Published online Dec 7, 2015. doi: 10.3748/wjg.v21.i45.12778
Peer-review started: June 19, 2015
First decision: July 10, 2015
Revised: July 22, 2015
Accepted: September 15, 2015
Article in press: September 15, 2015
Published online: December 7, 2015
AIM: To investigate the cytoprotective effects in hepatic ischemia-reperfusion injury, we developed a new formulation of hyaluronic acid (HA) and sphingosine 1-phophate.
METHODS: We divided Sprague-Dawley rats into 4 groups: control, HA, sphingosine 1-phosphate (S1P), and HA-S1P. After the administration of each agent, we subjected the rat livers to total ischemia followed by reperfusion. After reperfusion, we performed the following investigations: alanine aminotransferase (ALT), histological findings, TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining, and transmission electron microscopy (TEM). We also investigated the expression of proteins associated with apoptosis, hepatoprotection, and S1P accumulation.
RESULTS: S1P accumulated in the HA-S1P group livers more than S1P group livers. Serum ALT levels, TUNEL-positive hepatocytes, and expression of cleaved caspase-3 expression, were significantly decreased in the HA-S1P group. TEM revealed that the liver sinusoidal endothelial cell (LSEC) lining was preserved in the HA-S1P group. Moreover, the HA-S1P group showed a greater increase in the HO-1 protein levels compared to the S1P group.
CONCLUSION: Our results suggest that HA-S1P exhibits cytoprotective effects in the liver through the inhibition of LSEC apoptosis. HA-S1P is an effective agent for hepatic ischemia/reperfusion injury.
Core tip: We have developed a new formulation by directly combining hyaluronic acid and sphingosine 1-phosphate (HA-S1P), which targets liver sinusoidal endothelial cell (LSEC) by binding specifically to HA and HA receptors. This study demonstrated that HA-S1P protects the liver from hepatic ischemia/reperfusion (I/R) injury in rats. The strong protective effect of HA-S1P may be mediated by the anti-apoptotic effect of S1P on LSECs. These data indicate that HA-S1P is an effective agent for preventing hepatic I/R injury.
- Citation: Sano N, Tamura T, Toriyabe N, Nowatari T, Nakayama K, Tanoi T, Murata S, Sakurai Y, Hyodo M, Fukunaga K, Harashima H, Ohkohchi N. New drug delivery system for liver sinusoidal endothelial cells for ischemia-reperfusion injury. World J Gastroenterol 2015; 21(45): 12778-12786
- URL: https://www.wjgnet.com/1007-9327/full/v21/i45/12778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i45.12778
Hepatic ischemia/reperfusion (I/R) injury is a major problem in liver transplantation and liver resection[1]. A critical event during hepatic I/R injury is the death of liver sinusoidal endothelial cells (LSECs), which occurs within a few minutes of reperfusion and precedes hepatocyte death by several hours[2]. The apoptosis of LSECs is a pivotal mechanism of hepatic I/R[3]. Thus, apoptosis plays an important role in hepatic I/R injury and LSEC death[4] and therefore LSECs are the primary treatment target for hepatic I/R injury[5].
Sphingosine-1-phosphate (S1P) is a bioactive lipid that regulates diverse cellular functions, including proliferation, differentiation, migration and survival through interaction with different G protein-coupled S1P receptors[6,7]. S1P exhibits anti-apoptotic effects on human LSECs[8] and protects rat LSECs from ethanol-induced apoptosis[9]. In addition S1P also reduces hepatic I/R-induced acute kidney injury through the attenuation of endothelial injury in mice[10]. However, accumulating S1P (as a single-agent) on the LSECs is difficult, as S1P receptors are widely expressed in various different tissues, such as brain, heart, lung, spleen, kidney, intestine, testis, and liver[11-13].
In this study, we developed a new drug delivery system (DDS) for targeting the LSEC by combining S1P with HA, to make the formula HA-S1P. Circulating HA in the blood is taken up through receptor-mediated endocytosis into the LSECs[14] because the HA receptors, namely Stabilin-2 (STAB2), are highly and specifically expressed on LSECs. STAB2 is a type I transmembrane scavenger receptor that is highly expressed in LSECs and is the major clearance receptor for circulating HA[15-19]. We hypothesized that HA-S1P would accumulate in LSECs of liver via the STAB2 receptors. The aim of this study was to investigate whether the newly developed HA-S1P protects livers in the case of hepatic I/R injury.
S1P was purchased from Sigma Chemical Company (St. Louis, MO, United States) and HA [average molecular weight (MW) 8 kDa] was purchased from Food Chemifa (Tokyo, Japan).
A mixture was made of 95.85 μL 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (0.1 g/mL), 57.535 μL N-hydroxysuccinimide (NHS) (0.1 g/mL), HA (2 mg/mL, average MW 8 kDa), and water/DMF/CHCl3 (5/4/1). The mixture was stirred well at 55 °C with the addition of 67.378 μL S1P (25 mg/mL), and the reaction was allowed to proceed for 24 h. Dialysis was performed to remove the S1P, EDC, and NHS. Integrations of nuclear magnetic resonance (NMR) were used to confirm the amount of S1P introduced [comparison of peaks appeared at 1.2 ppm (methylene group of S1P) and 2.0 ppm (acetyl group of hyaluronic acid)]. Approximately 13.5%-40% of S1P bound to the carboxylic acid of HA.
Male Sprague-Dawley rats, weighing 200 to 250 g, were obtained from CLEA Japan (CLEA Corporation, Tokyo, Japan). Animal experiments were performed in a humane manner after receiving approval from the Institutional University Experiment Committee of the University of Tsukuba, and in accordance with the university’s Regulations for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions, under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
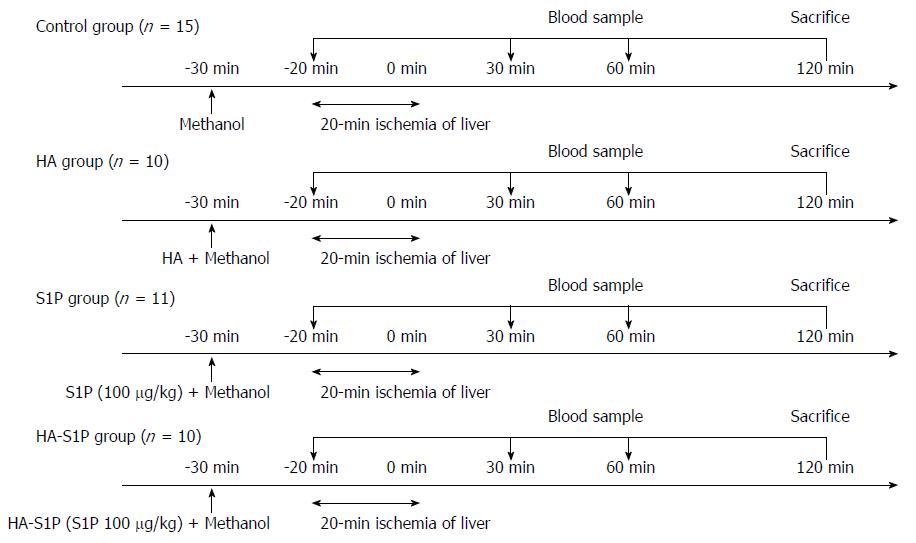
Total warm hepatic ischemia was induced in the rats for 20 min by clamping the portal triad. The rats were divided into 4 groups as follows: (1) methanol injection group (control group; n = 15); (2) HA injection group (HA group; n = 10); (3) single-agent S1P injection group (S1P group; n = 11); and (4) HA-S1P injection group (HA-S1P group; n = 10) (Figure 1). Methanol, S1P, HA, or HA-S1P were injected intravenously via the tail vein of the rats.
The rats were anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg/kg) and maintained by machine-regulated isoflurane. The rats were placed in a supine position on a heated pad to maintain a rectal temperature of 37 °C. After transverse laparotomy, the ligaments around the liver were dissected in order to clamp the portal triad. Concurrently, the hepatoduodenal ligament was taped in preparation for the subsequent clamping. Hepatic ischemia was induced via clamping of the portal triad, i.e., the hepatic artery, portal vein, and bile duct, by means of a microclip (B. Braun Aesculap Japan Co., Ltd, Tokyo, Japan) for 20 min. Surgical procedures were performed under sterile conditions. Blood samples were taken for the analysis of enzyme activities in serum before ischemia and at 30, 60, and 120 min after reperfusion. At the end of the experiments, liver tissue was obtained for histological examination. Finally, the experimental animals were euthanized by total blood collection via the abdominal aorta. During the period from drug administration until sacrificed, respiratory and circulatory dynamics of rats was stable. Moreover, all of rats did not die in this experiment.
To assess damage to the hepatic parenchyma, serum alanine transaminase (ALT) levels were measured using a Dry-Chem 7000 V autoanalyzer (Fujifilm Co, Tokyo, Japan). Blood samples were taken from the tail vein before the induction of ischemia and up to 120 min after reperfusion.
After 120 min of reperfusion, liver tissues were obtained from each group, fixed with 10% formaldehyde, and embedded in paraffin. Thin sections (4 μm) were prepared and stained with hematoxylin-eosin (HE). Tissue damage was evaluated in randomly selected high-power fields (× 200).
To detect apoptotic cells in liver tissue, a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (In situ Apoptosis Detection Kit; Takara Bio Inc., Otsu, Japan) was performed after 120 min of reperfusion. The ratio of TUNEL-positive/total cells in the microscope field was calculated at × 400.
Liver tissues were kept at -80 °C and were homogenized in a buffer consisting of 150 mmol/L NaCl, 50 mmol/L TrisCl, 1% NP-40, and proteinase inhibitors. The samples were centrifuged, and the supernatants collected for analysis. The samples were separated on 10% and 15% SDS-PAGE gels and transferred to nitrocellulose membranes (Millipore, Bedford, MA, United States). Anti-cleaved caspase-3 (9661), anti-heme oxygenase 1 (HO-1) (5141) (Cell Signaling Technology, Beverly, MA, United States) and anti-S1P antibodies (140592) (Abcam Ltd., Cambridge, United Kingdom) were used as primary antibodies. A secondary goat anti-rabbit antibody conjugated to horseradish peroxidase was purchased from Zymed Laboratories (San Francisco, CA, United States).
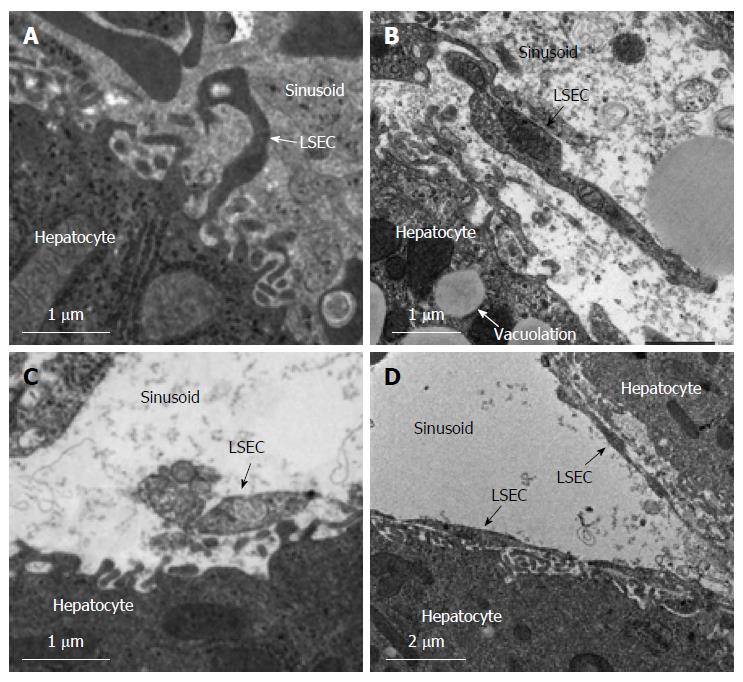
To assess the LSECs after hepatic I/R, we investigated the samples using transmission electron microscopy (TEM). After 120 min of reperfusion, the livers were resected. Tissue samples from the left hepatic lobe were cut into 1 mm3 cubes and stored in 2.5% glutaraldehyde. The specimens were postfixed with osmium tetroxide, dehydrated in graded alcohol series and embedded in Epon mixture. Ultrathin sections were prepared using an Ultracut S microtome (Leica Aktiengesellschaft, Vienna, Austria) and placed on copper grids. Sections were treated using uranyl acetate and lead citrate to enhance contrast. Specimens were examined using a Hitachi H-7000 transmission electron microscope (Hitachi Co., Tokyo, Japan).
All data are expressed as the mean ± SD. The Mann-Whitney U-test and Kruskal-Wallis H-test were used for statistical analysis, followed by the Mann-Whitney U-test with Bonferroni correction. P values of < 0.05 were considered statistically significant.
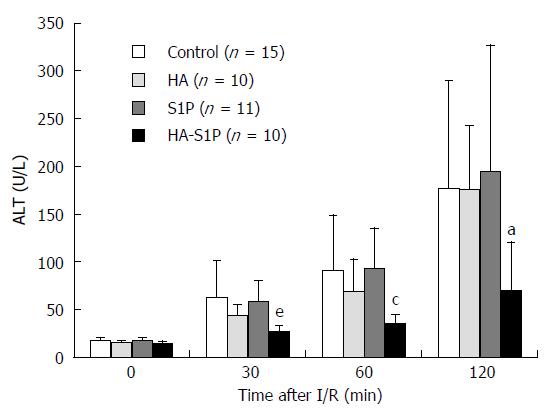
In the control, HA, and S1P groups, serum ALT (which reflects the degree of hepatic parenchymal injury) immediately increased after reperfusion. However, in the HA-S1P group serum ALT levels were unchanged up to 120 min (Figure 2).
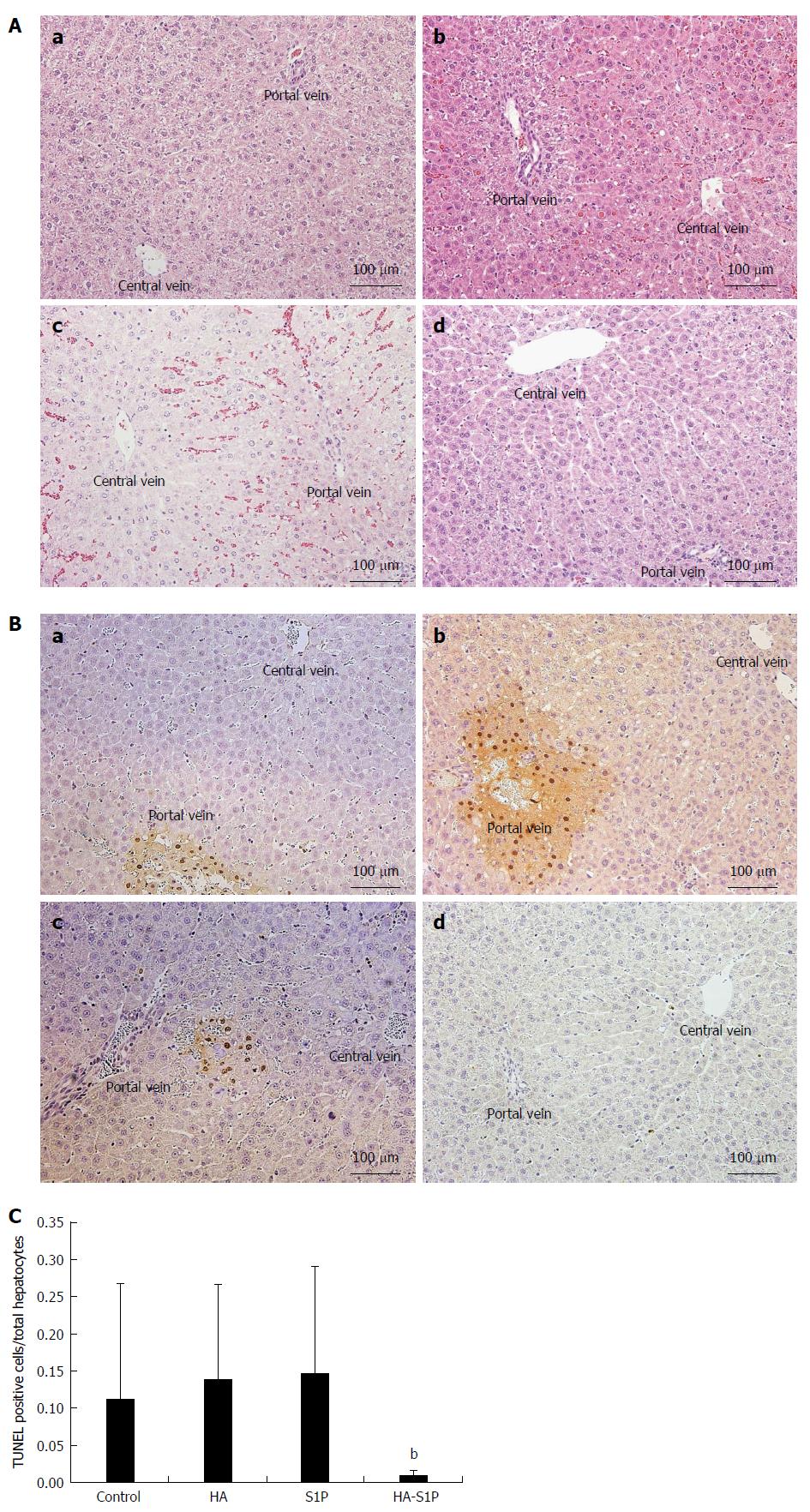
The light microscopy findings from the liver tissue, stained with HE, are shown in Figure 3A. In the control, HA and S1P groups, vacuolation of the hepatocytes and sinusoidal narrowing were observed after 120 min of reperfusion. These findings were more severely localized near the portal vein. On the other hand, no histological alteration was observed in the HA-S1P group.
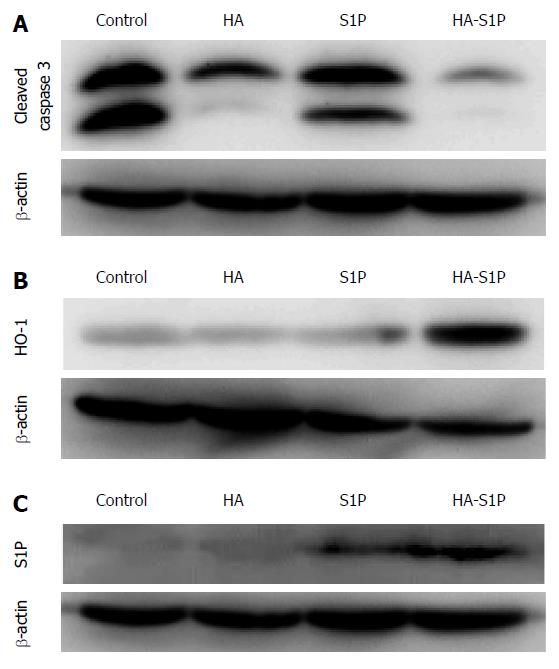
In the control, HA, and S1P groups, TUNEL-positive cells were observed after 120 min of reperfusion near the portal vein. However, TUNEL-positive cells were not observed in the HA-S1P group (Figure 3B). The ratio of TUNEL-positive to total hepatocytes was significantly lower in the HA-S1P group (Figure 3C). Western blot analysis of the liver tissue is presented in Figure 4. The expression of cleaved caspase-3 in the control, HA, and S1P groups was extremely higher than that in the HA-S1P group (Figure 4A). The expression of the HO-1 (a cytoprotective enzyme against hepatic I/R injury) in the HA-S1P group was higher than that in the control, HA, and S1P groups (Figure 4B).
In the control and HA groups, S1P did not accumulate in the liver. In the HA-S1P group, S1P accumulated in the liver to a greater degree than it did in the S1P group (Figure 4C).
Deterioration of the sinusoidal endothelial lining was observed and severe destruction of the perisinusoidal space structures were observed in the control, HA, and S1P groups (Figure 5). In the HA group vacuolation induced by hypoxia was observed. However, the structure of the endothelial lining in the HA-S1P group was well preserved. Moreover, it was no obvious mitochondrial disorders in the HA-S1P group.
LSECs play important functional roles in hepatic I/R injury, being particularly vulnerable to it[20,21]. S1P has an anti-apoptotic effect on LSECs[8,9], but S1P receptors are widely expressed in many organs[11-13]. Thus, it is difficult to deliver agents specifically to LSECs through a single-agent administration of S1P. However, HA receptors of STAB2 are specifically expressed on LSECs[15-19]. We have developed a new formulation of HA-S1P that targets LSECs by binding specifically to HA and HA receptors. This study demonstrates that HA-S1P protects the liver from hepatic I/R injury in rats and that the strong protective effect of HA-S1P may be mediated by the antiapoptotic effect of S1P on LSECs. Therefore, the results of this study strongly suggest that HA-S1P could be a useful agent for suppressing hepatic I/R injury.
In hepatic I/R injury, LSECs are injured and become apoptotic soon after reperfusion and this phenomenon occurs much earlier than it does in hepatocytes[22]. In addition, LSECs have important functional roles throughout hepatic injury in acute hepatitis[23] and hepatectomy[24]. In general, LSECs are known to be a septum between blood and hepatocytes and are involved in the stabilization of hepatocytes. In previously research we determined that LSEC injury results in microcirculatory blood flow disturbances[25]. In I/R injury, hepatocytes become seriously impaired after disorder of the LSECs, which subsequently induces liver dysfunction[26]. As such, LSECs are primary therapeutic targets of hepatic I/R injury[26]. In the present study we performed total hepatic I/R injury in rats and we found that serum alanine aminotransferase levels were also significantly relieved by HA-S1P administration. TEM findings supported our hypothesis that HA-S1P has a positive effect on LSEC injury. After HA-S1P administration there were no observations of apoptotic cells in the liver tissue thus preventing hepatic I/R injury. These results indicate that HA-S1P administration inhibits the deterioration of the LSEC lining structure and that this phenomenon leads to the suppression of hepatic injury.
S1P is a lipid mediator contained in platelets and is reported to regulate a broad variety of cellular processes, such as cell proliferation, apoptosis, calcium homeostasis, vascular maturation, or angiogenesis[27,28]. S1P is excreted in large amounts from activated platelets[29]. Zheng et al[9] reported that S1P protects LSECs from ethanol-induced injury through inhibition of apoptosis and we have previously reported that S1P prevents the apoptosis of LSECs by inhibiting the cleavage of caspase-3[8]. From these reports, we hypothesized that S1P is the most effective agent for inhibiting hepatic I/R injury, because apoptosis of LSEC is the primary mechanism underlying hepatic I/R injury[30,31]. S1P acts directly either on intracellular targets or activates G protein-coupled receptors[32]. Five S1P receptors have been identified, namely S1PR1-5[33]. S1PR1-3 are widely expressed in various tissues (including liver), whereas the expression of S1PR4 and S1PR5 are confined to lymphoid/hematopoietic tissue and to the central nervous system, respectively[33].
Recent investigations revealed that LSECs possess unique HA receptors of STAB2 that recognize and internalize HA[34]. We tried to utilize this feature of LSECs in the development of a novel DDS to achieve the accumulation of S1P[35]. Exogenous HA has been reported to rapidly integrate into the liver after in vivo administration[15,17]. HA has been widely used as a targeted delivery material for LSECs[36]. However, up to now, there has been no specific research focused on LSECs that has lead to the treatment of liver disease. To this end we developed HA-S1P formulation that is prepared by direct combination of HA and S1P. In the present study, we revealed that HA-S1P selectively accumulates in the liver to a greater extent than dose a single-agent administration of S1P.
HO-1 is widely expressed among the different liver cell populations, including LSECs, hepatocytes, Kupffer cells and hepatic stellate cells[37]. The cytoprotective effects of HO-1 have been shown to ameliorate hepatic I/R injury in various experimental models[38]. We have previously demonstrated that HO-1 overexpression exerts cytoprotective effects and improves hepatic I/R injury[39]. HO-1, also known as heat shock protein 32, is an inducible enzyme that converts heme into carbon monoxide (CO), billiverdin and free iron[40]. Moreover, induction of CO in the hepatic sinusoids before hepatic I/R may have prominent effects[39]. In the present study, we found that HA-S1P resulted in greater expression of HO-1 than single-agent administration of S1P. Consequently, these data suggest that by inducing HO-1 to maintain normal cellular function HA-S1P protects LSECs from hepatic I/R injury.
In conclusion, we have succeeded in specific delivery of S1P to LSECs for the first time by using HA as a carrier. Furthermore, HA-S1P exhibits a cytoprotective effect on the liver through the inhibition of LSEC apoptosis. HA-S1P attenuates hepatic I/R injury by protecting LSECs, which represent the primary therapeutic targets for treating hepatic I/R injury. Thus, HA-S1P is a promising new agent for hepatic I/R injury. Further investigations are needed to support our results and elucidate the molecular mechanisms.
The authors thank Ako Takahashi for providing technical assistance.
The apoptosis of liver sinusoidal endothelial cells (LSECs) is a pivotal mechanism of hepatic ischemia/reperfusion (I/R). Sphingosine 1-phosphate (S1P) exhibits antiapoptotic effects on human LSECs. However, accumulating S1P (as a single-agent) on the LSECs is difficult, as S1P receptors are widely expressed in various different tissues. The authors developed a new drug delivery system for targeting the LSEC by combining S1P with HA, to make the formula hyaluronic acid-S1P (HA-S1P). The aim of this study was to investigate whether the newly developed HA-S1P protects livers in the case of hepatic I/R injury.
LSECs play important functional roles in hepatic I/R injury. It is difficult to deliver protective agents specifically to LSECs through a single-agent administration. However, HA receptors of STAB2 are specifically expressed on LSECs. The authors have developed a new formulation of HA-S1P that targets LSECs by binding specifically to HA and HA receptors to protect the liver from hepatic I/R injury.
This is the first study to succeed in specific delivery of S1P to LSECs for the first time by using HA as a carrier. There has been no specific research focused on LSECs that has lead to the treatment of liver disease. HA-S1P attenuates hepatic I/R injury by protecting LSECs.
Hepatic I/R injury is a major problem in liver transplantation and liver resection. The authors suggest that HA-S1P is a promising new agent for hepatic I/R injury.
S1P is a bioactive lipid that regulates diverse cellular functions, including proliferation, differentiation, migration and survival through interaction with different G protein-coupled S1P receptors. The HA receptors, namely STAB2, are highly and specifically expressed on LSECs. STAB2 is a type I transmembrane scavenger receptor that is highly expressed in LSECs and is the major clearance receptor for circulating HA.
The authors have developed a new formulation of HA-S1P that targets LSECs by binding specifically to HA and HA receptors. This study demonstrates that HA-S1P protects the liver from hepatic I/R injury in rats and that the strong protective effect of HA-S1P may be mediated by the antiapoptotic effect of S1P on LSECs. Therefore, the results of this study strongly suggest that HA-S1P could be a useful agent for suppressing hepatic I/R injury.
P- Reviewer: Corrales FJ, Wu SL S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704-711; discussion 711-713. [PubMed] [Cited in This Article: ] |
| 2. | Selzner N, Liu H, Boehnert MU, Adeyi OA, Shalev I, Bartczak AM, Xue-Zhong M, Manuel J, Rotstein OD, McGilvray ID. FGL2/fibroleukin mediates hepatic reperfusion injury by induction of sinusoidal endothelial cell and hepatocyte apoptosis in mice. J Hepatol. 2012;56:153-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Banga NR, Prasad KR, Burn JL, Homer-Vanniasinkam S, Graham A. An in vitro model of warm hypoxia-reoxygenation injury in human liver endothelial cells. J Surg Res. 2012;178:e35-e41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Enomoto K, Nishikawa Y, Omori Y, Tokairin T, Yoshida M, Ohi N, Nishimura T, Yamamoto Y, Li Q. Cell biology and pathology of liver sinusoidal endothelial cells. Med Electron Microsc. 2004;37:208-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol. 1999;58:201-207. [PubMed] [Cited in This Article: ] |
| 7. | Igarashi Y, Yatomi Y. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim Pol. 1998;45:299-309. [PubMed] [Cited in This Article: ] |
| 8. | Nowatari T, Murata S, Nakayama K, Sano N, Maruyama T, Nozaki R, Ikeda N, Fukunaga K, Ohkohchi N. Sphingosine 1-phosphate has anti-apoptotic effect on liver sinusoidal endothelial cells and proliferative effect on hepatocytes in a paracrine manner in human. Hepatol Res. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Zheng DM, Kitamura T, Ikejima K, Enomoto N, Yamashina S, Suzuki S, Takei Y, Sato N. Sphingosine 1-phosphate protects rat liver sinusoidal endothelial cells from ethanol-induced apoptosis: Role of intracellular calcium and nitric oxide. Hepatology. 2006;44:1278-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Lee SY, Kim DH, Sung SA, Kim MG, Cho WY, Kim HK, Jo SK. Sphingosine-1-phosphate reduces hepatic ischaemia/reperfusion-induced acute kidney injury through attenuation of endothelial injury in mice. Nephrology (Carlton). 2011;16:163-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 558] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385-402. [PubMed] [Cited in This Article: ] |
| 14. | Shimizu H, He W, Guo P, Dziadkoviec I, Miyazaki M, Falk RE. Serum hyaluronate in the assessment of liver endothelial cell function after orthotopic liver transplantation in the rat. Hepatology. 1994;20:1323-1329. [PubMed] [Cited in This Article: ] |
| 15. | Fraser JR, Alcorn D, Laurent TC, Robinson AD, Ryan GB. Uptake of circulating hyaluronic acid by the rat liver. Cellular localization in situ. Cell Tissue Res. 1985;242:505-510. [PubMed] [Cited in This Article: ] |
| 16. | McCourt PA, Smedsrød BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology. 1999;30:1276-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Smedsrød B, Pertoft H, Eriksson S, Fraser JR, Laurent TC. Studies in vitro on the uptake and degradation of sodium hyaluronate in rat liver endothelial cells. Biochem J. 1984;223:617-626. [PubMed] [Cited in This Article: ] |
| 18. | Weigel JA, Raymond RC, McGary C, Singh A, Weigel PH. A blocking antibody to the hyaluronan receptor for endocytosis (HARE) inhibits hyaluronan clearance by perfused liver. J Biol Chem. 2003;278:9808-9812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Simpson MA, Weigel JA, Weigel PH. Systemic blockade of the hyaluronan receptor for endocytosis prevents lymph node metastasis of prostate cancer. Int J Cancer. 2012;131:E836-E840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, Geiger KK, Pannen BH. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg. 2007;245:931-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 22. | Kohli V, Selzner M, Madden JF, Bentley RC, Clavien PA. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation. 1999;67:1099-1105. [PubMed] [Cited in This Article: ] |
| 23. | Hisakura K, Murata S, Takahashi K, Matsuo R, Pak S, Ikeda N, Kawasaki T, Kohno K, Myronovych A, Nakano Y. Platelets prevent acute hepatitis induced by anti-fas antibody. J Gastroenterol Hepatol. 2011;26:348-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Hisakura K, Murata S, Fukunaga K, Myronovych A, Tadano S, Kawasaki T, Kohno K, Ikeda O, Pak S, Ikeda N. Platelets prevent acute liver damage after extended hepatectomy in pigs. J Hepatobiliary Pancreat Sci. 2010;17:855-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Tamura T, Kondo T, Pak S, Nakano Y, Murata S, Fukunaga K, Ohkohchi N. Interaction between Kupffer cells and platelets in the early period of hepatic ischemia-reperfusion injury--an in vivo study. J Surg Res. 2012;178:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, Gores GJ. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89-96. [PubMed] [Cited in This Article: ] |
| 27. | Kawasaki T, Murata S, Takahashi K, Nozaki R, Ohshiro Y, Ikeda N, Pak S, Myronovych A, Hisakura K, Fukunaga K. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J Hepatol. 2010;53:648-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1651] [Cited by in F6Publishing: 1643] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 29. | Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, Satoh K, Kume S, Tigyi G, Igarashi Y. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431-3438. [PubMed] [Cited in This Article: ] |
| 30. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. [PubMed] [Cited in This Article: ] |
| 31. | Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917-936. [PubMed] [Cited in This Article: ] |
| 32. | Adada M, Canals D, Hannun YA, Obeid LM. Sphingosine-1-phosphate receptor 2. FEBS J. 2013;280:6354-6366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 382] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 34. | Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem. 2000;275:37733-37741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Takei Y, Maruyama A, Ferdous A, Nishimura Y, Kawano S, Ikejima K, Okumura S, Asayama S, Nogawa M, Hashimoto M. Targeted gene delivery to sinusoidal endothelial cells: DNA nanoassociate bearing hyaluronan-glycocalyx. FASEB J. 2004;18:699-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Yamada Y, Hashida M, Hayashi Y, Tabata M, Hyodo M, Ara MN, Ohga N, Hida K, Harashima H. An approach to transgene expression in liver endothelial cells using a liposome-based gene vector coated with hyaluronic acid. J Pharm Sci. 2013;102:3119-3127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Bauer I, Wanner GA, Rensing H, Alte C, Miescher EA, Wolf B, Pannen BH, Clemens MG, Bauer M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Sun L, Shi T, Qiao H, Jiang X, Jiang H, Krissansen GW, Sun X. Hepatic overexpression of heme oxygenase-1 improves liver allograft survival by expanding T regulatory cells. J Surg Res. 2011;166:e187-e194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Tamura T, Kondo T, Ogawa K, Fukunaga K, Ohkohchi N. Protective effect of heme oxygenase-1 on hepatic ischemia-reperfusion injury through inhibition of platelet adhesion to the sinusoids. J Gastroenterol Hepatol. 2013;28:700-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |