Published online Jan 7, 2017. doi: 10.3748/wjg.v23.i1.25
Peer-review started: September 24, 2016
First decision: October 20, 2016
Revised: November 17, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: January 7, 2017
New technologies in endoscopic ultrasound (EUS) evaluation have been developed because of the need to improve the EUS and EUS-fine needle aspiration (EUS-FNA) diagnostic rate. This paper reviews the principle, indications, main literature results, limitations and future expectations for each of the methods presented. Contrast-enhanced harmonic EUS uses a low mechanical index and highlights slow-flow vascularization. This technique is useful for differentiating solid and cystic pancreatic lesions and assessing biliary neoplasms, submucosal neoplasms and lymph nodes. It is also useful for the discrimination of pancreatic masses based on their qualitative patterns; however, the quantitative assessment needs to be improved. The detection of small solid lesions is better, and the EUS-FNA guidance needs further research. The differentiation of cystic lesions of the pancreas and the identification of the associated malignancy features represent the main indications. Elastography is used to assess tissue hardness based on the measurement of elasticity. Despite its low negative predictive value, elastography might rule out the diagnosis of malignancy for pancreatic masses. Needle confocal laser endomicroscopy offers useful information about cystic lesions of the pancreas and is still under evaluation for use with solid pancreatic lesions of lymph nodes.
Core tip: Contrast-enhanced harmonic endoscopic ultrasound, elastography and needle confocal laser endomicroscopy represent new, emerging technologies for improving the diagnosis obtained using endoscopic ultrasonography. This paper reviews the principle, indications, main literature results, limitations and future expectations for each of these methods, such as their use in the guidance or orientation of endoscopic ultrasound fine needle aspiration, molecular imaging and neurophysiology assessment in gastroenterology.
- Citation: Seicean A, Mosteanu O, Seicean R. Maximizing the endosonography: The role of contrast harmonics, elastography and confocal endomicroscopy. World J Gastroenterol 2017; 23(1): 25-41
- URL: https://www.wjgnet.com/1007-9327/full/v23/i1/25.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i1.25
New technologies in endosonography assessment are under development due to the limitations of standard endoscopic ultrasound-fine needle aspiration (EUS-FNA): the diagnostic accuracy for pancreatic masses is as high as 95% for cytology[1,2] and 86% for histology[3], the sensitivity for diagnosing lymph nodes is 90%[4], correct diagnosis is difficult for submucosal neoplasms[5], the differential diagnosis of pancreatic cysts is approximately 20%-30%[6], and the malignancy potential is sometimes challenging[7].
The use of Doppler contrast-enhanced EUS using a high mechanical index has been abandoned due to artifacts such as blooming, motion artifacts, poor spatial resolution, and low sensitivity to slow-flow structures[8].
The principle of contrast-enhanced harmonic imaging is to selectively depict signals from the microbubbles of ultrasound contrast agents, which resonate non-linearly when exposed to ultrasonic beams[9]. Background tissue signals are automatically subtracted, and only signals from the contrast agent are enhanced. The mechanical index (MI), which represents the ratio between the peak negative pressure amplitude and the square of the frequency, is related to the oscillation of the microbubbles. For an MI value of lower than 0.1, the bubble oscillation is linear, and no harmonics are produced. For an MI value of higher than 0.6, the microbubbles are destroyed. For this reason, an MI value of 0.14-0.4 is used during contrast-enhanced harmonics EUS (CH-EUS). This method enables the dynamic observation of microvessels with slow flows that are not revealed by Doppler color, which differentiates perfused and non-perfused tissue; however, image resolution is reduced compared to that in B-mode harmonic images.
CH-EUS can be performed using dynamic contrast harmonic imaging (dCHI) implemented onthe Hitachi platform and using the extended pure harmonic (ExpH) as technique implemented on Aloka platforms. The principle behind the first method is based on the emission of consecutive waves in phase inversion, followed by non-linear oscillation of the microbubbles[10]. The second system produces two transmitted pulses that consecutively reach the microbubble, yielding a phase shift between the two received waves[10]. The acoustic power used is low to avoid rapid destruction of the microbubbles (0.2-0.4). A suitable dynamic range that enables good visualization of small differences between vessels and the parenchyma and the focus under the target lesion should be fixed before the contrast injection starts.
Two major contrast substances have been used. Sonovue (Bracco Imaging, Milan, Italy) contains microbubbles of sulfur hexafluoride gas enclosed in a lipid shell. Its injection is followed by an arterial phase (the first 25-30 s after the injection) and a venous phase (30-45 s after the injection). Sonazoid (Daiichi-Sankyo, Tokyo, Japan), which is unavailable in Europe and comprises perfluorobutane in a lipid shell, is uptaken by Kupffer cells, conferring a longer duration than Sonovue.
(1) Assessment of solid and cystic lesions of the pancreas; (2) characterization of submucosal neoplasms; (3) assessment of biliary neoplasms; and (4) assessment of lymph nodes.
CH-EUS helps in mass differentiation, the differentiation between vascular (solid) and avascular (liquid/necrotic) components of the lesion, and the depiction of the dimensions and margins of the pancreatic mass, including its relationship with adjacent vessels.
CH-EUS examination has to report three descriptors[11,12], and the resulting pattern differs between lesions, enabling differentiation before the pathology result is available (Tables 1 and 2).
| Descriptors | Enhancement | Pattern of distribution | Wash-out |
| Hyper/iso/hypoenhancement | Homogenous/inhomogenous | Slow/Fast | |
| Corresponding feature | Arteriolar density compared to the adjacent normal parenchyma | Vascularity architecture | Velocity of the venous blood flow |
| Phase | Arterial | Arterial | Venous |
| Enhancement | Pattern of distribution | Wash-out | ||
| Solid pancreatic lesion | Adenocarcinoma | Hypoenhanced | Homogenous/non-homogenous | Fast |
| NET | Hyperenhanced > hypoenhanced | Homogenous/non-homogenous | Slow > Fast | |
| Chronic pancreatitis | Isoenhanced/hyperenhanced > hypoenhanced | Homogenous/non-homogenous | Fast | |
| Autoimmune pancreatitis | Isoenhanced/hyperenhanced | Homogenous/non-homogenous | Fast | |
| Cystic pancreatic lesion | SCA | Hyperenhancement of the vascularized septae, | Homogenous | Slow |
| honeycomb aspect highlighted | ||||
| MCN | Hyperenhanced thick walls, thick septa and nodules are predictive for malignancy | Fast | ||
| Pseudocyst | Avascular wall + | - | ||
| solid component without any contrast uptake | ||||
| IPMN | Hyperenhanced septae and vascularized neoplastic nodules | Fast | ||
| NET cystic | Hyperenhanced wall and vascularized nodules | Slow |
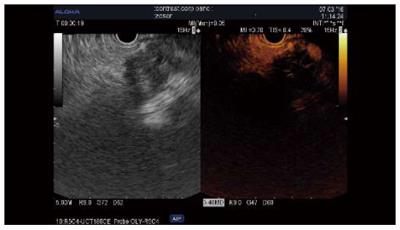
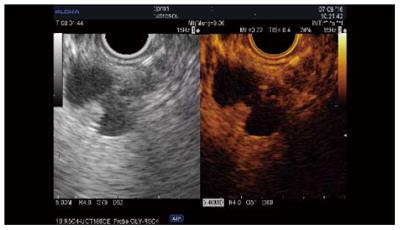
Qualitative assessment: Adenocarcinoma. Contrast uptake by small vessels reveals the low vascularity of these lesions. The hypoenhanced aspect has been reported as predictive for malignancy in several series of patients, with sensitivities of 84%-96%, specificities of 64%-94%, and accuracies 82%-92% (Table 3[12-21]); further, two studies reported superior results compared to standard EUS[12,13]. The pattern of contrast uptake can be inhomogenous when necrosis or intensive fibrosis is present, and fast wash-out is generally seen[12]. Some cases (4%-11% of cases) of isoenhanced/hyperenhanced adenocarcinoma aspect have been reported[14-17]. However, CH-EUS cannot yet replace EUS-FNA for the differentiation of solid masses[14,15,17,18] (Figure 1).
| Ref. | Type of study | Contrast agent | No. of patients | MI | Hypoenhancement as a sign of adenocarcinoma | EUS diagnostic rate | EUS-FNA diagnostic rate |
| Napoleon et al[18] 2010 | Endoscopy | Sonovue | 35 | 0.4 | Sn = 89% | Sn = 79% | |
| PC-18 | Sp = 88% | Sp = 100% | |||||
| NET-9 | PPV = 89% | PPV = 100% | |||||
| CP-7 | NPV = 88% | NPV = 54% | |||||
| Acc = 88.5% | Acc = 83% | ||||||
| Fusaroli et al[12] 2010 | Prospective | Sonovue | 90 | 0.36 radial | Sn = 96% | Sn = 86% | |
| PC-51, NET-13, CP-13 | 0.28 linear | Sp = 64% | Sp = 18% | ||||
| Ac = 82% | Ac = 57% | ||||||
| Ang et al[19] 2011 | Definity | 29 (PC-16, CP-4, Other-9) | 0.3 | Better detection of vascular invasion and tumor margins | - | ||
| Matsubara et al[20] 2011 | Retrospective | Sonazoid | 91 | 0.2 | Sn = 87.5% | - | - |
| Sp = 77.8% | |||||||
| Hocke et al[13] 2012 | Prospective | Sonovue | 58 | - | Sn = 84% | Sn = 73% | - |
| Sp = 76% | Sp = 61% | ||||||
| Kitano et al[15] 2012 | Prospective | Sonazoid | 277 (PC-204, NET-19, CrP-46, Other-8) | 0.3 | Sn = 95% | - | Sn = 92%1 |
| Sp = 89% | Sp = 100% | ||||||
| Lee et al[16] 2013 | Prospective | Sonovue | 37 (PC-28, NET-5, CP-2) | - | Sn = 93% | - | - |
| Sp = 86% | |||||||
| PPV = 93% | |||||||
| NPV = 75% | |||||||
| Acc = 92% | |||||||
| Gincul et al[14] 2014 | Prospective | Sonovue | 100 | 0.4 | Sn = 96% | Sn = 95% | |
| (PC-69, | Sp = 94% | Sp = 93% | |||||
| NET-10, CP-13, | PPV = 94% | PPV = 100% | |||||
| Other-8) | NPV = 97% | NPV = 100% | |||||
| Acc = 91% | Acc = 86% | ||||||
| Park et al[17] 2014 | Retrospective | Sonovue | 90 | - | Sn = 91.9% | - | Sn = 90% |
| Sp = 67.8% | Sp = 100% | ||||||
| Dietrich et al[21] 2016 | Retrospective | Sonovue | 394 | Sn = 92% | - | - | |
| PC-146 | |||||||
| NET-156 |
A meta-analysis of the diagnosis of adenocarcinoma published in 2012 proved that hypoenhancement has a global sensitivity of 94% and a specificity of 89%. However, the main bias of this study was the combination of Doppler and harmonic contrast EUS[22]. A second meta-analysis on contrast-enhanced ultrasound included a combination of endoscopic and transabdominal methods, and its results cannot be generalized for CH-EUS[23]. In a retrospective study of small pancreatic masses, adenocarcinoma was found in only 40%[21] of cases, and CH-EUS might be useful for the identification of such hypoenhanced lesions, which can be sent for surgery without EUS-FNA.
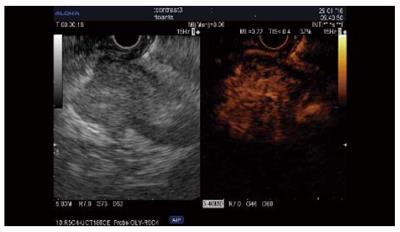
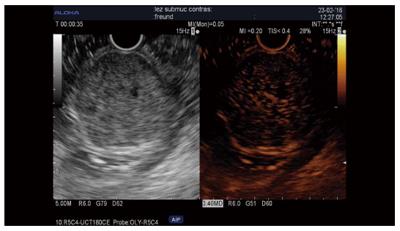
Tumor types other than adenocarcinoma, such as neuroendocrine tumors (NET), chronic pancreatitis, autoimmune pancreatitis, serous cystadenoma, and metastasis, are iso/hyperenhanced[12,14], with a sensitivity of 39%-86% and a specificity of 98%[12,15]. However, only 69%-100% of NET are hyperenhanced[12,14,15]. An inhomogenous pattern in these tumor types corresponding to hemorrhage or necrosis is suggestive of malignancy[12,24] and can be seen in 15% of cases[24]. Few data are available about the usefulness of CH-EUS compared to standard EUS and were acquired in a very limited number of patients (n = 19) (Sn = 78.9%, Sp = 98%), providing a similar value as CT scanning when the small lesions were taken into account[15] (Figure 2).
Focal inflammatory mass may have a hypoechoic appearance in standard EUS and may exhibit diffuse iso/hyperenhancement using CH-EUS[15,16,19] with homogenous or inhomogenous content and fast wash-out. Sometimes these masses present as hypoenhanced lesions (9%-17%) because they exhibit different degrees of fibrosis[14,15,25]. The presence of calcifications is a confounding factor and should be avoided in the region of interest while analyzing contrast uptake. Only one study on solid pancreatic masses compared the low mechanical index of CH-EUS with the high mechanical index of contrast Doppler CEUS, and the second method was found to be superior. The main reason for this was the lack of contrast enhancement in patients with chronic pancreatitis, which was perhaps related to the machine settings; however, this cannot be assessed because the settings were not fully reported[25]. Autoimmune pancreatitis is usually homogenously isoenhanced[26] or hyperenhanced[14].
Cancer metastases are hyperenhanced (renal and thyroid carcinomas, lymphoma, and colon cancer)[14,27,28] or are hypoenhanced (colon cancer, sarcoma, and breast and ovarian cancer)[27,28]; melanoma is isoenhanced[27].
Interobserver agreement for solid pancreatic mass diagnosis showed modest results for the examinations using Sonovue (k = 0.46-0.66) and for the degree of enhancement[14,29]. The k coefficient was 0.94 for Sonazoid, a value that was perhaps related to the signal intensity and its duration. The effect of the endosonographer in CH-EUS was similar for experienced and non-experienced doctors[14,29], except in one study[30].
Combined use of qualitative CH-EUS with EUS-FNA: increased the sensitivity of differential diagnosis using Sonazoid from 92% to 100%, and the specificity was maintained at 92%[15,17]; these findings were similar to results obtained using Sonovue[18]. In addition, the hypoenhanced aspect obtained during CH-EUS proved to be useful in false negative cases of adenocarcinoma[17,18].
Quantitative assessment: Several attempts were done to quantify the image obtained during contrast injection. The dedicated software installed with the instruments is difficult to use because respiratory movements cannot be corrected by the software, and the time-intensity curve (TIC) has many artifacts.
The first study using quantitative assessment and harmonics was based on a hue histogram analysis, and the uptake ratio index between the mass and the surrounding parenchyma was 0.17, with a sensitivity of 80%[28] (Table 4[20,25,31-33]). The post-processing analysis of the time-intensity curves using dedicated software showed that the inflammatory mass studied exhibited a dynamic enhancement pattern using CH-EUS that was similar to that obtained for the rest of the parenchyma, while an adenocarcinoma mass presented low contrast enhancement during the early arterial and late venous phases[25]. Two other studies showed that peak intensity and the rate of echo intensity decrease relative to the peak obtained at 1 min were useful for differentiating malignant tumors[13,26].
| Ref. | Type of study | Type of mass | Contrast agent | Type of echoendoscope | MI | Quantitative assessment | Features useful for differentiation | Diagnostic rate |
| Seicean et al[31], 2010 | Prospective | PC-15 | Sonovue | Radial | 0.36 | Hue histogram | Uptake index ratio | Sn = 80% |
| CP-12 | Sp = 91% | |||||||
| PPV = 92.8% | ||||||||
| NPV = 78% | ||||||||
| Matsubara et al[20], 2011 | Retrospective | PC-48 | Sonazoid | Linear | 0.20 | TIC | Echo intensity reduction rate relative to the peak at 1 min | Sn = 87.5% |
| AIP-14 | Sp = 88.9% | |||||||
| CP-13 | EUS + TIC | |||||||
| NET-16 | Sn = 95.8% | |||||||
| Sp = 92.6% | ||||||||
| Gheonea et al[25], 2012 | Prospective | CP-19 | Sonovue | Linear | 0.20 | Postprocessing TIC | Peak intensity intensity | Sn = 93.7% |
| PC-32 | TTP | Sp = 89.4% | ||||||
| AUC | ||||||||
| Imazu et al[32], 2014 | Prospective | AIP-8 | Sonazoid | Radial | 0.25-0.3 | TIC | Peak intensity | Sn = 100% |
| PC-22 | Maximum intensity gain | Sp = 100% | ||||||
| Săftoiu et al[33], 2015 | Prospective | PC-112 | Sonovue | Linear | 0.1-0.3 | TIC | Peak intensity | Sn = 87.5% |
| CP-55 | Radial | Wash-in AUC | Sp = 92.72% | |||||
| Wash-in rate | ||||||||
| Wash-in perfusion index |
A large multicentric study showed again that peak intensity and features related to the wash-in phase are good parameters for differentiating pancreatic masses, presenting a similar diagnostic rate to that obtained in studies using Sonazoid. However, the time-to-peak value revealed no significance[33]. Post-processing analysis of the TIC in a neural network showed even better diagnostic value, but further results are expected due to the extensive use of this method[33]. However, the software available for use with ultrasound machines awaits further refinement.
Mass detection is improved only in cases that are poorly seen using standard EUS, such as chronic pancreatitis or biliary stents[12]. CH-EUS is superior to CT for the detection of small tumors (Sn = 91.2%, Sp = 94.4%) but not for the detection of all tumors[15].
Tumor staging appears to be better assessed using Sonazoid[32], in particular because the portal vein wall is more clearly seen[11]. No superiority in staging was observed when Sonovue was used[28], although vessel invasion and tumor size were more effectively seen in contrast-enhanced images[28].
The orientation of the EUS-FNA after contrast injection in the hypoenhanced areas was first described by Kitano and then applied in 26% of cases with mixed adenocarcinoma to aid needle placement in the hypoenhanced area[14].
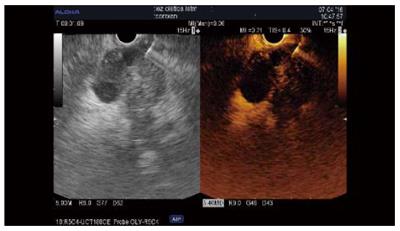
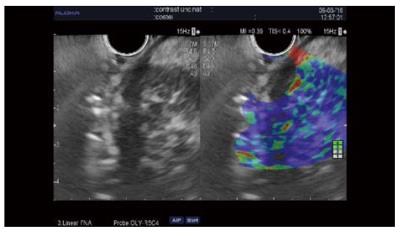
The guidance of the needle during the venous phase of contrast EUS was reported in some case reports and in three series[34]. The diagnostic value was similar to that of standard EUS-FNA in all three studies[28,35,36]. In one randomized control study, the first pass provided better cytology results than standard puncture and limited the number of passes[35]. Our results showed that a combination of two passes under contrast and two standard passes improved core histology-based diagnosis[28] (Figure 3). However, further larger multicenter studies are needed to establish the value of this method, which is safe, rapid, and entails minimal extra costs.
In cystic lesions of the pancreas (PCL), the cystic wall, septae and nodules are assessed for vascularization using the contrast-enhancing bubble movement[37], with the goal of obtaining a differential diagnosis of PCL and identifying malignancy risk features (vascularized wall nodules and the intracystic solid component).
The CH-EUS degree and pattern of enhancement aids in the differentiation of PCL when used as an additional examination (Table 2). The method cannot replace EUS-FNA, except for with typical serous cystadenoma (SCA), because in 86% of cases, the aspect is hyperenhanced, with slow wash-out in 78% of cases[38]. Mucinous cystadenoma with neoplastic transformation presents thick septa and hyperenhanced mural nodules but cannot be differentiated from macrocystic SCA using CH-EUS[38]. Pancreatic pseudocysts have an avascular wall. However, in a series of 46 pseudocysts, three exhibited some wall vascularity[37]. These cysts can have a solid component without any contrast uptake during CH-EUS, thus avoiding EUS-FNA with a potential risk of infection[38]. Neuroendocrine tumors (NET) with a cystic aspect, despite the presence of a hyperenhanced rim, should be sampled by EUS-FNA to differentiate them from cystic adenocarcinoma.
The wall nodules of mucinous cystic neoplasms (MCNs) or intraductal papillary mucinous neoplasms (IPMNs) at risk of becoming malignant appear as hyper/isoechoic without a hyperechoic rim or are smooth edged, and the Doppler vascularity is seldom positive (2 of 14 cases were resected)[39]. CH-EUS is superior to EUS because it reveals small vessels by intense uptake of the contrast substance and differentiates the vessels from mucus or debris, which are not enhanced. Moreover, the CH-EUS may orientate/guide the EUS-FNA[38].
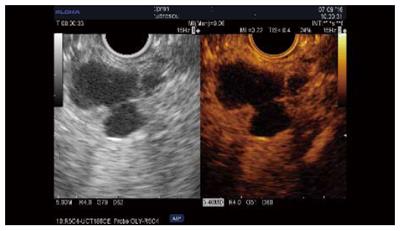
CH-EUS differentiates unenhanced mucus or debris from the malignant nodules of MCNs or IPMNs, which are hyperenhanced, and fast wash-out has been reported in some retrospective studies (Table 5; Figure 4). The CH-EUS detection rate of malignant nodules (84%-98%) was found to be superior to that of standard EUS and CT scan in three studies using Sonazoid[40-42]. The quantitative analysis of contrast uptake may add supplementary data for use in nodule assessment[43]. Several parameters have been described in a retrospective study using Sonazoid, such as the echo intensity change, echo intensity reduction rate and nodule/pancreatic parenchyma contrast ratio. The nodule size on CH-EUS was found to be predictive of malignancy (4 mm and 8 mm)[41,42]; however, another study found that size did not have predictive value[43]. Hyperenhancement of the solid component might orientate/guide EUS-FNA and help the operator to avoid puncturing debris, sludge and mucus plugs[38] (Figure 5).
| Ref. | Type of study | MI | No. of patients | Type of cystic lesions | Contrast substance | Detection of mural nodules accuracy | Diagnosis of malignancy | Cut-off height for malignancy diagnosis(mm) |
| Yamashita et al[40] 2013 | Retrospective | 0.36 | 17 | IPMN | Sonazoid | EUS-0 | ||
| CT-71% | ||||||||
| CH-EUS-94% | ||||||||
| Hocke et al[37] 2014 | Retrospective | 0.02-0.18 | 125 | 1 MCN | Sonovue | Not defined | Not defined | - |
| 6 MD-IPMN | ||||||||
| 16 BD-IPMN | ||||||||
| 103 others | ||||||||
| Harima et al[41] 2015 | Retrospective | - | 50 | IPMN BD | Sonazoid | CT-92% | 8.8 (AUROC = 0.93) | |
| EUS-72% | ||||||||
| CH-EUS-98% | ||||||||
| Kamata et al[42] 2016 | Retrospective | 0.30 | 70 | 6 MCN 42 BD-IPMNs | Sonazoid | EUS-73% | EUS-64 | EUS-8 mm (AUROC = 0.84) |
| 4 SCN | CH-EUS-84% | CH-EUS-84 | CH-EUS-4 mm (AUROC = 0.93) | |||||
| 18 other | ||||||||
| Yamamoto et al[43] 2016 | Retrospective | 0.20 | 30 | 6/18/2006 | Sonazoid | Echo intensity change-0.8 | No effect on malignancy rate | |
| MD/BD/Mixt IPMN | Echo intensity reduction | |||||||
| rate-0.9 | ||||||||
| Nodule/pancreatic parenchyma contrast ratio-0.89 |
Interobserver agreement: For cyst assessment was moderate for the uptake (k = 0.557), slight for the pattern (k = 0.083), and fair for the washout (k = 0.350)[29]. Considering mural nodules, interobserver agreement was excellent using Sonazoid as the contrast agent[42].
Submucosal neoplasms: Hyperenhancement of the submucosal neoplasm during contrast injection was considered suggestive of gastrointestinal stromal tumor (GIST) and is useful for differentiation from benign hypoenhanced lesions, such as lipoma and leiomyoma[44]. The consideration of irregular vessels as predictors of GIST malignancy has shown a sensitivity of 100% and a specificity of 63%[45]. Interobserver agreement was substantial (k = 0.63) for the uptake, slight for the pattern (k = 0.18), and fair for the washout (k = 0.39)[29] (Figure 6).
Biliary tumors: Biliary polyps have been assessed using CH-EUS. Cholesterol polyps revealed heterogenous enhancement, and adenoma exhibited homogenous enhancement with a sensitivity and specificity of 75% and 66%, respectively[46]. However, the heterogenous aspect of a cholesterol polyp could be mistaken for adenoma due to the presence of microvessels with hyaline fibrosis[46].
Ampullary carcinoma and biliary tumors are hyperenhanced with fast wash-out[19,46]. The thick wall of the gallbladder makes it difficult to differentiate between malignant tumors and inflammatory modifications using standard EUS; however, CH-EUS improves the accuracy (94% vs 73%)[47]. The interobserver accuracy obtained when Sonazoid was used to examine the gallbladder wall was substantial (k = 0.77)[47].
Lymph node assessment: Round shape, sharp edge, and a short axis exceeding 8.3 mm are significantly associated with malignant cytology in LNs[48]. A retrospective study of CH-EUS use in LNs showed that 83% of malignant nodes presented a heterogenous pattern with distorted vessels and that a homogenous enhancement was suggestive of reactive lymph nodes[43]. The diagnostic value of CH-EUS for malignancy was characterized by a sensitivity, a specificity and an accuracy of 83%, 91% and 88% respectively[49]. Similar results have been reported for the characterization of intra-abdominal lesions of unknown origin[50]. The interobserver agreement obtained for LN assessment was excellent (k = 0.81)[49].
Limitations: (1) the duration of contrast enhancement is short, especially for Sonovue; (2) quantitative assessment is difficult due to respiratory movements; and (3) technical standardization is lacking.
Future perspectives: The contrast guidance of EUS-FNA could become a routine technique. Because CH-EUS has proven its role to show the change of size and tumor vascularity during chemotherapy for gastric cancers[51], it can be used also assess therapy in other digestive tumors.
Principle: This technique assesses the hardness of the tissue by measuring its elasticity, similar to a virtual palpation. The compression of a target tissue by an echo-endoscopic probe produces a displacement of the tissue called “strain”, which correlates with the hardness of the structure.
Technique: It is essential to establish a large region of interest (ROI) that half comprises the lesion and half comprises the surrounding tissues such that the hardness of the lesion and that of the surrounding tissue can be compared. The probe of the echoendoscope, when upright, creates some pressure, and very small additional movements are important for obtaining the image.
Qualitative assessment is based on superimposing a colored image over the conventional gray-scale EUS image in a region of interest. The strain level of the hard tissue is colored in blue, and the soft tissue is colored in green. An elastic score has been proposed for the pancreas: homogenously hard, heterogenously hard, mixed, heterogenously soft, and homogenously soft[52].
Two semiquantitative approaches are included in the software and can be accessed during the EUS procedure. One approach calculates the hue histogram (strain histogram) as the ratio of the strain between two areas that are selected by the investigator, which are situated at the same distance from the transducer to obtain a similar compression by the probe[13]. The new generation of EUS elastography enables the operator to calculate the mean strain ratio (SR) within a selected area inside the ROI as the difference in elasticity between the targeted lesion and the surrounding tissue, yielding an objective numeric value. However, it is important to obtain a still image, and for this reason, multiple measurements are performed in each patient[53].
Indications: (1) differentiation of the pancreatic masses; (2) differentiation of the lymph nodes; and (3) assessment of fibrosis.
Differentiation of pancreatic masses: Normal pancreas tissue appears as soft tissue (green color) in E-EUS. A pancreatic mass, which is usually hypoechoic in standard EUS, appears as homogenously or inhomogenously green or blue, depending on tissue hardness[54-57].
Based on this qualitative assessment, the global sensitivity and specificity for pancreatic mass assessment were considered to be 100% and 67%, respectively[58]; however, a later multicentric European study found a sensitivity of 93.4% and a specificity of 66%, with a global accuracy of 85.4%[58]. Other studies obtained similar sensitivities and lower specificities for discriminating malignant pancreatic masses (Table 6). It was hoped that this examination could discriminate inflammatory changes from tumor involvement of the vessel wall[59], which has not yet been demonstrated (Figure 7).
| Ref. | Type of study | Final diagnosis | No. of patients | E-EUS assessment | Main results |
| Giovannini et al[58] 2006 | Prospective | Surgery | 24 | Color pattern | Sn = 100% |
| Single center | EUS-FNA | Sp = 67% | |||
| Janssen et al[75] 2007 | Prospective | Surgery | 73 | Color pattern | - |
| Single center | EUS-FNA | ||||
| Săftoiu et al[60] 2008 | Prospective | Surgery | 43 | Hue histogram cut-off value=175 | Sn = 91%, Sp = 87%, PPV = 88%. NPV = 90%, Acc = 89% |
| Single center | EUS-FNA | ||||
| Iglesias-Garcia et al[72] 2009 | Prospective | Surgery | 130 | Color pattern | Sn = 100%, Sp = 85%, PPV = 90%, NPV= 100%, Acc = 94% |
| Single center | EUS-FNA | ||||
| Giovannini et al[79] 2009 | Prospective | Surgery | 121 | Color pattern | Sn = 92% |
| Multicenter | EUS-FNA | Sp = 80% | |||
| Iglesias-Garcia et al[57] 2010 | Prospective | Surgical | 86 | SR = 4.62 | Sn = 100%, Sp = 92% |
| Single center | FNA | ||||
| Săftoiu et al[59] 2011 | Prospective | Surgery | 258 | Hue histogram cut-off value = 175 | Sn = 93%, Sp = 66%, PPV = 92%, NPV = 68%, Acc = 85% |
| Multicenter | EUS-FNA | ||||
| Itokawa et al[73] 2011 | Retrospective | 109 | SR=39.08 | - | |
| Hocke et al[13] 2012 | Prospective | Surgical | 58 | Color pattern | Sn = 94.7% |
| Single center | EUS-FNA | Sp = 33.4% | |||
| Follow up | |||||
| Figueiredo et al[71] 2012 | Prospective | Surgical | 47 | SR = 8 | Sn = 90% Sp = 75% |
| Single center | EUS-FNA | ||||
| Follow up | |||||
| Dawwas et al[70] 2012 | Prospective | Surgical | 111 | SR = 4.69 (AUC = 0.69) | Sn = 100%, Sp = 16.7%, PPV = 86%, NPV = 100%, Acc = 86% |
| Single center | EUS-FNA | Masks elasticity (AUC= 0.72) | Sn = 95%, Sp = 22%, PPV = 86%, NPV = 50%, Acc = 83% | ||
| Lee et al[74] 2013 | Retrospective | - | 15 | Color pattern | - |
| SR = 0.02% | |||||
| Havre et al[54] 2014 | Prospective | Surgery | 48 | SR = 4.4 | Sn = 67%, Sp = 71% |
| EUS-FNA | |||||
| Follow-up | |||||
| Rustemovic et al[93] 2014 | Prospective | Surgery | 149 | SR = 7.59 | Sn = 100% |
| Single center | EUS-FNA | Sp = 45% | |||
| Kongkam et al[69] 2015 | Prospective | Surgery | 38 | SR=3.17 | Sn = 86%, Sp = 66% |
| Single center | EUS-FNA | ||||
| Opačić et al[94] 2015 | Prospective | Surgery | 105 pancreatic mass | Hue histogram | Sn = 98%, Sp = 50%, PPV = 92%, NPV = 100%, Ac = 69% |
| Single center | EUS-FNA | 44 controls | |||
| Mayerle et al[68] 2016 | Prospective | Surgery | 85 | SR = 24.82 or 10 | Sn = 77%, Sp = 65% |
| Single center | EUS-FNA | Sn = 96%, Sp = 43% | |||
| Follow-up |
Using the hue histogram, a value of 175 was found to be suggestive of malignancy[60,61]. However, artifacts related to the presence of surrounding structures with excessive or insufficient stiffness[61] are very important; therefore, the selection of appropriate regions of interest is of great importance.
Postprocessing analysis of the hue histogram using an artificial neural network enabled an optimal prediction of all types of pancreatic lesions and provided better results than hue histogram analysis[60,62]. However, the procedure is complex, and further studies on its practical applicability are warranted.
Multiple studies were performed to measure the mean SR and cut-off value for malignancy. No optimal cut-off value has yet been established for use in malignancy diagnosis (Table 6) due to the inter-observer variability of the method and the difficulty of standardizing the compression.
Two meta-analyses on the differentiation of malignant pancreatic tumors from inflammatory pancreatic masses, each including 13 studies with 1042 and 1044 patients, showed a sensitivity of 95% and a specificity of 67%-69%, with an AUC of 0.86-0.90[63,64]. A third meta-analysis included 7 studies and 752 patients, with a global sensitivity of 97%, a specificity of 76% and an AUC of 0.95[65]. This meta-analysis serves as a reminder that it is difficult to differentiate adenocarcinoma and neuroendocrine tumor, which are both hard lesions, using elastography[65]. A fourth meta-analysis found that the use of a color pattern for elastography EUS interpretation was associated with a sensitivity of 99% and a specificity of 69%-76%[65-67]; moreover, using a hue histogram, the sensitivity was 92% and the specificity was lower, 86%[66]. Both of the semiquantitative assessments evaluated by other meta-analyses showed a sensitivity of 96% and a specificity of 76%[67].
A comparison of B mode EUS, EUS-FNA and EUS elastography favored the standard EUS; the accuracies were 87%, 85% and 73%, respectively[68]. A combination of EUS-FNA with SR was not superior to EUS-FNA alone in a study involving 28 patients[69]. Due to the low negative value of LR (0.09), this method should be indicated to rule out a diagnosis of malignancy and to avoid unnecessary EUS-FNA[63].
Limitations: (1) The control of tissue compression (the degree of compression and the angulation) and motion artifacts determined by respiratory and heart movements is difficult[69-72]; (2) adjacent structures with very low or very high density should be avoided (the heart, major vessels). The interposition of cysts or dilated ducts should be avoided because these may impair the SR[73,74]; (3) the size and depth of the region of interest should be similar when SR is calculated[73]. This improves with the experience of the endosonographer, and modern equipment automatically selects the best still image that is representative of the mean SR of the lesion; (4) The negative predictive value remains low, at 60%-70%[69]; and (5) The procedure is poorly reproducible, and the coefficient of variance is greater than 0.3[69].
Interobserver variability is good in the case of experienced EUS elastography operators (k = 0.8) and is fair in the case of unexperienced EUS elastography operators (k = 0.24)[73]. Intraobserver variability was also good (k = 0.86-0.94)[59].
Differentiation between benign and malignant lymph nodes: The main problem in assessing lymph nodes is to determine when to apply EUS-FNA. Using EUS elastography, benign LNs appear homogenous and are colored green, whereas malignant LNs are colored blue[75]. The same color differentiation also proved efficient while applying elastography to endobronchial ultrasound[76]. Parts of LNs that appear blue can be targeted by the needle to prove the presence of micrometastasis.
The cut-off SR value for differentiating between malignant and benign LNs in 55 patients was considered to be 3.81[77]; a value of 7.5 was found in another group of 53 LNs[78]. Interobserver agreement was good to excellent, with a K value for ES of 0.58-0.84 and a value of 0.35 for the ES scoring system[79-81](Table 7).
| Ref. | Type of study | Final diagnosis | No. of patients | E-EUS assessment | Main results |
| Giovannini et al[58] 2006 | Prospective | EUS-FNA | 31 | Color pattern | Sn = 100% |
| Single center | Sp = 50% | ||||
| Janssen et al[75] 2007 | Prospective | EUS-FNA | 66 | Color pattern | Hard - Acc = 81%-86% |
| Single center | Soft - Acc = 84%-86% | ||||
| Săftoiu et al[95] 2007 | Prospective Single center | Surgery | 78 | Hue histogram | Sn = 85% |
| EUS-FNA | Sp = 91% | ||||
| Giovannini et al[79] 2009 | Prospective | Surgery | 101 | Color pattern | Sn = 91.8% |
| Multicenter | EUS-FNA | Sp = 82.5% | |||
| Larsen et al[81] 2012 | Prospective Single center | Surgery | 56 | Color pattern | Sn = 55%-59% |
| Sp = 82%-85% | |||||
| Paterson et al[78] 2012 | Prospective | EUS-FNA | 53 | Strain ratio for malignancy = 7.5 | Sn = 83%, Sp = 96%, PPV = 95%, NPV = 86%, Acc = 90% |
| Single center | |||||
| Knabe et al[83] 2013 | Prospective | EUS-FNA | 40 | Color pattern | Sn = 100% |
| Computed analysis | Sp = 64% | ||||
| Computed analysis | |||||
| Sn = 88.9% | |||||
| Sp = 86.7% |
A meta-analysis, including seven studies and 368 patients, revealed a sensitivity of 88% and a specificity of 85% using elastography EUS for differentiating between malignant and benign LNs[82].
Results concerning the superiority of elastography EUS over EUS are conflicting. The color pattern[79,83] and strain ratio were found to be of superior value to conventional EUS criteria[78]. However, these results were not sustained in another study that included a surgical pathology comparison[77].
Assessment of pancreatic fibrosis: The usefulness of elastography for assessing pancreatic fibrosis distal to a tumor and to differentiate chronic pancreatitis from healthy pancreas was previously proven[84,85]. Using a radial scope, an SR cut-off value of 2.25 yielded a diagnosis accuracy of 91% for chronic pancreatitis[1] and increased the EUS evaluation yield in 18% of cases. Autoimmune pancreatitis exhibits a blue pattern involving both mass-forming autoimmune pancreatitis and surrounding tissue[86].
A direct relationship was found between the SR and the probability of pancreatic exocrine insufficiency, as measured using the 13C-MTG breath test, and a probability of 87% was found in patients with an SR of higher than 4.5[87]. The results were similar for patients with calcifying and non-calcifying chronic pancreatitis[87]. No elastographic studies have compared this finding using radial and linear echoendoscopes.
Other indications: Few cases of gastric submucosal tumors have been assessed using elastography[53,88]. A GIST may have a non-homogenous blue-green structure, and a typical lipoma is mostly soft, green and homogenous; however, differentiation between benign and malignant lesions using elastography EUS remains difficult.
Sessile rectal adenoma and adenocarcinoma were better differentiated using elastography compared to standard rectal endosonography, with an SR cut-off mean of 1.25, resulting in a sensitivity of 96% and a specificity of 86%; these results are superior to the use of EUS alone[89,90]. This could be important for the local resection of rectal tumors, but multiple studies confirming this indication are needed.
The discrimination between T2 and T3 tumors based on identifying “softer” inflammatory tissue and “harder” tumor tissue has not yet been published.
Elastography was not found to be more useful compared to rectal EUS in patients with fecal incontinence, previously irradiated or not[91], where the sphincter appears as an interrupted inhomogenous thickened layer[53], or for the discrimination of Crohn strictures from adenocarcinoma.
Sclerosing primary cholangitis might have a hard or mixed type common bile duct wall compared to controls; this might be related to an extension of the fibrotic change of the wall[92].
Principle: Standard confocal laser endomicroscopy (CLE) allows the real-time visualization of cellular and subcellular structures with up to 1000 times magnification and a penetration of 100 μm below the mucosal surface[96]. The studied tissue is illuminated with a low-power laser, and the fluorescence of light reflected from the tissue is subsequently detected through a pinhole[96]. The returned light is reflected by the same lens and reaches the detector of the confocal system. The illumination and detection systems are “confocal”, meaning that they are aligned in the same focal plane[96]. A contrast agent is administered intravenously (fluorescein) or topically (acriflavine and cresyl violet) to emphasize the cellular, subcellular and vasculature elements.
In clinical practice, two CLE systems are used: an endoscope-integrated CLE and a probe-based CLE (p-CLE), the latter being the widest system used for the assessment of colonic polyps, neoplastic lesions in inflammatory bowel diseases or Barrett’s esophagus[97-100].
Needle-CLE (nCLE) represents an improved version of CLE and is performed during EUS; the organs within or adjacent to the GI tract are assessed using a miniprobe, which is passed through an endoscopic needle. nCLE allows in vivo, real-time histological diagnosis, thus enhancing EUS performance, mainly in the setting of pancreatic and lymph node lesions[101]. Inconclusive diagnostic procedures can be decreased using this technique, termed “optical needle biopsy”[93].
Technique: The system comprises an AQ-Flex 19 miniprobe, which is inserted through a 19-gauge EUS needle while a fluorescence contrast agent (acriflavine, fluorescein) provides tissue architecture imaging, similar to standard histological examination (depth 40-70 mm, field of view 325 microns, lateral resolution 3.5 mm).
Indications: (1) cystic lesions of the pancreas larger than 1 cm; and (2) solid pancreatic masses and lymph node nCLE remain under evaluation.
Pancreatic cystic lesions: The management of cystic lesions is currently suboptimal mainly due to a lack of accuracy in discriminating among different types of pancreatic cystic lesions.
The nCLE patterns for pancreatic cystic lesions were recently published[96,102,103] and provide a global accuracy for diagnosis ranging from 46% to 90%[102-106], and studies have underlined the importance of nCLE acting as an optical needle biopsy[93] (Table 8). Serous cystadenomas presents a superficial vascular network, which can stop their follow-up[103] or avoid an unnecessary resection (reported as 60% in a multicentric study of 2622 patients[105]). Benign IPMNs appear as finger-like papillary projections with an epithelial border and a vascular core, while malignant IPMNs appear as dark clumps with fluorescent substance leakage due to tumor neo-vascularization[103].
| Type of lesion | nCLE features | Diagnostic rate, references |
| SCA | A vascular network of the cystic wall | Sn = 69%, Sp = 100%, PPV = 100%, NPV = 82%[100] |
| MCN | A gray band delineated by a thin dark line | Sn = 80%, Sp = 100%[103] |
| Sn = 67%, Sp = 96%[100] | ||
| IPMN | Papillary projections: characterized by the alternation of vascular cores (white) and epithelial borders | Sn = 59%, Sp = 100%[102] |
| Sn = 80%, Sp = 92%[100] | ||
| Pseudocyst | Inflammatory cells bright, gray and black particles | Sn = 43%, Sp = 100%, Acc = 87%[100] |
| Cystic NET | Dark irregular clusters of compact cells + gray tissue of fibrovascular stroma | Sn = 67%, Sp = 96%, Acc = 90%[100] |
The multicentric INSPECT study[106] showed that the accuracy of differentiating between different types of PCL using nCLE was 41.9%, which is greater than that obtained using a carcinoembryonic antigen (CEA) level > 192 ng/mL (28.6%) or cytology results (29.6%). Epithelial villous structures were found to be predictive of PCL with a specificity of 100%, but the sensitivity and negative predictive value were only 59% and 50%, respectively[106].
In the DETECT trial[107], nCLE was combined with cystoscopy using a SpyGlass fiberoptic probe in 18 patients with a high probability of having PCNs. Cystoscopy and nCLE were reported to have sensitivities of 90% and 80%, respectively; the combination of the two methods reached a sensitivity of 100% for the clinical diagnosis of mucinous cysts. In addition, both cystoscopy and nCLE exhibited higher sensitivity and accuracy than CEA levels (33% and 61%, respectively) in the entire study population[107,108].
Limitations: Inter-observer agreement is considered as globally low[104] and fair for MCN, moderate for IPMN, and very good for PC and SCA[102]; The operator learning curve of the technique influences the results obtained[103]; Sampling error is limited by the location and size of the cyst, the angulation of the needle, and the use of a transgastric or transduodenal approach; Incomplete evaluation - needle entry site, a solid mass inside the cyst; Better in combination with cystoscopy for cyst evaluation[107]; Complications of nCLE are seen in up to 3.29%-9% of cases such as pancreatitis or bleeding[102,106,107].
Solid pancreatic lesions: Few data are available for nCLE assessment in pancreatic solid lesions, and difficulties have been encountered, especially when using the transduodenal approach.
Normal pancreas has been described as having “an appearance of coffee beans corresponding to acinis”[108]. Adenocarcinoma has an aspect of dark cell aggregates and irregular vessels with the leakage of fluorescein[108]. Chronic pancreatitis presents as residual regular glandular pancreatic structures[108] and white fibrous bands[109,110]. Neuroendocrine tumors appear as black cell aggregates surrounded by vessels and fibrotic areas[108].
The sensitivity, specificity and accuracy of this technique were 77%, 100% and 85%, respectively, supporting the use of nCLE instead of repeating EUS-FNA after a previous inconclusive biopsy[108].
Another study used nCLE as optical guidance for EUS-FNA and found an accuracy rate of 90.9% with good inter-observer agreement (k = 0.82)[109].
Lymph nodes: On nCLE, benign lymph nodes have a reticular background with lymphocytes[111]. Clusters of dark pleomorphic tumor cells are consistent with carcinoma features, while enlarged follicles are less convincing for malignancy[112]. Additionally, significant leakage of fluorescent dye due to tumor angiogenesis is suggestive of malignant nodes[111].
Future: Molecular imaging of the pancreas might be feasible using nCLE: pancreatic histology assessment after the use of a fluorescence-labeled anti-EGF-R antibody[113] has been reported. One study recently used nCLE for the visualization of the Meissner and Auerbach plexus after the submucosal injection of NeuroTrace[114], a new step in the assessment of functional and motility disorders of the gastrointestinal tract.
In vitro imaging of pancreatic carcinogenesis using nCLE combined with molecular markers, such as cathepsin E[96], has also been reported, with important future clinical implications for the monitoring of pancreatic ductal carcinoma.
Despite its high accuracy and the existence of several clinical applications, nCLE is still used only in research trials, probably due to a lack of standardization, availability and reimbursement in some countries, a lengthy physician learning curve and the broad range of histologic diagnoses. A recent study showed the benefit of using nCLE in cases of “diagnostic doubts”, impacting diagnosis and management in 40% of cases and the performance of target biopsies in 100% of cases[115].
The new derivative modalities described above represent a step forward in maximizing the results of endoscopic ultrasonography procedures. The complementary role of these techniques is becoming clearer, and elastography and harmonic contrast-enhanced EUS are suitable for routine use in the future. However, none of these techniques is yet able to replace EUS-FNA.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Santoro GA, Slomiany BL, Tarnawski AS S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 251] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 2. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest. 2007;131:539-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Sekine M, Imaoka H, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Tanaka T, Ishihara M, Ito S. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc. 2015;27:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [PubMed] [Cited in This Article: ] |
| 7. | Wang QX, Xiao J, Orange M, Zhang H, Zhu YQ. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: a Meta-Analysis. Cell Physiol Biochem. 2015;36:1197-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590-597.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Kitano M, Kamata K, Imai H, Miyata T, Yasukawa S, Yanagisawa A, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27 Suppl 1:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Alvarez-Sánchez MV, Napoléon B. Contrast-enhanced harmonic endoscopic ultrasound imaging: basic principles, present situation and future perspectives. World J Gastroenterol. 2014;20:15549-15563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Kitano M, Kudo M, Sakamoto H, Komaki T. Endoscopic ultrasonography and contrast-enhanced endoscopic ultrasonography. Pancreatology. 2011;11 Suppl 2:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-634.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Hocke M, Ignee A, Dietrich CF. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma--elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z Gastroenterol. 2012;50:199-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Gincul R, Palazzo M, Pujol B, Tubach F, Palazzo L, Lefort C, Fumex F, Lombard A, Ribeiro D, Fabre M. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: a prospective multicenter trial. Endoscopy. 2014;46:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Lee TY, Cheon YK, Shim CS. Clinical role of contrast-enhanced harmonic endoscopic ultrasound in differentiating solid lesions of the pancreas: a single-center experience in Korea. Gut Liver. 2013;7:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Park JS, Kim HK, Bang BW, Kim SG, Jeong S, Lee DH. Effectiveness of contrast-enhanced harmonic endoscopic ultrasound for the evaluation of solid pancreatic masses. World J Gastroenterol. 2014;20:518-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Ang TL, Teo EK, Ang D, Kwek AB, Fock KM. A pilot study of contrast harmonic endosonography using DEFINITY™ in the evaluation of suspected pancreatic and peri-ampullary malignancies. J Interv Gastroenterol. 2011;1:160-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Dietrich CF, Sahai AV, D’Onofrio M, Will U, Arcidiacono PG, Petrone MC, Hocke M, Braden B, Burmester E, Möller K. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | D’Onofrio M, Biagioli E, Gerardi C, Canestrini S, Rulli E, Crosara S, De Robertis R, Floriani I. Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: a systematic review and meta-analysis. Ultraschall Med. 2014;35:515-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Nakamura M, Miyahara R, Hayashi K. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Gheonea DI, Streba CT, Ciurea T, Săftoiu A. Quantitative low mechanical index contrast-enhanced endoscopic ultrasound for the differential diagnosis of chronic pseudotumoral pancreatitis and pancreatic cancer. BMC Gastroenterol. 2013;13:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Imazu H, Kanazawa K, Mori N, Ikeda K, Kakutani H, Sumiyama K, Hino S, Ang TL, Omar S, Tajiri H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand J Gastroenterol. 2012;47:853-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Fusaroli P, D’Ercole MC, De Giorgio R, Serrani M, Caletti G. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video). Pancreas. 2014;43:584-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Seicean A, Badea R, Moldovan-Pop A, Vultur S, Botan EC, Zaharie T, Săftoiu A, Mocan T, Iancu C, Graur F. Harmonic Contrast-Enhanced Endoscopic Ultrasonography for the Guidance of Fine-Needle Aspiration in Solid Pancreatic Masses. Ultraschall Med. 2015; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Fusaroli P, Kypraios D, Mancino MG, Spada A, Benini MC, Bianchi M, Bocus P, De Angelis C, De Luca L, Fabbri C. Interobserver agreement in contrast harmonic endoscopic ultrasound. J Gastroenterol Hepatol. 2012;27:1063-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Soares JB, Iglesias-Garcia J, Gonçalves B, Lindkvist B, Lariño-Noia J, Bastos P, Caetano AC, Ferreira A, Pimentel-Nunes P, Lopes L. Interobserver agreement of contrast-enhanced harmonic endoscopic ultrasonography in the evaluation of solid pancreatic lesions. Endosc Int Open. 2015;3:E205-E209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Seicean A, Badea R, Stan-Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Imazu H, Uchiyama Y, Matsunaga K, Ikeda K, Kakutani H, Sasaki Y, Sumiyama K, Ang TL, Omar S, Tajiri H. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010;45:732-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Săftoiu A, Vilmann P, Dietrich CF, Iglesias-Garcia J, Hocke M, Seicean A, Ignee A, Hassan H, Streba CT, Ioncică AM. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2015;82:59-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 34. | Ueda K, Yamashita Y, Itonaga M. Real-time contrast-enhanced endoscopic ultrasonography-guided fine-needle aspiration (with video). Dig Endosc. 2013;25:631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, Kikuchi H, Konno N, Waragai Y, Watanabe H. Conventional versus contrast-enhanced harmonic endoscopic ultrasonography-guided fine-needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology. 2015;15:538-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Hou X, Jin Z, Xu C, Zhang M, Zhu J, Jiang F, Li Z. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic lesions: a retrospective study. PLoS One. 2015;10:e0121236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Hocke M, Cui XW, Domagk D, Ignee A, Dietrich CF. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound. 2014;3:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Fusaroli P, Serrani M, De Giorgio R, D’Ercole MC, Ceroni L, Lisotti A, Caletti G. Contrast Harmonic-Endoscopic Ultrasound Is Useful to Identify Neoplastic Features of Pancreatic Cysts (With Videos). Pancreas. 2016;45:265-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Clain JE, Deutsch JC, DeWitt J, Eloubeidi MA, Gleeson FC. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192-198, 198.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61-68. [PubMed] [Cited in This Article: ] |
| 41. | Harima H, Kaino S, Shinoda S, Kawano M, Suenaga S, Sakaida I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2015;21:6252-6260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 55] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 42. | Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Yamao K, Imai H, Sakamoto H, Harwani Y, Chikugo T. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Yamamoto N, Kato H, Tomoda T, Matsumoto K, Sakakihara I, Noma Y, Horiguchi S, Harada R, Tsutsumi K, Hori K. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy. 2016;48:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Kannengiesser K, Mahlke R, Petersen F, Peters A, Ross M, Kucharzik T, Maaser C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, Dote K, Kudo M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2011;73:227-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Park CH, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Kim H, Hwang HK, Lee WJ, Song SY. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Imazu H, Mori N, Kanazawa K, Chiba M, Toyoizumi H, Torisu Y, Koyama S, Hino S, Ang TL, Tajiri H. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2014;59:1909-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Gill KR, Ghabril MS, Jamil LH, Hasan MK, McNeil RB, Woodward TA, Raimondo M, Hoffman BJ, Hawes RH, Romagnuolo J. Endosonographic features predictive of malignancy in mediastinal lymph nodes in patients with lung cancer. Gastrointest Endosc. 2010;72:265-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Miyata T, Kitano M, Omoto S, Kadosaka K, Kamata K, Imai H, Sakamoto H, Nisida N, Harwani Y, Murakami T. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J Gastroenterol. 2016;22:3381-3391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Xia Y, Kitano M, Kudo M, Imai H, Kamata K, Sakamoto H, Komaki T. Characterization of intra-abdominal lesions of undetermined origin by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2010;72:637-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Matsui S, Kudo M, Kitano M, Asakuma Y. Evaluation of the Response to Chemotherapy in Advanced Gastric Cancer by Contrast-Enhanced Harmonic EUS. Hepatogastroenterology. 2015;62:595-598. [PubMed] [Cited in This Article: ] |
| 52. | Iglesias-Garcia J, Domínguez-Muñoz JE, Castiñeira-Alvariño M, Luaces-Regueira M, Lariño-Noia J. Quantitative elastography associated with endoscopic ultrasound for the diagnosis of chronic pancreatitis. Endoscopy. 2013;45:781-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Dietrich CF, Săftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Havre RF, Ødegaard S, Gilja OH, Nesje LB. Characterization of solid focal pancreatic lesions using endoscopic ultrasonography with real-time elastography. Scand J Gastroenterol. 2014;49:742-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, Hirche H, Dietrich CF. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Hu DM, Gong TT, Zhu Q. Endoscopic ultrasound elastography for differential diagnosis of pancreatic masses: a meta-analysis. Dig Dis Sci. 2013;58:1125-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 58. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 59. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 60. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 61. | Săftoiu A, Iordache SA, Gheonea DI, Popescu C, Maloş A, Gorunescu F, Ciurea T, Iordache A, Popescu GL, Manea CT. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2010;72:739-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol. 2012;10:84-90.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 63. | Pei Q, Zou X, Zhang X, Chen M, Guo Y, Luo H. Diagnostic value of EUS elastography in differentiation of benign and malignant solid pancreatic masses: a meta-analysis. Pancreatology. 2012;12:402-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 64. | Mei M, Ni J, Liu D, Jin P, Sun L. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc. 2013;77:578-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Xu W, Shi J, Li X, Zeng X, Lin Y. Endoscopic ultrasound elastography for differentiation of benign and malignant pancreatic masses: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Li X, Xu W, Shi J, Lin Y, Zeng X. Endoscopic ultrasound elastography for differentiating between pancreatic adenocarcinoma and inflammatory masses: a meta-analysis. World J Gastroenterol. 2013;19:6284-6291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 47] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Ying L, Lin X, Xie ZL, Hu YP, Tang KF, Shi KQ. Clinical utility of endoscopic ultrasound elastography for identification of malignant pancreatic masses: a meta-analysis. J Gastroenterol Hepatol. 2013;28:1434-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Mayerle J, Beyer G, Simon P, Dickson EJ, Carter RC, Duthie F, Lerch MM, McKay CJ. Prospective cohort study comparing transient EUS guided elastography to EUS-FNA for the diagnosis of solid pancreatic mass lesions. Pancreatology. 2016;16:110-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Kongkam P, Lakananurak N, Navicharern P, Chantarojanasiri T, Aye K, Ridtitid W, Kritisin K, Angsuwatcharakon P, Aniwan S, Pittayanon R. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single-blinded study. J Gastroenterol Hepatol. 2015;30:1683-1689. [Cited in This Article: ] |
| 70. | Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76:953-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 71. | Figueiredo FA, da Silva PM, Monges G, Bories E, Pesenti C, Caillol F, Delpero JR, Giovannini M. Yield of Contrast-Enhanced Power Doppler Endoscopic Ultrasonography and Strain Ratio Obtained by EUS-Elastography in the Diagnosis of Focal Pancreatic Solid Lesions. Endosc Ultrasound. 2012;1:143-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 73. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 74. | Lee TH, Cho YD, Cha SW, Cho JY, Jang JY, Jeong SW, Choi HJ, Moon JH. Endoscopic ultrasound elastography for the pancreas in Korea: a preliminary single center study. Clin Endosc. 2013;46:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 76. | Izumo T, Sasada S, Chavez C, Matsumoto Y, Tsuchida T. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol. 2014;44:956-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 77. | Larsen MH, Fristrup CW, Mortensen MB. Intra- and interobserver agreement of endoscopic sonoelastography in the evaluation of lymph nodes. Ultraschall Med. 2011;32 Suppl 2:E45-E50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol. 2012;18:889-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [PubMed] [Cited in This Article: ] |
| 80. | Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Larsen MH, Fristrup C, Hansen TP, Hovendal CP, Mortensen MB. Endoscopic ultrasound, endoscopic sonoelastography, and strain ratio evaluation of lymph nodes with histology as gold standard. Endoscopy. 2012;44:759-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Xu W, Shi J, Zeng X, Li X, Xie WF, Guo J, Lin Y. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001-1009; quiz 1115.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Knabe M, Günter E, Ell C, Pech O. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc. 2013;27:1196-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Itoh Y, Itoh A, Kawashima H, Ohno E, Nakamura Y, Hiramatsu T, Sugimoto H, Sumi H, Hayashi D, Kuwahara T. Quantitative analysis of diagnosing pancreatic fibrosis using EUS-elastography (comparison with surgical specimens). J Gastroenterol. 2014;49:1183-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Janssen J, Papavassiliou I. Effect of aging and diffuse chronic pancreatitis on pancreas elasticity evaluated using semiquantitative EUS elastography. Ultraschall Med. 2014;35:253-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Dominguez-Muñoz JE, Iglesias-Garcia J, Castiñeira Alvariño M, Luaces Regueira M, Lariño-Noia J. EUS elastography to predict pancreatic exocrine insufficiency in patients with chronic pancreatitis. Gastrointest Endosc. 2015;81:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Jenssen C, Dietrich CF. Endoscopic ultrasound of gastrointestinal subepithelial lesions. Ultraschall Med. 2008;29:236-256; quiz 257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Waage JE, Leh S, Røsler C, Pfeffer F, Bach SP, Havre RF, Haldorsen IS, Ødegaard S, Baatrup G. Endorectal ultrasonography, strain elastography and MRI differentiation of rectal adenomas and adenocarcinomas. Colorectal Dis. 2015;17:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Azuma M, Kusano C, Gotoda T. Diagnostic potential of endoscopic ultrasonography-elastography for gastric submucosal tumors. Dig Endosc. 2015;27 Suppl 1:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 91. | Allgayer H, Ignee A, Zipse S, Crispin A, Dietrich CF. Endorectal ultrasound and real-time elastography in patients with fecal incontinence following anorectal surgery: a prospective comparison evaluating short- and long-term outcomes in irradiated and non-irradiated patients. Z Gastroenterol. 2012;50:1281-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Rustemovic N, Cukovic-Cavka S, Opacic M, Petrovecki M, Hrstic I, Radic D, Ostojic R, Pulanic R, Vucelic B. Endoscopic ultrasound elastography as a method for screening the patients with suspected primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2010;22:748-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 93. | Rustemovic N, Opacic D, Ostojic Z, Opacic M, Ledinsky I, Višijić A, Ravić KG, Iveković H, Markoš P. Comparison of elastography methods in patients with pancreatic masses. Endosc Ultrasound. 2014;3:S4. [PubMed] [Cited in This Article: ] |
| 94. | Opačić D, Rustemović N, Kalauz M, Markoš P, Ostojić Z, Majerović M, Ledinsky I, Višnjić A, Krznarić J, Opačić M. Endoscopic ultrasound elastography strain histograms in the evaluation of patients with pancreatic masses. World J Gastroenterol. 2015;21:4014-4019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Săftoiu A, Vilmann P, Ciurea T, Popescu GL, Iordache A, Hassan H, Gorunescu F, Iordache S. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 96. | Chapman CG, Siddiqui UD. New Scopes, New Accessories, New Stents for Interventional Endoscopic Ultrasound. Clin Endosc. 2016;49:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Kuiper T, van den Broek FJ, van Eeden S, Fockens P, Dekker E. Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol. 2012;107:543-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Neumann H, Vieth M, Atreya R, Grauer M, Siebler J, Bernatik T, Neurath MF, Mudter J. Assessment of Crohn’s disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis. 2012;18:2261-2269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | van den Broek FJ, van Es JA, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Fockens P, Dekker E. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy. 2011;43:116-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 101. | Bhutani MS, Koduru P, Joshi V, Karstensen JG, Saftoiu A, Vilmann P, Giovannini M. EUS-Guided Needle-Based Confocal Laser Endomicroscopy: A Novel Technique With Emerging Applications. Gastroenterol Hepatol (N Y). 2015;11:235-240. [PubMed] [Cited in This Article: ] |
| 102. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 103. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 104. | Karia K, Waxman I, Konda VJ, Gress FG, Sethi A, Siddiqui UD, Sharaiha RZ, Kedia P, Jamal-Kabani A, Gaidhane M. Needle-based confocal endomicroscopy for pancreatic cysts: the current agreement in interpretation. Gastrointest Endosc. 2016;83:924-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 106. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 107. | Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 108. | Giovannini M, Caillol F, Monges G, Poizat F, Lemaistre AI, Pujol B, Lucidarme D, Palazzo L, Napoléon B. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy in solid pancreatic masses. Endoscopy. 2016;48:892-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 109. | Kongkam P, Pittayanon R, Sampatanukul P, Angsuwatcharakon P, Aniwan S, Prueksapanich P, Sriuranpong V, Navicharern P, Treeprasertsuk S, Kullavanijaya P. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions (ENES): a pilot study. Endosc Int Open. 2016;4:E17-E23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Karstensen J, Cartana T, Pia K, Saftoiu A, Vilmann P. Endoscopic ultrasound-guided needle confocal laser endomicroscopy in pancreatic masses. Endosc Ultrasound. 2014;3:S2-S3. [PubMed] [Cited in This Article: ] |
| 111. | Giovannini M, Caillol F, Lemaistre A, Monges G, Napoleon B, Pujol B. Endoscopic ultrasound guided confocal microscopy: Atlas of cystic pancreatic lesions. Endosc Ultrasound. 2014;3:S19-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Benias PC, D’Souza LS, Papafragkakis H, Kim J, Harshan M, Theise ND, Carr-Locke DL. Needle-based confocal endomicroscopy for evaluation of malignant lymph nodes - a feasibility study. Endoscopy. 2016;48:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Nakai Y, Shinoura S, Ahluwalia A, Tarnawski AS, Chang KJ. In vivo visualization of epidermal growth factor receptor and survivin expression in porcine pancreas using endoscopic ultrasound guided fine needle imaging with confocal laser-induced endomicroscopy. J Physiol Pharmacol. 2012;63:577-580. [PubMed] [Cited in This Article: ] |
| 114. | Samarasena JB, Ahluwalia A, Shinoura S, Choi KD, Lee JG, Chang KJ, Tarnawski AS. In vivo imaging of porcine gastric enteric nervous system using confocal laser endomicroscopy & amp; molecular neuronal probe. J Gastroenterol Hepatol. 2016;31:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (2)] |
| 115. | Robles-Medranda C. Confocal endomicroscopy: Is it time to move on? World J Gastrointest Endosc. 2016;8:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |