Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7359
Peer-review started: June 16, 2017
First decision: July 13, 2017
Revised: July 26, 2017
Accepted: August 25, 2017
Article in press: August 25, 2017
Published online: November 7, 2017
To investigate the intestinal segment-specific effects of diabetes and insulin replacement on the density of different subpopulations of submucous neurons.
Ten weeks after the onset of type 1 diabetes samples were taken from the duodenum, ileum and colon of streptozotocin-induce diabetic, insulin-treated diabetic and sex- and age-matched control rats. Whole-mount preparations of submucous plexus were prepared from the different gut segments for quantitative fluorescent immunohistochemistry. The following double-immunostainings were performed: neuronal nitric oxide synthase (nNOS) and HuC/D, heme oxygenase (HO) 1 and peripherin, as well as HO2 and peripherin. The density of nNOS-, HO1- and HO2-immunoreactive (IR) neurons was determined as a percentage of the total number of submucous neurons.
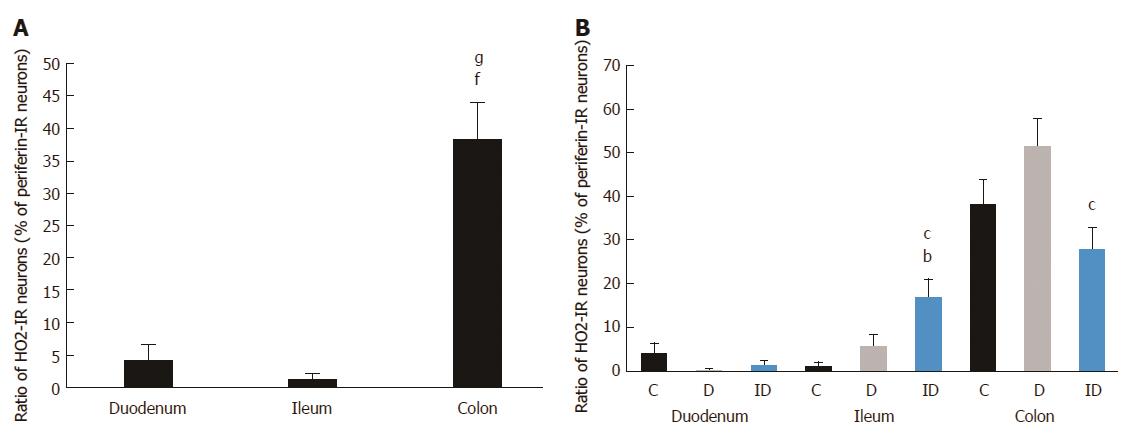
The total number of submucous neurons and the proportion of nNOS-, HO1- and HO2-IR subpopulations were not affected in the duodenal ganglia of control, diabetic and insulin-treated rats. While the total neuronal number did not change in either the ileum or the colon, the density of nitrergic neurons exhibited a 2- and 3-fold increase in the diabetic ileum and colon, respectively, which was further enhanced after insulin replacement. The presence of HO1- and HO2-IR submucous neurons was robust in the colon of controls (38.4%-50.8%), whereas it was significantly lower in the small intestinal segments (0.0%-4.2%, P < 0.0001). Under pathophysiological conditions the only alteration detected was an increase in the ileum and a decrease in the colon of the proportion of HO-IR neurons in insulin-treated diabetic animals.
Diabetes and immediate insulin replacement induce the most pronounced region-specific alterations of nNOS-, HO1- and HO2-IR submucous neuronal density in the distal parts of the gut.
Core tip: Our present findings demonstrate for the first time the segment–specific alterations of the proportion of nitrergic, heme oxygenase (HO)1-immunoreactive (IR) and HO2-IR neurons in the submucous plexus of control, diabetic and insulin-treated diabetic rats. Our results suggest that duodenal nitrergic neurons are not affected, but ileal and colonic ones are induced to change their neurochemical character in diabetes and insulin replacement. The colonic ganglia are abundant in HO-IR neurons in both controls and diabetics, whereas insulin treatment decreases their proportion. In contrast to the colon, the low amount of HO-IR ileal neurons was increased by insulin-treatment.
- Citation: Bódi N, Szalai Z, Chandrakumar L, Bagyánszki M. Region-dependent effects of diabetes and insulin-replacement on neuronal nitric oxide synthase- and heme oxygenase-immunoreactive submucous neurons. World J Gastroenterol 2017; 23(41): 7359-7368
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7359.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7359
Diabetes has widespread effects on gastrointestinal (GI) function causing different GI symptoms, including nausea, vomiting, abdominal pain, constipation or diarrhoea[1]. The functional impairments of the GI tract in diabetes are studied intensively; however, the complexity of diabetes-related pathophysiological consequences hinders the attainment of clear findings about the underlying mechanisms and alterations. An increasing body of evidence confirms that the impaired function of the enteric nervous system (ENS) is closely related to the GI syndromes of diabetic patients[2-4]. The ENS is an autonomous entity structured as two major ganglionated plexi, i.e., the myenteric and the submucosal plexi. The myenteric plexus regulates intestinal motility[5]. It is more difficult to determine the functions of submucous neurons because of differences in the organization and function of the submucous plexus: it is more complex in large mammals with at least two distinct nerve networks than in small rodents. Submucous neurons regulate electrolyte absorption across the mucosa, blood flow and mucosal secretion in small rodents[6,7], moreover in large mammals it is involved both in mucosal and motility processes[8,9].
The alterations in the number of inhibitory neurons including nitrergic neurons in diabetes and their responsiveness to insulin treatment have been intensively studied, but these investigations mainly target the myenteric plexus. Furthermore, a region-specific susceptibility of the nitrergic neurons to diabetes- as well as insulin-related damages was identified in the myenteric plexus[10-12].
In contrast to the intense interest in the neurochemical characterization of myenteric neurons in non-diabetic and diabetic state, the submucous plexus is poorly investigated in diabetes. A decreased total and an unchanged nitrergic submucous neuronal density were found in the jejunum and ileum in streptozotocin-induced (STZ) diabetes as compared to the controls[13,14], but we have no information about the effects of insulin treatment on the submucous neurons.
Long-lasting hyperglycemia can lead to oxidative stress, an imbalance between the increased production of reactive oxygen and nitrogen species (RONS) and the decrease of antioxidant defence mechanisms, which have been implicated in the damage of the ENS such as quantitative and neurochemical changes of the enteric neurons. The systemic oxidative state in type 2 diabetic patients was proved by the determination of serum oxidative stress markers including increased malondialdehyde level and superoxide dismutase activity, decreased glutathione reductase, glutathione peroxidase, glucose-6-phosphate dehydrogenase enzyme activities and glutathione level[15]. This oxidative state was further confirmed by the results from diabetic duodenum and colon of human patients or rats, where it led to the apoptosis of enteric neurons and to neuronal remodelling in the ENS[16,17].
The heme catabolizing heme oxygenase (HO) enzyme plays an essential role in antioxidant defence mechanisms. This enzyme has two main isoforms including the inducible HO1 and the constitutive HO2. Of the two, HO2 is described as the isoform widely expressed in the neuronal and non-neuronal cell types of the healthy GI tract, and both HO1 and HO2 can be co-localized with neuronal nitric oxide synthase (nNOS)[18-21]. In diabetic rat ileum, the nitrergic myenteric neurons containing HO2 were more resistant to the effect of experimentally induced diabetes on cell body size[22]. However, the detailed characterization of the changes of HO1-immunoreactive (HO1-IR) and HO2-IR submucous neurons in the different gut regions of diabetic and insulin-treated diabetic rats has not been accomplished.
In view of the interest in understanding the gut segment-dependent changes in the submucous plexus in diabetes and insulin-treated diabetes, our study aimed to reveal the region-specific alterations in the density of neurons containing nNOS as well as HO1 or HO2 enzymes in the different segments of the small and large intestine of control, diabetic and insulin-treated diabetic rats.
Adult male Wistar rats (Crl:WI BR; Toxi-Coop Zrt.) weighing 210–260 g, kept on standard laboratory chow (Farmer-Mix Kft., Zsámbék) and with free access to drinking water were used throughout the experiments. The rats were divided randomly into three groups: STZ-induced diabetics (diabetics; n = 6), insulin-treated STZ-induced diabetics (insulin-treated diabetics; n = 4) and sex- and age-matched controls (n = 5). Hyperglycaemia was induced as described previously[10,23]. The animals were considered diabetic if the non-fasting blood glucose concentration was 18 mmol/L. From this time on, one group of hyperglycaemic rats received a subcutaneous injection of insulin (Humulin M3, Eli Lilly Nederland) each morning (2 IU) and afternoon (2 IU). The blood glucose level and weight of each animal were measured weekly. In all procedures involving experimental animals, the principles of laboratory animal care (NIH Publication No. 85-23, revised 1985) were strictly followed, and all the experiments were approved in advance by the Local Ethics Committee for Animal Research Studies at the University of Szeged.
Ten weeks after the onset of diabetes, the animals were killed by cervical dislocation under chloral hydrate anaesthesia (375 mg/kg i.p.). The gut segments of control, diabetic and insulin-treated diabetic rats were dissected and rinsed in 0.05 mol/L phosphate buffer (PB, pH 7.4). Samples were taken from the duodenum (1 cm distal to the pylorus), the ileum (1 cm proximal to the ileocecal junction), and the proximal colon and processed for immunohistochemical studies. For double-labelling fluorescent immunohistochemistry, the intestinal segments were cut along the mesentery, pinched flat and fixed overnight at 4 °C in 4% paraformaldehyde solution buffered with 0.1 mol/L PB (pH 7.4). The samples were then washed, and to obtain the whole-mounts of the submucous plexus, the muscular layers of the gut wall were removed, and the mucosa was extracted by scrapping with a small spatula under an Olympus SD30 stereomicroscope.
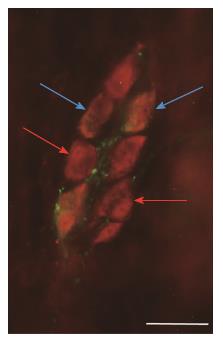
For quantitative immunohistochemistry, whole-mount preparations derived from different gut segments were double-immunostained with nNOS and HuC/D, HO1 and peripherin, as well as HO2 and peripherin. Briefly, after blocking the preparations in PB containing 0.1% bovine serum albumin, 10% normal goat serum and 0.3% Triton X-100, they were incubated overnight with anti-nNOS (rabbit; Cayman Chemical, Ann Arbor, MI, the United States of America; final dilution 1:200) in combination with anti-HuC/D (mouse; Molecular Probes, Eugene, OR, the United States of America; final dilution 1:50), anti-HO1 (mouse; Novus Biologicals Europe, Abington, United Kingdom; final dilution 1:200) or anti-HO2 (mouse; Santa Cruz Biotechnology, Inc., Dallas, TX, the United States of America; final dilution 1:50) in combination with anti-peripherin (rabbit; Millipore, Temecula, CA, the United States of America; final dilution 1:400) primary antibodies. After washing in PB, whole-mounts were incubated with anti-rabbit Alexa Fluor 488 (Life Technologies Corporation, Molecular Probes, Inc., Eugene, OR, the United States of America; final dilution 1:200) and anti-mouse CyTM3 (Jackson ImmunoResearch Laboratories, Inc., Baltimore Pike, PA, the United States of America; final dilution 1:200) secondary antibodies for 4 h. All incubations were carried out at room temperature. Negative controls were performed by omitting the primary antibody, when no immunoreactivity was observed. Whole-mounts were mounted on slides in EverBriteTM Mounting Medium (Biotium, Inc., Hayward, CA, the United States of America), observed and photographed with an Olympus BX51 fluorescent microscope equipped with an Olympus DP70 camera. One hundred submucous ganglia were taken from each intestinal segment in each experimental group and we counted the nNOS-, HO1- and HO2-IR neurons and the HuC/D- and peripherin-IR neurons to yield the total number of submucous neurons. We determined the nNOS-, HO1- and HO2-IR neurons as a percentage of the total number of neurons.
Statistical analysis was performed with one-way ANOVA and the Newman–Keuls test. All analyses were carried out with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, the United States of America). A probability of P < 0.05 was set as the level of significance. All data were expressed as mean ± SE.
According to the data shown in Table 1, STZ promoted hyperglycaemia in the diabetic rats (23.31 ± 0.53 mmol/L), but the insulin treatment prevented the extremely high blood glucose concentration (9.48 ± 0.14 mmol/L). The weight of the animals significantly increased in all groups during the ten weeks of the experiment, but the final body weight of diabetic rats was less elevated compared to the control and the insulin-treated diabetic animals.
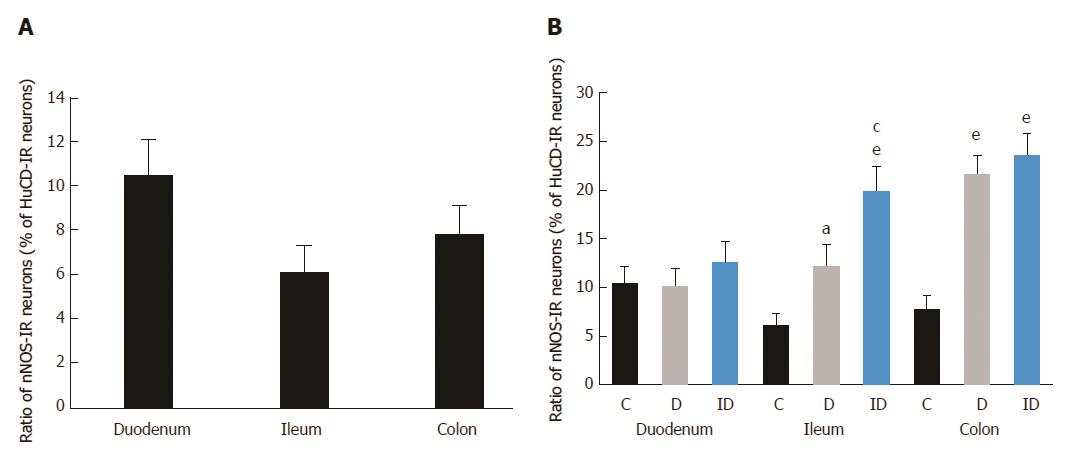
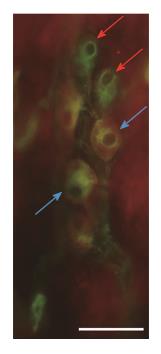
To determine the proportion of nNOS-IR neurons in the total submucous neuronal number, nNOS and HuC/D double-labelling fluorescent immunohistochemistry was applied, as shown in the representative micrograph (Figure 1). In the total number of neurons, no changes were found between the different gut segments within the control group or among the three experimental groups in the duodenal, ileal or colonic intestinal regions (data are not shown). The proportion of nitrergic neurons in the total neuronal number did not show any differences between the three investigated gut segments of controls (Figure 2A). STZ treatment resulted in an increase in the proportion of nitrergic neurons in the distal intestinal segments (in the ileum and colon) but not in the duodenum. The proportion of the nitrergic subpopulation exhibited a 2-fold increase in the ileum (12% vs 6% in controls), whereas a 3-fold increase was observed in the colon of diabetics (22% vs 8% in controls; Figure 2B). The insulin treatment had no effect on the proportion of duodenal and colonic nitrergic submucous neurons which, however, was found to increase significantly in the ileal segment as compared to the diabetic rats.
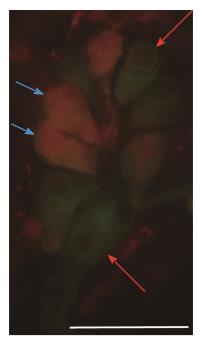
To visualize and quantify the occurrence of HO1-IR submucous neurons, HO1 immunohistochemistry was used combined with peripherin as pan-neuronal marker (Figure 3).
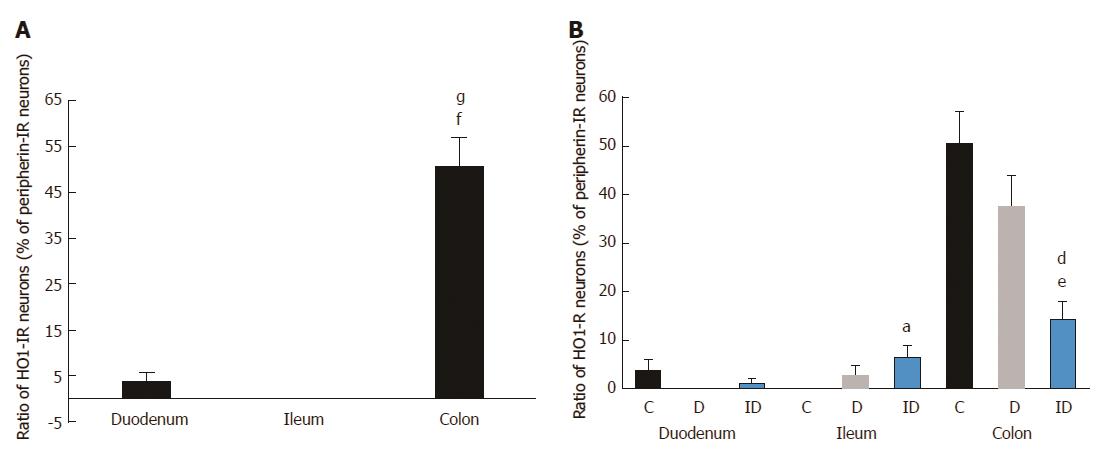
The distribution of HO1-IR submucous neurons as compared to total neuronal number demonstrated region-dependent differences in the three examined segments even in the controls. The proportion of duodenal and ileal HO1-IR submucous neurons was only 4% and 0%, respectively, but the occurrence of HO1-IR neurons was extremely robust in the colon (51%; P < 0.0001; Figure 4A). In the diabetics, results similar to the controls were obtained along the intestinal tract, with HO1-IR neurons seldom occurring in the small intestine and present in abundance in the large intestine (Figure 4B). In the duodenal segment no change was induced by the insulin treatment in the presence of HO1-IR neurons, whereas in the ileum quantitative analysis of the HO1-IR submucous subpopulation revealed a significant increase in the proportion of these neurons in the insulin-treated diabetic group (6%) as compared to the control group (0%). In the colonic submucous ganglia we found a significant decrease in the insulin-treated diabetics (14%) as compared to the control (51%) or the diabetic (38%) groups (Figure 4B).
The occurrence of HO2-IR submucous neurons was also calculated as the proportion of peripherin-stained total submucous neuronal number (Figure 5). In the controls, the HO2-IR neurons demonstrated a distribution similar to that of the HO1-IR submucous neurons. In the segments of small intestine, a low level of HO2-IR neurons was detected (4% in the duodenum, 1% in the ileum), but in the colon this proportion was significantly higher (38%; P < 0.0001; Figure 6A). In the diabetics, results similar to the controls were obtained along the intestine; the marked differences in the ratio of HO2-IR neurons between the different gut segments remained unchanged (Figure 6B).
Immediate insulin replacement had no effect on the abundance of HO2-IR neurons in the duodenum. Although the ileal segment showed the lowest proportion of HO2-IR neurons compared to the other two control regions, a significant increase in their numbers was found in the ileum after insulin replacement (17%) compared to the control (1%) and the diabetic rats (6%). The proportion of colonic HO2-IR neurons showed a pattern similar to that of the HO1-IR neurons in the same segment. The ten-week insulin treatment reduced the proportion of HO2-IR neurons (28%) below control level, and this decrease was significant as compared to the diabetic group (52%; Figure 6B).
In the present study we investigated the nitrergic subpopulation of submucous neurons similarly to our earlier study on the myenteric plexus. In that study we published the gut region-dependent quantitative changes of the total population and the nitrergic subpopulation of myenteric neurons in STZ-induced diabetic and insulin-treated diabetic rats[10]. In the present study we demonstrate that the total submucous neuronal number is similar in the duodenum, ileum and colon in the controls, and remains unchanged in diabetes as well as after insulin replacement. This is in agreement with the observation of da Silva et al[24], however their investigations are limited to the ileum. Our data reflect that STZ-induced diabetes does not induce degenerative changes in the total population of submucous neurons, unlike our previous data about the myenteric plexus. Oxidative stress is known to be induced by hyperglycaemia in diabetes via the mitochondrial overproduction of RONS, which leads to an imbalance between free radical production and antioxidant defense molecules or mechanisms[15-17]. We suggest, in accordance with the opinion of Lopes et al[13], that submucous neurons may have greater resistance to RONS under diabetic conditions.
In non-diabetic state, nitrergic myenteric and submucous neurons have been examined along the gut in different rodent species, and it has been described that the myenteric plexus has more nitrergic neurons as compared to the submucous plexus[25-27]. Using the same rat model of type 1 diabetes as Izbéki et al[10], the gut segment-specific alterations in the two ganglionated plexi can be compared. According to the investigation of the myenteric plexus in the controls, the ileum contained the lowest proportion of nitrergic neurons[10], but we found no region-specific differences in the submucous plexus in accordance with other findings[28,29]. We have revealed different effects of hyperglycaemia on the ratio of nitrergic neurons in the two plexi; a significant decrease was seen in all segments of the myenteric plexus, but, with the exception of the duodenum, an increase was found in the ileal and colonic submucous ganglia. Our results demonstrate that not only total neuronal density but also the nitrergic subpopulation of the two plexi are affected in a different way by the diabetic state.
Because of the unchanged total neuronal number in submucous ganglia, alterations of the neurochemical character as an adaptation to diabetes-related oxidative stress is suggested to be in the background of these modifications. Neurochemical changes as an answer of the ENS to pathological conditions including Crohn’s disease, ulcerative colitis or Parkinson’s disease are known[30-32]. Moreover, there is evidence that diabetic neuropathy and changes in neurochemical coding can occur in the submucous plexus of the ENS[13,14]. We found a similar result in another pathological rat model, in chronic ethanol-consumption, where the total myenteric neuronal number remained constant, but the density of nitrergic neurons was changed[33,34]. Previous studies showed that there are few nitrergic neurons in the submucous ganglia, the submucous plexus is more abundant in vasoactive intestinal polypeptide (VIP)-IR neurons as compared to the myenteric plexus, and there is a high proportion of VIP and nNOS co-localizing neurons in both plexi[14,35]. Lin et al[36] demonstrated that colonic VIP-IR submucous neurons start to express nNOS during culturing as a form of functional plasticity.
Our results reported here show gut segment-specific alterations of not only the nitrergic, but also the HO1-IR and HO2-IR submucous neurons, and these alterations become increasingly pronounced along the proximo-distal axis of the gut. An important finding of this study is that HO1-IR and HO2-IR neurons are more abundant in the colon (about half of the colonic neurons was HO-IR) than in the small intestinal segments (about 0%-5%) in the controls, and STZ treatment did not result in any significant changes in the investigated regions. The question arises as to what the reasons underlying these differences are. In non-diabetic state, more RONS are generated in the colon then in the small intestine[37]. It is possible that under worsening oxidative circumstances the pro-oxidant basal state in the colon acts as a preconditioning factor inducing the higher physiological activity of both isoforms of the antioxidant HO enzymes. This mechanism may play a role in the protection of this segment against neuronal cell loss regarding the total submucous neuronal number in diabetic condition, in contrast to earlier findings in the colonic myenteric ganglia. The neuroprotective, anti-apoptotic effects of the HO1 enzyme in oxidative stress were proved in the central ENS[38,39]. However, we did not investigate the co-localization of nNOS with HO in these neurons, despite earlier findings demonstrating the beneficial effects of the coexistence of these enzymes on the myenteric neurons[22]. Moreover, former studies visualized nNOS-HO2-IR neurons in the submucous ganglia[19,20,40], which suggests a protective role of HO, and may contribute to our results revealing the increasing proportion of nitrergic neurons in the diabetic ileum and colon.
The data about the effects of insulin are contradictory. While early insulin replacement was preventive to the total population of colonic myenteric neurons[10], we found no changes in the three intestinal regions in the total number of submucous neurons of insulin-treated diabetic rats. The density of nitrergic neurons showed similar patterns in the two plexi after insulin replacement, an increase was found from proximal to distal direction, which means significant changes in the ileum and the colon as compared to untreated diabetic rats.
As in the case of the nitrergic subpopulation, not the duodenal, but the ileal and colonic HO1- and HO2-IR submucous neurons responded to insulin treatment, but in an opposite way. However, there were no differences between the control and diabetic groups in these two regions and we found an increased density in the ileum, but a decreased abundance in the colon of insulin-treated diabetic rats.
Our results suggest that the three investigated gut segments have different levels of responsiveness to immediate insulin replacement in diabetes regarding the nitrergic and HO-IR subpopulation of submucous neurons. This is confirmed by our earlier findings in the myenteric neurons and in their microenvironment including the capillary endothelium adjacent to the myenteric ganglia[23]. The environmental changes of submucous ganglia were described, such as the alteration of the faeces-associated microbiota in diabetes and insulin-treatment[41]. Moreover, microangiopathy as a complication of diabetes in submucosal vessels of the GI tract was identified on duodenal and colon samples from diabetic patients[42,43]. On the other hand, our results are in accordance with the previous contradictory data about the beneficial or harmful effects of insulin. Insulin was shown to induce DNA damage through the enhanced production of RONS and to increase the risk of cancer in different cell types[44,45]. But insulin therapy decreased vascular superoxide overproduction in diabetic rats generated by NADPH oxidase, NOS or xanthine oxidase[46]. It is important to mention that the nitric oxide produced by NOS and the carbon monoxide generated by HO enzymes may have either pro-oxidant or antioxidant effects depending on the microenvironment, the duration of production and the amount of these gases[47-49].
This complex background makes it difficult to understand the details of our recent findings, and further experiments are needed to explore the mechanisms of physiological and pathological events in the submucous neurons in diabetes and insulin replacement, their relationships and communication with their environment.
In summary, our present study provides evidence for the first time that the neurochemical character of the nitrergic submucous neurons exhibits gut region-dependent changes in diabetic and insulin-treated diabetic rats. In addition, we prove that HO1-IR and HO2-IR submucous neurons are present in small amounts in the small intestine, but in high abundance in the colon of control and diabetic rats, and they have segment-specific responsiveness to immediate insulin replacement.
In diabetic rat ileum, the nitrergic myenteric neurons containing heme oxygenase (HO) 2 were more resistant to the effect of experimentally induced diabetes on cell body size. However, the detailed characterization of the changes of HO1-immunoreactive (HO1-IR) and HO2-IR submucous neurons in the different gut regions of diabetic and insulin-treated diabetic rats has not been accomplished.
This study aimed to reveal the region-specific alterations in the density of neurons containing nNOS as well as HO1 or HO2 enzymes in the different segments of the small and large intestine of control, diabetic and insulin-treated diabetic rats.
The authors prove that HO1-IR and HO2-IR submucous neurons are present in small amounts in the small intestine, but in high abundance in the colon of control and diabetic rats, and they have segment-specific responsiveness to immediate insulin replacement.
This is a good experimental research and suitable for publication. The findings are of interest and original.
We thank our teacher, Professor Éva Fekete for her valuable support and guidance in the research work.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dinc M, Tomkin GH S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
| 1. | Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia. 2016;59:404-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Horváth VJ, Putz Z, Izbéki F, Körei AE, Gerő L, Lengyel C, Kempler P, Várkonyi T. Diabetes-related dysfunction of the small intestine and the colon: focus on motility. Curr Diab Rep. 2015;15:94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26:611-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Uranga-Ocio JA, Bastús-Díez S, Delkáder-Palacios D, García-Cristóbal N, Leal-García MÁ, Abalo-Delgado R. Enteric neuropathy associated to diabetes mellitus. Rev Esp Enferm Dig. 2015;107:366-373. [PubMed] [Cited in This Article: ] |
| 5. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 856] [Cited by in F6Publishing: 922] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 6. | Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20 Suppl 1:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Andres H, Rock R, Bridges RJ, Rummel W, Schreiner J. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985;364:301-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 103] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec. 2001;262:71-78. [PubMed] [Cited in This Article: ] |
| 9. | Krueger D, Michel K, Zeller F, Demir IE, Ceyhan GO, Slotta-Huspenina J, Schemann M. Neural influences on human intestinal epithelium in vitro. J Physiol. 2016;594:357-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, Bagyánszki M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | de Mello ST, de Miranda Neto MH, Zanoni JN, Furlan MM. Effects of insulin treatment on HuC/HuD, NADH diaphorase, and nNOS-positive myoenteric neurons of the duodenum of adult rats with acute diabetes. Dig Dis Sci. 2009;54:731-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Lopes CR, Ferreira PE, Zanoni JN, Alves AM, Alves EP, Buttow NC. Neuroprotective effect of quercetin on the duodenum enteric nervous system of streptozotocin-induced diabetic rats. Dig Dis Sci. 2012;57:3106-3115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Hermes-Uliana C, Panizzon CP, Trevizan AR, Sehaber CC, Ramalho FV, Martins HA, Zanoni JN. Is L-glutathione more effective than L-glutamine in preventing enteric diabetic neuropathy? Dig Dis Sci. 2014;59:937-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Aouacheri O, Saka S, Krim M, Messaadia A, Maidi I. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes. 2015;39:44-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Jancsó Z, Bódi N, Borsos B, Fekete É, Hermesz E. Gut region-specific accumulation of reactive oxygen species leads to regionally distinct activation of antioxidant and apoptotic marker molecules in rats with STZ-induced diabetes. Int J Biochem Cell Biol. 2015;62:125-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131-138, e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol. 2004;556:325-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Donat ME, Wong K, Staines WA, Krantis A. Heme oxygenase immunoreactive neurons in the rat intestine and their relationship to nitrergic neurons. J Auton Nerv Syst. 1999;77:4-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Battish R, Cao GY, Lynn RB, Chakder S, Rattan S. Heme oxygenase-2 distribution in anorectum: colocalization with neuronal nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2000;278:G148-G155. [PubMed] [Cited in This Article: ] |
| 21. | Ny L, Alm P, Larsson B, Andersson KE. Morphological relations between haem oxygenases, NO-synthase and VIP in the canine and feline gastrointestinal tracts. J Auton Nerv Syst. 1997;65:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Shotton HR, Lincoln J. Diabetes only affects nitric oxide synthase-containing myenteric neurons that do not contain heme oxygenase 2. Brain Res. 2006;1068:248-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Bódi N, Talapka P, Poles MZ, Hermesz E, Jancsó Z, Katarova Z, Izbéki F, Wittmann T, Fekete É, Bagyánszki M. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation. 2012;19:316-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | da Silva GG, Zanoni JN, Buttow NC. Neuroprotective action of Ginkgo biloba on the enteric nervous system of diabetic rats. World J Gastroenterol. 2011;17:898-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Ekblad E, Mulder H, Uddman R, Sundler F. NOS-containing neurons in the rat gut and coeliac ganglia. Neuropharmacology. 1994;33:1323-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Ekblad E, Alm P, Sundler F. Distribution, origin and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience. 1994;63:233-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Costa M, Furness JB, Pompolo S, Brookes SJ, Bornstein JC, Bredt DS, Snyder SH. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992;148:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 215] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Chino Y, Fujimura M, Kitahama K, Fujimiya M. Colocalization of NO and VIP in neurons of the submucous plexus in the rat intestine. Peptides. 2002;23:2245-2250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Sayegh AI, Ritter RC. Morphology and distribution of nitric oxide synthase-, neurokinin-1 receptor-, calretinin-, calbindin-, and neurofilament-M-immunoreactive neurons in the myenteric and submucosal plexuses of the rat small intestine. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:209-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn’s disease. Neurogastroenterol Motil. 2001;13:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, Li Q, Accary D, Bézard E, Bruley des Varannes S, Derkinderen P. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motil. 2009;21:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Krecsmarik M, Izbéki F, Bagyánszki M, Linke N, Bódi N, Kaszaki J, Katarova Z, Szabó A, Fekete E, Wittmann T. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin Exp Res. 2006;30:967-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Bagyánszki M, Bódi N. Gut region-dependent alterations of nitrergic myenteric neurons after chronic alcohol consumption. World J Gastrointest Pathophysiol. 2015;6:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 120] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Lin Z, Sandgren K, Ekblad E. Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton Neurosci. 2004;114:29-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Carroll RJ, Turner ND, Chapkin RS, Lupton JR. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 2004;208:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Al-Owais MM, Dallas ML, Boyle JP, Scragg JL, Peers C. Heme Oxygenase-1 Influences Apoptosis via CO-mediated Inhibition of K+ Channels. Adv Exp Med Biol. 2015;860:343-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Chen L, Wang L, Zhang X, Cui L, Xing Y, Dong L, Liu Z, Li Y, Zhang X, Wang C. The protection by octreotide against experimental ischemic stroke: up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res. 2012;1475:80-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001;13:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Wirth R, Bódi N, Maróti G, Bagyánszki M, Talapka P, Fekete É, Bagi Z, Kovács KL. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS One. 2014;9:e110440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Sasor A, Ohlsson B. Microangiopathy is common in submucosal vessels of the colon in patients with diabetes mellitus. Rev Diabet Stud. 2014;11:175-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | De Las Casas LE, Finley JL. Diabetic microangiopathy in the small bowel. Histopathology. 1999;35:267-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Othman EM, Kreissl MC, Kaiser FR, Arias-Loza PA, Stopper H. Insulin-mediated oxidative stress and DNA damage in LLC-PK1 pig kidney cell line, female rat primary kidney cells, and male ZDF rat kidneys in vivo. Endocrinology. 2013;154:1434-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Othman EM, Leyh A, Stopper H. Insulin mediated DNA damage in mammalian colon cells and human lymphocytes in vitro. Mutat Res. 2013;745-746:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Malardé L, Rebillard A, Le Douairon-Lahaye S, Vincent S, Zguira MS, Lemoine-Morel S, Gratas-Delamarche A, Groussard C. Superoxide production pathways in aortas of diabetic rats: beneficial effects of insulin therapy and endurance training. Mol Cell Biochem. 2014;389:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Rivera LR, Poole DP, Thacker M, Furness JB. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil. 2011;23:980-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Chen S, Khan ZA, Barbin Y, Chakrabarti S. Pro-oxidant role of heme oxygenase in mediating glucose-induced endothelial cell damage. Free Radic Res. 2004;38:1301-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | da Silva JL, Morishita T, Escalante B, Staudinger R, Drummond G, Goligorsky MS, Lutton JD, Abraham NG. Dual role of heme oxygenase in epithelial cell injury: contrasting effects of short-term and long-term exposure to oxidant stress. J Lab Clin Med. 1996;128:290-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |