72.34) | ||
IHC staining for Arpin was performed in the obtained specimens, which consisted of serial 4-μm-thick sections of 10% formalin-fixed, paraffin-embedded tissue. After blocking endogenous peroxidase activity to reduce nonspecific binding by boiling in a pressure cooker for 3 min, the samples were placed in 3% hydrogen peroxide (H2O2) for 10 min. Then the samples washed with buffer and incubated with a goat polyclonal antibody specific to Arpin (sc-242049; Santa Cruz Biotechnology Inc., Bergheimer, Heidelberg, Germany, final dilution 1:25) at 4 °C for 24 h, followed by three washes in buffer. The secondary antibody consisted of biotinylated anti-goat immunoglobulin. Then, the slides were incubated with avidin-biotin-conjugated peroxidase. After washing and staining with 3,3-diaminobenzidine tetrahydrochloride and H2O2, a brown pigment was obtained after counterstaining with hematoxylin.
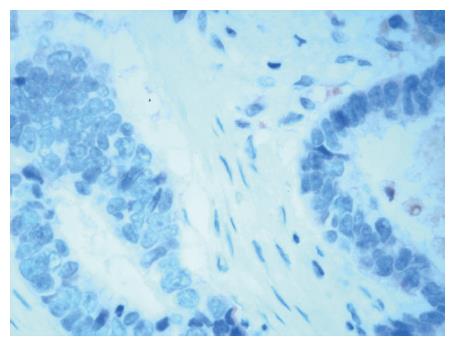
Because Arp2 exhibits the same distribution as Arp3 in tumorous tissue, we concluded that identical expression of Arp2 and Arp3 indicates the formation of Arp2/3 complex. Therefore, Arp3 IHC staining (Figure 1) represented the distribution of the Arp2/3 complex[11]. The sections were incubated with an anti-Arp3 polyclonal antibody (diluted to 1:5000). Sections of GC tissue, in which Arp3 was confirmed to be expressed by immunoblot analysis, were used as the positive control for Arp3 immunostaining. For the negative control, normal rabbit serum was substituted for the primary antibody. Smooth muscle cells of small blood vessels were used as endogenous-negative controls in each section. The secondary antibody consisted of biotinylated anti-goat immunoglobulin.
Tumor cells in which the cytoplasm was stained dark brown under light microscopy were considered positive for Arpin IHC staining. Both the staining intensity and the percentage of stained cells were evaluated for the quantification of Arpin expression. Cells with no staining were scored as 0 points, 1 point represented weak staining intensity, 2 points represented moderate staining intensity, and 3 points represented strong staining intensity. Additionally, we assessed the percentage of stained tumor cells: 0% corresponded to 0 points, less than 25% corresponded to 1 point, 25%-50% corresponded to 2 points, and more than 50% corresponded to 3 points. The final score for Arpin expression was equal to the sum of the two types of scores. A staining score ranging from 0 to 3 points represented low expression, and a score more than 3 points was considered a high expression level[19].
If more than 10% of the tumor cells expressed Arp3, expression by the tumor cells was considered positive. Otherwise, Arp3 expression was considered negative.
SPSS 16.0 software (SPSS, Chicago Statistical IL, United States) was used for the statistical analysis. The differences in clinicopathological variables were analyzed by the χ2 test. The McNemar test was applied to determine the correlation between expression of Arpin and the Arp2/3 complex. Survival curves for 3-year disease-free survival (DFS) were constructed using the Kaplan-Meier method and compared by a log-rank test. The significance of survival variables was evaluated using a multivariate Cox proportional hazard regression analysis, which further showed the independent effect of Arpin expression on DFS. A value of P < 0.05 represented a significant difference.
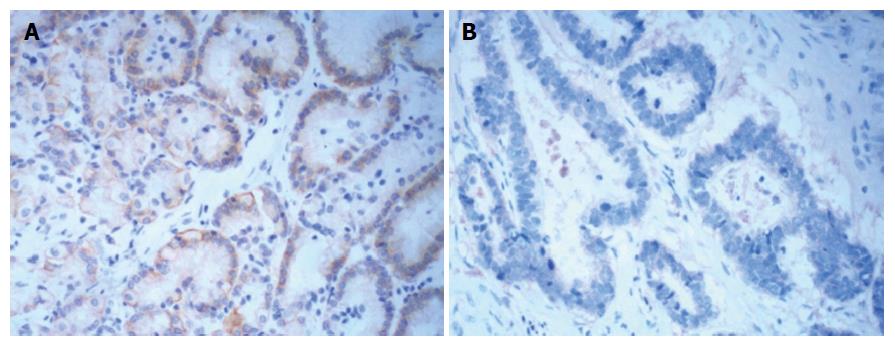
The expression of Arpin in GC tissues was lower than that in normal gastric mucosa (30.68% vs 60.47%, P < 0.001, Figure 2 and Table 2).
| Group | No. of patients | Arpin expression | χ2 | P value | |
| (n = 219) | High (n = 80) | Low (n = 139) | |||
| Normal | 43 | 26 (60.47) | 17 (39.53) | 13.221 | < 0.001 |
| Cancer | 176 | 54 (30.68) | 122 (69.32) | ||
Correlations between Arpin expression and clinicopathological characteristics are shown in Table 1. Analysis of the IHC results from 176 tumor samples showed that Arpin protein expression was lower in stage III (82.14%) than in stage I (57.14%) or II (72.00%). Low Arpin protein expression was significantly associated with advanced TNM stage (stage III vs I, P < 0.01, Table 3). The protein expression level of Arpin in tumor tissues was significantly correlated with the presence or absence of lymph node metastasis (80.92% vs 35.56%, P < 0.001). In addition, we found no significant correlation between the expression of Arpin protein and other clinical parameters, such as tumor location and size, patient age, gender, and histological type.
The expression levels of Arpin and the Arp2/3 complex in gastric carcinoma were significantly correlated (McNemar χ2 = 30.535, P < 0.001, Table 4).
| Arp2/3 complex | Arpin | χ2 | P value | |
| High (n = 54) | Low (n = 122) | |||
| Positive (n = 114) | 27 | 87 | 30.535 | 0.000 |
| Negative (n = 62) | 27 | 35 | ||
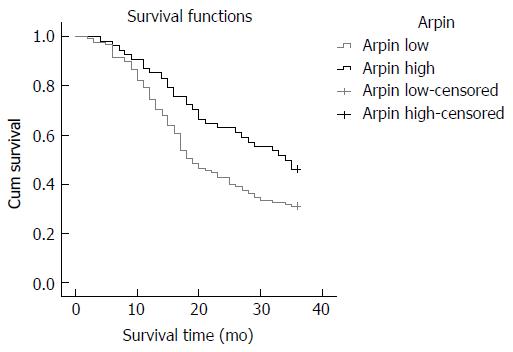
The effects of Arpin expression and clinicopathological characteristics on DFS were evaluated by Kaplan-Meier analysis and log-rank tests. The results showed that GC patients in the low Arpin expression group had a higher recurrence rate (median DFS 19 mo) than those in the high Arpin expression group (median DFS 34 mo, P = 0.022, Figure 3). We found that the 3-year DFS was 31.00% in the low Arpin expression group and 46.00% in the high Arpin expression group. Univariate analyses of clinical variables considered as potential predictors of survival are shown in Table 5. Further analysis using a multivariate Cox proportional hazards model showed that Arpin expression, together with TNM stage, was strongly associated with DFS. Arpin expression [hazard ratio (HR) = 0.551, P = 0.029] and TNM stage (HR = 5.344, P = 0.001) were independent prognostic indicators of DFS in GC patients (Table 5).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR | P value | HR | P value | |
| Age | 1.14 | 0.488 | ||
| Gender | 1.373 | 0.098 | ||
| tumor size | 1.411 | 0.08 | ||
| Depth of invasion | 1.818 | 0.017 | 1.117 | 0.732 |
| Lymph node metastasis | 2.498 | 0.035 | 1.413 | 0.225 |
| tumor site | 1.336 | 0.612 | ||
| TNM stage | 4.985 | < 0.001 | 5.344 | 0.001 |
| Arpin expression | 0.494 | 0.005 | 0.551 | 0.029 |
To our knowledge, the present study is the first to report the clinical significance of Arpin expression in GC patients, although reduced expression of Arpin and its influence on poor prognosis need to be confirmed in further follow-up studies with larger samples.
In this study, all participants with GC were treated surgically. The sections were obtained from postoperative gastric specimens. In addition, the Arpin and Arp3 IHC analysis was conducted using postoperative gastric slides. The results showed that the Arpin protein was significantly decreased in tumor samples relative to the normal tissues in the control group. Therefore, we speculate that due to the decreased expression of Arpin, tumor cells acquire the ability to invade and metastasize in GC, and the degree of malignancy of tumors is closely related to the clinical stage. We then assessed the correlation between Arpin expression and tumor staging. We found that low Arpin expression was significantly associated with advanced TNM stage, and we concluded that Arpin could play a significant role in tumor biology. In the later stage, lower Arpin expression levels indicate greater malignancy. Whether patients in TNM stage IV, with liver metastasis and peritoneal metastasis are correlated with the expression of Arpin deserves intensive study. Further research may accompany with the expression of Arpin with sensitivity of anti-cancer drug and the correlation of the expression of Arpin with cadherin. Because tumor invasion depth, lymph node metastasis, and distant metastasis are the main pathological basis of malignant tumor staging, we further analyzed the correlation between Arpin expression and local invasion of regional lymph nodes. The IHC results showed that low Arpin expression was significantly associated with the depth of invasion and local lymph node metastases. The results of this research may provide new approaches for determining whether loss of Arpin function is associated with increased cell motility in the epithelial-mesenchymal transition during the progression of cancer. Moreover, the inhibitory effect of Arpin on cell migration could potentially be used to control metastasis. However, the tumor size had no significant effect on the test results. Due to the characteristics of the samples and other factors, our results may be different from previous experimental results.
The expression of the Arp2/3 complex was detected in gastric carcinoma tissue in our experiments. Some observations suggest that stromal cells that express Arp2/3 move and grow in advance of the cancer cells to prepare an environment that facilitates cancer cell invasion. Myofibroblasts, which are considered cancer-induced stromal cells, have been shown to affect the adhesion and movement of cancer cells[19]. Additionally, Arpin inhibits the Arp2/3 complex at the lamellipodium tip. Our research results showed that the expression of Arpin and Arp2/3 complex expression in gastric carcinoma were significantly correlated. Determination of whether a negative correlation exists between these two proteins and the specific mechanism concerning how these proteins affect cell migration will require further study with larger samples. Our experiments may have also indirectly shown a decrease in Arpin expression in GC tissues.
More significantly, in order to investigate the effect of Arpin on the prognosis of GC patients, we compared the 3-year DFS in the Arpin low expression group and the high expression group. Consistently, our study demonstrated that Arpin expression and the TNM stage were independent prognostic indicators of DFS. We propose that Arpin likely plays a role in GC metastasis and the prognosis. However, the exact molecular events leading to cancer metastasis and a poor prognosis have not yet been well elucidated, and further research is required.
The present study had some limitations. We could not visualize the specific signal transduction and regulation mechanism of Arpin and the Arp2/3 complex in GC tissue due to technical difficulties. Arpin is a newly discovered protein, its impact on GC patients and the specific mechanism that it guides cell migration may help us develop new therapeutic target. The study of mechanism about signal path is the direction of our future research. Thus, more work is required in the future.
In summary, the present study showed that Arpin was decreased in GC tissues. Additionally, this low expression pattern was found to be significantly correlated with aggressive clinicopathological features. In addition, the specific regulatory mechanisms of Rac, WAVE, Arpin and the Arp2/3 complex in the development of GC are still unclear. In recent years, targeted therapeutics for key molecular drivers of cancer progression have been developed[20]. Arpin may be used as a potential biomarker that could provide important information about tumor progress and could even be a possible target for GC therapy.
Invasion and metastasis are the two main characteristics of malignant tumors. Gastric cancer (GC), the fourth most common malignancy, and is the third leading cause of cancer-related death worldwide. Arpin, a newly found Arp2/3 complex inhibitor reported in Nature, 2013, which was involved in the development and migration of cancer cells for its key role in the filopodia initiation. Thus, further investigations have been carried out about the role of the Arpin protein in GC.
Arpin, a newly found Arp2/3 complex inhibitor, simultaneously acts on cell speed and directional persistence, which are strongly coupled parameters. Loss of the Arp2/3 inhibitory protein Arpin produces a similar poor outcome in breast cancer as high expression of the NCKAP1 subunit of the Arp2/3 activatory WAVE complex. Moreover, Arpin downregulation may contribute to the initiation and development of breast cancer metastasis. Therefore, as a potential predictive marker, Arpin deserves future studies.
In this study, the authors for the first time investigated the expression of Arpin in GC tissue and its relationship with clinical pathological characteristics and prognosis of GC patients, preliminarily determined the correlations between Arpin and GC, Arpin and Arp2/3 complex, and provided a theoretical and experimental basis for further research of the specific signal transduction mechanism of Arpin and Arp2/3 complex in advanced gastric carcinoma. The authors could not visualize the specific signal transduction and regulation mechanism of Arpin and Arp2/3 complex in GC due to a lack of technical assistance.
Arpin protein expression was significantly decreased in tumor samples. Low Arpin associates with clinicopathological variables and poor prognosis in GC patients. Arpin may be regarded as potential prognosis indicator of GC. This provides us a more powerful tool and technical guidance to evaluate the clinicopathologic features and prognosis of patients with GC. Further studies are needed to clarify the detailed mechanisms involved.
The authors detected the expression of Arpin, and evaluated its correlation with clinicopathologic characteristics and prognosis of GC patients. They concluded that low Arpin occurs and associates with clinicopathological characteristics and poor prognosis in GC patients. This is a well written interesting study, which is the first report conducted to determine the value of Arpin in patients with GC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aoyagi K, Bang YJ, Gotoda T, Raffaniello R, Yashiro M S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20838] [Article Influence: 2315.3] [Reference Citation Analysis (2)] |
| 2. | Mayer RJ, Venook AP, Schilsky RL. Progress against GI cancer during the American Society of Clinical Oncology’s first 50 years. J Clin Oncol. 2014;32:1521-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 4. | Lee JH, Kim KM, Cheong JH, Noh SH. Current management and future strategies of gastric cancer. Yonsei Med J. 2012;53:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 6. | Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 465] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 749] [Cited by in F6Publishing: 793] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 8. | Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 698] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 9. | Rauhala HE, Teppo S, Niemelä S, Kallioniemi A. Silencing of the ARP2/3 complex disturbs pancreatic cancer cell migration. Anticancer Res. 2013;33:45-52. [PubMed] [Cited in This Article: ] |
| 10. | Kaneda A, Kaminishi M, Sugimura T, Ushijima T. Decreased expression of the seven ARP2/3 complex genes in human gastric cancers. Cancer Lett. 2004;212:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Otsubo T, Iwaya K, Mukai Y, Mizokami Y, Serizawa H, Matsuoka T, Mukai K. Involvement of Arp2/3 complex in the process of colorectal carcinogenesis. Mod Pathol. 2004;17:461-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Kim DH, Bae J, Lee JW, Kim SY, Kim YH, Bae JY, Yi JK, Yu MH, Noh DY, Lee C. Proteomic analysis of breast cancer tissue reveals upregulation of actin-remodeling proteins and its relevance to cancer invasiveness. Proteomics Clin Appl. 2009;3:30-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Veltman D. Actin dynamics: cell migration takes a new turn with arpin. Curr Biol. 2014;24:R31-R33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Dang I, Gorelik R, Sousa-Blin C, Derivery E, Guérin C, Linkner J, Nemethova M, Dumortier JG, Giger FA, Chipysheva TA. Inhibitory signalling to the Arp2/3 complex steers cell migration. Nature. 2013;503:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Zech T, Calaminus SD, Caswell P, Spence HJ, Carnell M, Insall RH, Norman J, Machesky LM. The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J Cell Sci. 2011;124:3753-3759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Yang C, Svitkina T. Filopodia initiation: focus on the Arp2/3 complex and formins. Cell Adh Migr. 2011;5:402-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Liu X, Zhao B, Wang H, Wang Y, Niu M, Sun M, Zhao Y, Yao R, Qu Z. Aberrant expression of Arpin in human breast cancer and its clinical significance. J Cell Mol Med. 2016;20:450-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Mullins RD, Stafford WF, Pollard TD. Structure, subunit topology, and actin-binding activity of the Arp2/3 complex from Acanthamoeba. J Cell Biol. 1997;136:331-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 186] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Smith AD, Roda D, Yap TA. Strategies for modern biomarker and drug development in oncology. J Hematol Oncol. 2014;7:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |