Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4397
Peer-review started: January 30, 2016
First decision: February 18, 2016
Revised: March 3, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: May 7, 2016
AIM: To evaluate the association between serum concentrations of S100β in patients with cirrhosis and the presence of low grade hepatic encephalopathy (HE).
METHODS: This was a cross-sectional study. The population was categorized into four groups healthy subjects, cirrhosis without HE, cirrhosis with covert hepatic encephalopathy (CHE) and cirrhosis with overt HE. Kruskal-Wallis, Mann Whitney’s U with Bonferroni adjustment Spearman correlations and area under the ROC were used as appropriate.
RESULTS: A total of 61 subjects were included, 46 cirrhotic patients and 15 healthy volunteers. S100β values were different among all groups, and differences remained significant between groups 1 and 2 (P < 0.001), and also between groups 2 and 3 (P = 0.016), but not between groups 3 and 4. In cirrhotic patients with HE S100β was higher than in patients without HE [0.18 (0.14-0.28) ng/mL vs 0.11 (0.06-0.14) ng/mL, P < 0.001]. There was a close correlation between serum concentrations of S100β and psychometric hepatic encephalopathy score in patients with cirrhosis without HE compared to the patients with cirrhosis with CHE (r = -0.413, P = 0.019). ROC curve analysis yielded > 0.13 ng/mL as the best cutoff value of S100β for the diagnosis of HE (sensitivity 83.3%, specificity 63.6%).
CONCLUSION: Serum concentrations of S100β are higher in patients with cirrhosis than in healthy volunteers, and are further increased in the presence of hepatic encephalopathy. The results suggest that serum biomarkers such as S100β could help in the correct characterization of incipient stages of HE.
Core tip: Hepatic encephalopathy is a complication present in 30%-80% of the patients with cirrhosis and it is associated with increased mortality, adverse clinical outcomes, and poor quality of life. An increased concern about early recognition of this complication has risen in recent years; however, no biochemical marker is available to date. In this paper we evaluated the performance of S100β as a biochemical marker to identify the early stages of HE, and its correlation with neuropsychometric tests.
- Citation: Duarte-Rojo A, Ruiz-Margáin A, Macias-Rodriguez RU, Cubero FJ, Estradas-Trujillo J, Muñoz-Fuentes RM, Torre A. Clinical scenarios for the use of S100β as a marker of hepatic encephalopathy. World J Gastroenterol 2016; 22(17): 4397-4402
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4397
Hepatic encephalopathy (HE) reflects a spectrum of neuropsychiatric abnormalities seen in patients with liver dysfunction after exclusion of other brain diseases[1,2]. Covert hepatic encephalopathy (CHE) is the initial stage in the clinical spectrum of HE and involves subtle changes in cognitive and motor function, and electroencephalographic patterns. In contrast to patients with symptomatic HE, patients with CHE have no recognizable clinical symptoms of brain dysfunction and need to be diagnosed on the basis of neuropsychologic tests[3]. The prevalence of CHE in cirrhotics with good liver function (Child A) is 15%, while in those with advanced cirrhosis (Child B/C) it approaches 50%[4-6]. Multiple pathways are involved in the pathophysiology of HE, however, hyperammonemia plays a central role. Astroglial swelling in HE occurs if the load of glutamine accumulating during ammonia detoxification is not compensated by a decrease in other osmolytes like myo-inositol[7]. This is followed by development of low-grade cerebral edema, which is accompanied by an increased production of reactive oxygen and nitrogen species, triggering multiple protein and RNA modifications, thereby affecting brain function[8]. Associated abnormalities in the homeostasis of cerebral neurotransmitters and blood-brain barrier (BBB) disruption have not been well characterized yet. Different molecules have been addressed as markers of brain injury, some of them being linked to astrocyte damage, but to date there is no useful serologic marker that can aid the diagnosis of HE or CHE.
S100β is a 10.4 kDa protein that is primarily synthesized in the brain by the endfeet processes of the astrocytes, and it belongs to a superfamily of low molecular weight acidic calcium binding proteins of the EF-hand type[9]. This protein is primarily metabolized in the kidney and excreted in the urine, its expression across ethnic groups seems to be consistent, differences between genders are not found, and it does not seem to show circadian variation[10]. Although S100β is not entirely specific for the central nervous system (CNS), it is present in the brain at much higher concentrations than in other tissues (80%-90% of the total pool is found within the brain), and as such, this protein can be used as an early marker of brain damage[9].
Astrocytes are key in homeostasis regulation in the CNS, being activated after brain injury and releasing S100β[11]. Some studies have shown increased levels of S100β in patients with acute or chronic liver failure and HE which might reflect an early stage of intracerebral changes before the development of marked cerebral edema[12,13]. Moreover it has been proposed that increased serum concentrations of S100β could predict CHE[14]. However, evidence associating S100β levels and the presence of HE is scarce. The aim of this study was to evaluate the association between serum concentrations of S100β and the presence of low-grade HE in patients with cirrhosis.
This was a cross-sectional analytic study. Healthy volunteers and ambulatory patients with cirrhosis were invited to participate. Cirrhosis was confirmed on the basis of liver biopsy, or presence of biochemical, ultrasonographic, and/or endoscopic features of portal hypertension and liver dysfunction. High-grade HE, use of psychoactive drugs, alcohol during the 3 mo prior to the start of the study, presence of renal failure, respiratory or central nervous system (CNS) disease, cardiac failure, or severe malnutrition were all considered exclusion criteria. History and physical examination, as well as psychometric hepatic encephalopathy score (PHES) test, and critical flicker frequency (CFF) analysis (Hepatonorm Analyzer; R&R Medi-Business Freiburg GMBH, Freiburg, Germany) were performed in all patients. Healthy volunteers did not show any neurologic abnormality on exam.
Overt HE was diagnosed on clinical grounds and classified according to West Haven criteria (all grade I or II). Covert HE (CHE)was identified in patients without overt manifestations of HE, when a PHES score was more than 4 SD below the Mexican population norms[15]. Using above criteria, 4 groups were formed: (1) healthy subjects; (2) cirrhosis without HE; (3) cirrhosis with CHE; and (4) cirrhosis with overt HE. Blood samples were drawn after an 8-h fasting period, and plasma collected and stored at -70 °C for further determination. S100β was measured using a human S100β enzyme-linked immunoassay (ELISA) according to manufacturer’s instructions (BioVendor, Candler, United States). All tests were run in duplicate.
All results HE are expressed in medians and interquartile range, except for PHES, for which we used maximum and minimum values. The Kruskal-Wallis test was used to compare differences between groups for each of the variables of interest, while the U of Mann-Whitney with Bonferroni adjustment (α/κ = 0.016) was used to evaluate differences between pairs of groups. Spearman test was used for bivariate correlations. ROC curve analysis was used to obtain a cutoff value for S100β. A P value < 0.05 was considered significant. SPSS version 21.0 was used for statistical analysis (IBM, Armonk, NY).
A total of 61 subjects were included, 46 cirrhotic patients and 15 healthy volunteers. The main characteristics of the population are shown in Table 1. Median age was higher in cirrhotics compared to the healthy subjects, most patients were males. The main etiology of cirrhosis was HCV and there was a stepwise increase in Child-Pugh and MELD score according to the presence and severity of HE.
| Healthy subjects | C + non-HE | C + CHE | C + HE | |
| n | 15 | 22 | 10 | 14 |
| Gender (M/F) | (8/7) | (11/11) | (6/4) | (9/5) |
| Age | 51.5 (46-64.5) | 53 (45-60) | 65.5 (59-71.5) | 66 (57-71) |
| Years of education | 14 (6-19) | 12 (6-16) | 6 (4-9.7) | 9 (6-16) |
| Main etiology (%) | -- | HCV (34%) | HCV (40%) | HCV (66%) |
| MELD score | -- | 9.5 (8.5-11.5) | 10 (7.7-15.2) | 11.5 (10-17.5) |
| Child-Pugh (points) | -- | 7 (6-8) | 7.5 (6-9) | 10 (8-12) |
| Serum sodium | -- | 136 (129-143) | 135 (126-139) | 138 (122-141) |
| Ammonia | -- | 179 (113.5-224) | 217 (194-250) | 134 (95-277) |
| Total bilirubin | -- | 1.81 (1.11-2.48) | 1.50 (1.26-2.58) | 2.81 (2.09-5.94) |
| ALT | -- | 34 (28-55.2) | 31 (24.5-95.5) | 42.5 (32.2-65.5) |
| AST | -- | 62.5 (41-98) | 49 (37.5-115) | 77 (44-105.5) |
| Albumin | -- | 2.9 (2.6-3.3) | 2.9 (2.6-3.2) | 2.2 (2.0-2.7) |
| Alkaline phosphatase | -- | 141.5 (89.7-211) | 140 (85-184) | 154 (100.5-80.5) |
| INR | -- | 1.1 (1.1-1.2) | 1 (0.9-1.2) | 1.2 (1.1-1.7) |
| Creatinine | -- | 0.82 ± 0.19 | 1.11 ± 0.38 | 0.92 ± 0.31 |
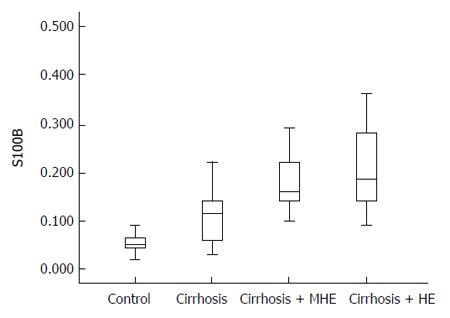
The distribution of S100β serum values according from each group is shown in Figure 1. S100β values were different among all groups, and differences remained significant between groups 1 and 2 (P < 0.001), and also between groups 2 and 3 (P = 0.016), but not between groups 3 and 4. In cirrhotic patients with HE S100β was higher than in patients without HE [0.18 (0.14-0.28) ng/mL vs 0.11 (0.06-0.14) ng/mL, P < 0.001].
The results of the HE psychometric evaluation can be observed in Table 2. There was a clear deterioration in the performance of the CFF and PHES tests in the group with cirrhosis and cirrhosis with CHE or HE, as well as worsening in the individual PHES tests with a statistically significant difference.
| Healthy subjects | C + non-HE | C + CHE | C + HE | P value | |
| CFF | 45.0 (43.0-48.6) | 39.9 (39.2-41.4) | 36 (33-39) | 36 (35-40) | 0.000 |
| PHES | 0 (-2 to 1) | -2 (-3 to -1) | -9 (-13 to -5) | -9.5 (-12 to –3) | 0.000 |
| Digit symbol | 33.8 ± 18.3 | 28.91 ± 9.06 | 18.9 ± 8.8 | 13.4 ± 7.8 | 0.000 |
| Number connection A | 43.6 ± 21.1 | 49.5 ± 19.4 | 102.8 ± 66.5 | 148.8 ± 85.7 | 0.000 |
| Number connection B | 112.9 ± 39.1 | 147.2 ± 82.8 | 318.5 ± 153.03 | 435.7 ± 209.9 | 0.000 |
| Serial dotting | 62.3 ± 22.6 | 77.43 ± 18.1 | 127 ± 46.9 | 147.6 ± 64.3 | 0.000 |
| Line tracing | 95.2 ± 27.45 | 95.6 ± 28.6 | 193.8 ± 93.5 | 167.5 ± 37.8 | 0.000 |
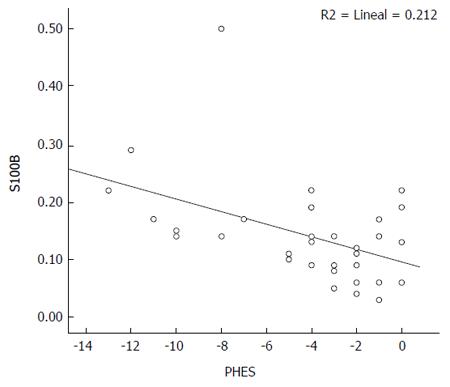
In the total population, S100β was found to have a moderate correlation both with PHES (rho = -0.624, P < 0.001) and CFF (rho = -0.516, P < 0.001). In the subgroup of patients with cirrhosis, S100β showed moderate correlations with PHES (rho = -0.528, P < 0.001), as well as with CTP score (rho = -0.515, P < 0.001), and MELD score (rho = -0.440, P < 0.001), but not with CFF (rho = 0.240, P = 0.108) (Figure 2).
ROC curve analysis yielded > 0.13 ng/mL as the best cutoff value of S100β for the diagnosis of CHE and HE, with sensitivity of 83.3% and specificity of 63.6%. Area under the ROC curve (AUROC) was 0.801 (P < 0.0001).
HE and CHE are associated with decreased quality of life, impaired working performance, and patients become unfit to safely drive motor vehicles[16-21]. Cirrhotic patients with CHE are prone to develop episodes of overt HE when compared to those without CHE (incidence of 56% for those with CHE vs 8% for those without CHE at 3 years of follow up)[22]. Moreover, CHE is associated with a decreased survival, and thus it might be considered within the spectrum of complications definying advanced liver failure[23]. It is not known whether treatment of CHE may prevent overt HE or improve survival, but it is tempting to hypothesize that early identification of CHE followed by proper treatment should translate into improved quality of life, and have a favorable impact in prognosis. Psychometric tests are the preferred tool to diagnose CHE, and, since the final PHES score is adjusted to age and years of education, the differences of these two variables in our population did not have an impact in the results of our study.
S100β is not entirely specific for the CNS, but it is present in the brain at much higher concentrations than other tissues (80%-90% of the total pool is found within the brain), and as such, this protein can be used as a marker of brain damage[9]. Other places where S100β synthesis has been identified are adipose tissue[24], skin and melanoma tumors[25,26] and T-lymphocytes[27]. S100β protein is secreted by activated astrocytes and the mechanism of secretion remains unknown but astrocyte expression has been reported to be stimulated by interleukin-1 (IL-1) and by cyclic-AMP. The effects of secreted glial S100β depends on its concentration, being neurotrophic at low levels (nanomolar) and neurotoxic at high levels (micromolar)[9]. Nanomolar levels of S100β exert a stimulatory effect on astrocytes, causing glial proliferation in vitro[28]. S100β also accumulates in the extracellular space after astrocyte death or after cellular disintegration of the damaged parenchyma. Under these conditions, the S100β concentration may well be in the micromolar range and the protein may become toxic. This has originated the hypothesis that extracellular accumulation of S100β in ongoing brain insults can, in combination with other unknown factors, cause a shift in its neuronal attributes from protective to hazardous[9].
We found significant differences in the serum concentrations of S100β between healthy volunteers and patients with cirrhosis. Moreover, in patients with cirrhosis, the levels of S100β increase in the presence of CHE and overt HE. The correlation between S100β and PHES indicates that at lower PHES scores, indicating a higher degree of HE, there is an increased concentration of S100β in serum. This suggests increased permeability of the BBB as the cognitive deterioration of HE progresses, what would determine not only leaking of S100β into the blood circulation, but increased ammonia delivery to the brain, leading to astrocyte activation and swelling. All of these changes (astrocyte activation and swelling, increased BBB permeability) seem to be present in patients with cirrhosis, and, moreover, seem to be more notorious in the presence of CHE and HE. However, our results would suggest that even in the absence of overt HE and CHE, the cirrhotic patient is at a state of astrocyte activation and swelling, and has increased BBB permeability. Brain adaptation to ammonia insults during early stages of cirrhosis and portal hypertension might consitute the counteracting condition preventing CHE or overt HE[29-31]. Our understanding of the cellular mechanisms leading to astrocyte dysfunction and neurotoxicity is limited, and it is unclear whether BBB disruption is crucial in the etiopathogenesis of HE, or an inevitable consequence of the disease itself. More studies are needed to better understand the role of S100β in the origin and perpetuation of HE in cirrhotic patients.
When we compared the cirrhotic patients without HE and CHE we found that S100β could differentiate both groups of patients with a fair accuracy, as shown by an AUROC curve of 0.801. Moreover, S100β correlated with the neuropsychometric tests (PHES and CFF), and with the hepatic functional status (MELD and CTP), attesting for its possible role in the evaluation of HE. Our results suggest that S100β can be useful in the diagnosis of HE, precisely in clinical scenarios where the diagnosis of HE remains unclear because behavior changes being subtle, evaluation of highly-educated patients, or when other psychiatric abnormalities (i.e., depression) coexist or cannot be ruled out.
In conclusion, serum concentrations of S100β are higher in patients with cirrhosis when compared to healthy volunteers, and are further increased in the presence of CHE and overt HE. The results suggest the presence of astrocyte dysfunction, and maybe BBB disruption too, in patients with cirrhosis that seems to be more severe and progressive in the presence of HE. S100β showed significant differences between patients with cirrhosis but no encephalopathy at all, and the subgroup presenting with CHE, suggesting its usefulness to identify CHE. Although the AUROC curve was modest, S100β would potentially be useful in the diagnosis of low-grade HE whenever neuropsychometric tests are suboptimal, or when competing psychiatric differential diagnoses are in place.
The authors would like to thank Dr. Mick Frissen for IT support and Dr. Norberto Chavez Tapia for his assistance in the initial conceptualization of this project.
Hepatic encephalopathy (HE) is a frequent complication of cirrhosis, present in 30%-80% of patients. Covert HE has a profound impact in the quality of life of these patients, especially in daily functioning and driving ability, and it affects the sense of well-being. Thus, covert HE is very relevant to patients who suffer from it.
The diagnosis and characterization of HE, especially in its low-grade forms, is difficult and requires specialized tests. Serum levels of ammonia were initially considered as a suitable marker of HE; however, they do not correlate with the degree of HE. Therefore, different serum markers for the diagnosis of HE, and particularly covert HE, are urgently needed.
Little attention has been paid to the use of biochemical markers as a means to detect early stages of HE. The data presented in the current study, indicate that S100β - a molecule released after astrocyte damage - might be a potential marker for covert HE in patients with cirrhosis. Interestingly, the association between high levels of S100β and presence of HE, as well as with the psychometric hepatic encephalopathy score, points towards a novel finding in the field.
The determination of S100β levels in serum, together with the standard clinical and psychometric evaluations, might help clinicians to establish the diagnosis of covert HE and therefore a timely treatment for these patients.
S100β is a protein that is primarily synthesized in the brain by the processes of the astrocytes. Although S100β is not entirely specific to the central nervous system, it is present in the brain at much higher concentrations than in other tissues (80%-90% of the total pool is found within the brain), and as such, this protein could be used as an early marker of damage.
This manuscript mainly describes the association between serum concentrations of S100β and the presence of low-grade HE in patients with liver cirrhosis. The results suggest that S100β might help in the correct characterization of early stages of HE. The content is interesting and meaningful.
P- Reviewer: Jia L, Kumar R, Montagnese S S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1468] [Cited by in F6Publishing: 1364] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 2. | Torre A. Update in the physiopathology and diagnosis of hepatic encephalopathy. Rev Invest Clin. 2008;60:321-331. [PubMed] [Cited in This Article: ] |
| 3. | Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748-753. [PubMed] [Cited in This Article: ] |
| 5. | Quero JC, Hartmann IJ, Meulstee J, Hop WC, Schalm SW. The diagnosis of subclinical hepatic encephalopathy in patients with cirrhosis using neuropsychological tests and automated electroencephalogram analysis. Hepatology. 1996;24:556-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Rikkers L, Jenko P, Rudman D, Freides D. Subclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology. 1978;75:462-469. [PubMed] [Cited in This Article: ] |
| 7. | Cordoba J, Gottstein J, Blei AT. Glutamine, myo-inositol, and organic brain osmolytes after portocaval anastomosis in the rat: implications for ammonia-induced brain edema. Hepatology. 1996;24:919-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007;85:1373-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Ikeda Y, Umemura K. Analysis of reference values of serum S100B concentrations of Japanese adults. Rinsho Byori. 2005;53:395-399. [PubMed] [Cited in This Article: ] |
| 11. | Petzold A, Keir G, Lim D, Smith M, Thompson EJ. Cerebrospinal fluid (CSF) and serum S100B: release and wash-out pattern. Brain Res Bull. 2003;61:281-285. [PubMed] [Cited in This Article: ] |
| 12. | Strauss GI, Christiansen M, Møller K, Clemmesen JO, Larsen FS, Knudsen GM. S-100b and neuron-specific enolase in patients with fulminant hepatic failure. Liver Transpl. 2001;7:964-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Isobe-Harima Y, Terai S, Segawa M, Uchida K, Yamasaki T, Sakaida I. Serum S100b (astrocyte-specific protein) is a useful marker of hepatic encephalopathy in patients with fulminant hepatitis. Liver Int. 2008;28:146-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Wiltfang J, Nolte W, Otto M, Wildberg J, Bahn E, Figulla HR, Pralle L, Hartmann H, Rüther E, Ramadori G. Elevated serum levels of astroglial S100beta in patients with liver cirrhosis indicate early and subclinical portal-systemic encephalopathy. Metab Brain Dis. 1999;14:239-251. [PubMed] [Cited in This Article: ] |
| 15. | Duarte-Rojo A, Estradas J, Hernández-Ramos R, Ponce-de-León S, Córdoba J, Torre A. Validation of the psychometric hepatic encephalopathy score (PHES) for identifying patients with minimal hepatic encephalopathy. Dig Dis Sci. 2011;56:3014-3023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Curran S, Wattis J. Critical flicker fusion threshold: a potentially useful measure for the early detection of Alzheimer’s disease. Hum Psychopharmacol. 2000;15:103-112. [PubMed] [Cited in This Article: ] |
| 17. | Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 252] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, de Madaria E, Montoliu C, Nuñez D, Flavia M. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, Schalm SW. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 348] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37-41. [PubMed] [Cited in This Article: ] |
| 22. | Hartmann IJ, Groeneweg M, Quero JC, Beijeman SJ, de Man RA, Hop WC, Schalm SW. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029-2034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Amodio P, Del Piccolo F, Marchetti P, Angeli P, Iemmolo R, Caregaro L, Merkel C, Gerunda G, Gatta A. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Hidaka H, Endo T, Kawamoto S, Yamada E, Umekawa H, Tanabe K, Hara K. Purification and characterization of adipose tissue S-100b protein. J Biol Chem. 1983;258:2705-2709. [PubMed] [Cited in This Article: ] |
| 25. | Cocchia D, Michetti F, Donato R. Immunochemical and immuno-cytochemical localization of S-100 antigen in normal human skin. Nature. 1981;294:85-87. [PubMed] [Cited in This Article: ] |
| 26. | Kindblom LG, Lodding P, Rosengren L, Baudier J, Haglid K. S-100 protein in melanocytic tumors. An immunohistochemical investigation of benign and malignant melanocytic tumors and metastases of malignant melanoma and a characterization of the antigen in comparison to human brain. Acta Pathol Microbiol Immunol Scand A. 1984;92:219-230. [PubMed] [Cited in This Article: ] |
| 27. | Takahashi K, Isobe T, Ohtsuki Y, Sonobe H, Yamaguchi H, Akagi T. S-100 protein positive human T-lymphocyte. Am J Clin Pathol. 1985;83:69-72. [PubMed] [Cited in This Article: ] |
| 28. | Selinfreund RH, Barger SW, Pledger WJ, Van Eldik LJ. Neurotrophic protein S100 beta stimulates glial cell proliferation. Proc Natl Acad Sci USA. 1991;88:3554-3558. [PubMed] [Cited in This Article: ] |
| 29. | Marchi N, Cavaglia M, Fazio V, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood-brain barrier damage. Clin Chim Acta. 2004;342:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Kapural M, Krizanac-Bengez Lj, Barnett G, Perl J, Masaryk T, Apollo D, Rasmussen P, Mayberg MR, Janigro D. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102-104. [PubMed] [Cited in This Article: ] |
| 31. | Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806-2813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |