Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.12114
Peer-review started: March 22, 2015
First decision: April 13, 2015
Revised: April 28, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: November 14, 2015
Matrix metalloproteinases (MMPs) are a family of proteases using zinc-dependent catalysis to break down extracellular matrix (ECM) components, allowing cell movement and tissue reorganization. Like many other proteases, MMPs are produced as zymogens, an inactive form, which are activated after their release from cells. Hepatic ischemia/reperfusion (I/R) is associated with MMP activation and release, with profound effects on tissue integrity: their inappropriate, prolonged or excessive expression has harmful consequences for the liver. Kupffer cells and hepatic stellate cells can secrete MMPs though sinusoidal endothelial cells are a further source of MMPs. After liver transplantation, biliary complications are mainly attributable to cholangiocytes, which, compared with hepatocytes, are particularly susceptible to injury and ultimately a major cause of increased graft dysfunction and patient morbidity. This paper focuses on liver I/R injury and cholestasis and reviews factors and mechanisms involved in MMP activation together with synthetic compounds used in their regulation. In this respect, recent data have demonstrated that the role of MMPs during I/R may go beyond the mere destruction of the ECM and may be much more complex than previously thought. We thus discuss the role of MMPs as an important factor in cholestasis associated with I/R injury.
Core tip: Induction of matrix metalloproteinases (MMPs) modulates the progression of liver damage such as ischemia/reperfusion (I/R) injury and acute allograft rejection. The high incidence of biliary complications, after liver transplantation, is due to a cascade of events leading to biliary lesions to which cholangiocytes are particularly susceptible. This paper, while focusing on liver I/R and cholestasis, reviews factors and mechanisms implicated in MMP activation/regulation together with the role of MMPs in biliary complications following I/R injury. Recent data support the view that MMPs play a dual role, both good and bad, in liver I/R depending on the length of time after damage.
- Citation: Palladini G, Ferrigno A, Richelmi P, Perlini S, Vairetti M. Role of matrix metalloproteinases in cholestasis and hepatic ischemia/reperfusion injury: A review. World J Gastroenterol 2015; 21(42): 12114-12124
- URL: https://www.wjgnet.com/1007-9327/full/v21/i42/12114.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i42.12114
Liver fibrosis arises from chronic damage to the liver associated with the over-accumulation of extracellular matrix (ECM) proteins, a characteristic of most types of chronic hepatic diseases[1] including: cholestatic liver diseases; primary biliary cirrhosis and secondary biliary cirrhosis; hepatotoxic diseases such as hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver disease (ALD), and non-alcoholic steatohepatitis (NASH)[2].
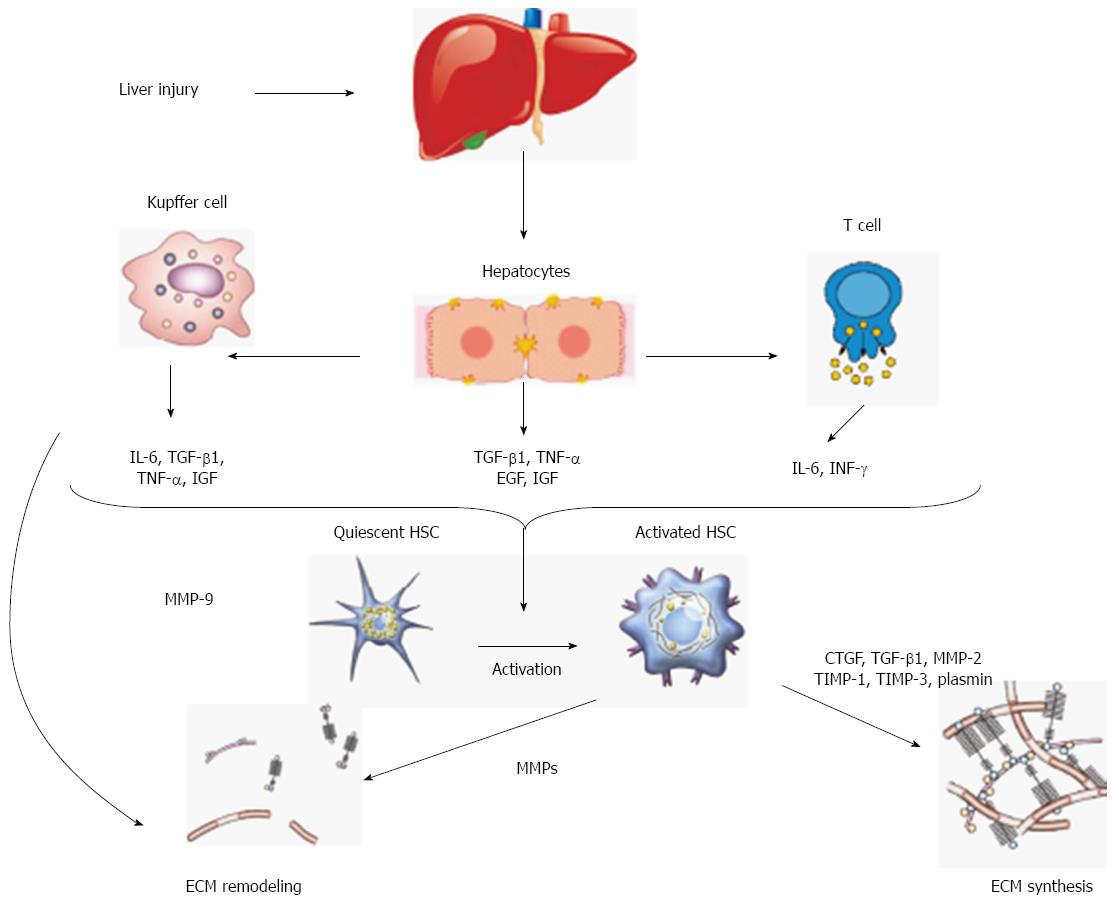
Advanced liver fibrosis disrupts the liver’s normal architecture; hepatocytes are replaced with abundant ECM causing hepatocellular dysfunction and portal hypertension. Hepatic stellate cells (HSCs) are a major fibrogenic cell type in the liver[3]. Following liver injury, HSCs undergo an activation process and change their phenotype from quiescent retinoid storing HSCs to collagen-producing and contractile myofibroblast-like cells[4]. Activated HSCs migrate and accumulate at the sites of tissue repair, secreting large amounts of ECM and regulating ECM degradation.
While the classic liver injury paradigm asserts that HSCs produce, remodel and turn over abnormal ECMs of fibrosis via MMPs, a recent paper by Calabro et al[5] has shown that MMPs are also secreted by other intrahepatic cell populations including hepatocytes.
Major changes in both quantity and composition of ECMs[6] and excessive ECM remodeling arises from a balance between increased synthesis and decreased degradation[7]. One class of zinc and calcium-dependent endopeptidases - matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) - plays a major role in the ECM remodeling[8]. Analysis of human and experimental animal fibrotic liver demonstrates an increase in a number of MMPs with a wide activity spectrum. Like many other proteases, MMPs are produced by activation of zymogens, which are released from cells[9-11]. Several different kinds of MMP have been identified (Table 1). Most of them can act on different collagen types, fibronectin, laminin, elastin, proteoglycans, and surface molecules such as growth factors or selectins.
| MMP (class and number) | Name | Extracellular Matrix substrate |
| Collagenases | ||
| MMP-1 | Collagenase 1 | Collagen I, II, III, VII, VIII and X, gelatin, proteoglycans, tenascin, entactin |
| MMP-8 | Collagenase 2 | Collagen I, II, III, V, VIII and X, gelatin, aggrecan |
| MMP-13 | Collagenase 3 | Collagen I, II, III, IV, IX and X, fibronectin, gelatin, tenascin, aggrecan, osteonectin |
| Gelatinases | ||
| MMP-2 | Gelatinase A | Collagen I, IV, V, VII, IX and X, gelatin, proteoglycans, elastin, fibronectin, laminin, aggrecan, versican, osteonectin |
| MMP-9 | Gelatinase B | Collagen IV, V, VII, X and XIV, gelatin, proteoglycans, elastin, aggrecan, versican, osteonectin |
| Stromelysins | ||
| MMP-3 | Stromelysins 1 | Collagen III, IV, V, and IX, gelatin, proteoglycans, tenascin, fibronectin, laminin, aggrecan, versican, osteonectin |
| MMP-10 | Stromelysins 2 | Collagen III, IV and V, gelatin, proteoglycans, aggrecan, elastin, casein |
| MMP-11 | Stromelysins 3 | Collagen IV, fibronectin, laminin, gelatin, transferrin |
| Membrane type | ||
| MMP-14 | MT1-MMP | Collagen I, II and III, fibronectin, vitronectin, tenascin, laminin, proteoglycans, aggrecan, elastin, casein, entactin |
| MMP-15 | MT2-MMP | Fibronectin, tenascin, laminin |
| MMP-16 | MT3-MMP | Collagen III, fibronectin, casein, gelatin |
| MMP-17 | MT4-MMP | ND |
| MMP-24 | MT5-MMP | Activator of proMMP-2 |
| MMP-25 | MT6-MMP | Collagen IV, fibronectin, gelatin, fibrinogen |
| Others | ||
| MMP-7 | Collagen IV and X, gelatin, proteoglycans, tenascin, fibronectin, laminin, aggrecan, osteonectin, entactin, casein, tranferrin, integrin b4 | |
| MMP-12 | Collagen IV, gelatin, proteoglycans, fibronectin, laminin, entactin, casein, vibronectin, elastin | |
| MMP-19 | ND | Aggrecan, cartilage oligomeric matrix protein (COMP) |
| MMP-20 | Enamelysin | Amelogenin |
| MMP-23A | MMP-21 | ND |
| MMP-23B | MMP-22 | ND |
| MMP-26 | Matrilysin 2 | Collagen IV, fibronectin, casein, fibrinogen |
| MMP-27 | ND | ND |
| MMP-28 | Epilysin | Casein |
MMP activity is regulated at three levels: gene transcription; posttranslational activation of zymogens, and interactions of secreted MMPs with specific inhibitors TIMP[12]. However, specific MMP inhibitors do not simply block protease activity but, on the contrary, the role of TIMPs is to modulate MMP functioning. Different protease activation occurs as a response to liver injury[13]. Usually, while injured cells release proteases, healthy cells release TIMPs; put another way, inhibitors are secreted by the cells surrounding these producing proteases[14-17]. Thus, high level of TIMPs occur simultaneously to an increase in proteases; in other words, both proteases and inhibitors could be produced by the same cell type at the same time[13]. TIMP concentrations and MMP/TIMP ratios are critical in this respect: a high MMP/TIMP ratio activate MMPs, while a low MMP/TIMP ratio lead to the opposite effect[18].
The uncontrolled ECM remodeling plays a central role in pathological changes leading to fibrosis[1,7]. A change in quality and quantity of matrix proteins occurs during fibrogenesis, resulting in excessive accumulation of fibrous tissue and an increase in ECM density[19] (Figure 1).
Several animal models of liver fibrosis have been developed, each of these with its strengths and weaknesses[20]. Bile duct ligation (BDL) has been used as an experimental model for chronic liver injury because of its closeness to hepatocyte damage, hepatic stem cell activation and the liver fibrosis found in human cholestatic liver disease[21].
The present study reviews and discusses the published articles searched on PubMed, MEDLINE, Google Scholar, and Google databases using specific keywords to identify articles related to MMPs in cholestasis and I/R injury. These keywords were “liver” and “MMPs,”“cholestasis” and “ischemia/reperfusion”. The search included letters to the editor, case reports, review articles, original articles, and meeting presentations published in the English-language literature from January 2000 to February 2015.
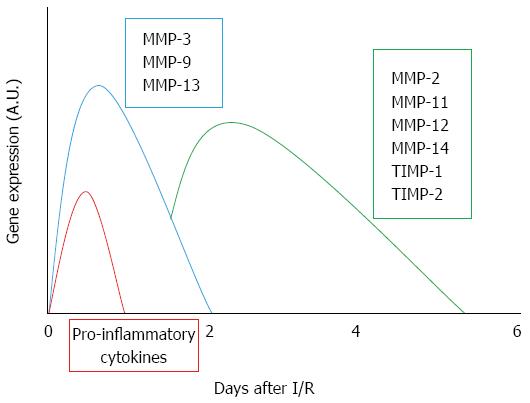
During liver resection, transplantation and trauma a prolonged oxygen deficiency is observed; the following oxygen restoration always induces reperfusion injury. In particular, the sequence of events that occurs during I/R injury is represented by an early increase in oxidative stress, liver sinusoidal endothelial cell damage, Kupffer cell activation and further release of reactive oxygen species, all of which in turn leads to marked tissue damage and liver remodeling[22]. MMPs are enzymes primarily involved in connective tissue remodeling; their inappropriate, prolonged or excessive expression has harmful consequences[23]. I/R is associated with gene expression, activation and release of MMPs, which have profound effects on tissue integrity[22,24] (Figure 2). A main role seems to be played by MMP-2 (gelatinase A; EC 3.4.24.24) and MMP-9 (gelatinase B; EC 3.4.24.35). These two gelatinases are the two main components of the space of Disse[25] and they are critically involved in the degradation of collagen IV and fibronectin[26]. Hence, increased activity of these MMPs may cause liver injury, with alterations of the sinusoidal cells and remodeling of the stromal structure. Already in 1997, Upadhya et al[27] demonstrated that MMP content, following release of MMP-2 and MMP-9 during cold preservation using rat and human liver perfusates, was dependent on the length of cold storage. Other data have since confirmed and extended the role of MMPs in hepatocyte cell death after prolonged cold storage and subsequent reperfusion[28]: the protective effects obtained using MMP inhibitors led the authors to suggest their addition to the liver preservation solution[28] (Table 2).
| Compounds | Mechanism involved | MMPs involved | I/R model | Ref. |
| Bortezomib | Downregulation of pro-inflammatory (IL-1β, TNF-α and IFN-γ) and pro-fibrotic (VEGF, TGF-β, HGF, bFGF) factors | MMP-2MMP-9 | Steatotic orthotopic liver transplant | Tiriveedhi et al[45],Transpl Immunol 2014 |
| KMUP-1 (NO-donor) | Protects from apoptosis-associated free radical generation and pro-inflammation | MMP-9 | Hypoxic HepG2 cells | Kuo et al[90], Int J Imm Pharm 2013 |
| CS-1 peptide | Blocks fibronectin α4β1 and decreases the release of pro-inflammatory mediators | MMP-9MT-1-MMP/MMP-14 | Cold ischemia | Duarte et al[91],Am J Transpl 2012 |
| CTT peptide | Reduction in TNF-α, IL-1, IL-2 and IFN-γ | MMP-9 | Acute small-for-size liver graft | Ma et al[34], Am J Transpl 2010 |
| Cyclic RGD peptide | Depresses inflammatory mediators (IFN-γ) | MMP-9 | Steatotic Cold ischemia | Fondevila et al[92],Am J Transpl 2009 |
| ONO-1714 | iNOS inhibitor | MMP-9 | Warm ischemia | Hamada et al[46], Am J Pathol 2009 |
| RXP409 | Inhibitory effects on MMP activity | MMPs | Cold ischemia | Defamie et al[28],Hepatology 2008 |
| Anti-MMP-9 | Decrease in expression of TNF-α, IL-2 and IFN-γ | MMP-9 | Warm ischemia | Hamada et al[23], Hepatology 2008 |
| CS-1 peptide | Blocks FN α4β1 integrin and its FN ligand | MMP-9 | Steatotic orthotopic liver transplant | Moore et al[29], Am J Pathol 2007 |
| ONO-4817 | Reduction in TNF-α, IL-1β | MMP-2 and MMP-9 | Warm ischemia | Shirahane et al[33],Surgery 2006 |
| NAC | Reduction in free radicals | MMP-9 | Warm ischemia | Chen et al[93], Transpl Proc 2005 |
| BB3103 | Prostaglandin PGE(1) protection | MMP-2 | Cold ischemia | Yang et al[94], Microvasc Res 2002 |
| RXPO3 | Protects from necrosis/apoptosis | MMP-3-9-11-13 | Warm ischemia | Cursio et al[24], FASEB J 2002 |
I/R injury is also typical of other pathological conditions in which the ischemic phase takes place under normothermic conditions. In particular, increased liver MMP-9 expression has been reported after normothermic I/R injury[29]. Moreover, serum MMP-9 has been found to be associated with the progression of liver damage in I/R injury[30], acute allograft rejection[31] and chronic viral hepatitis[32]. Some reports have suggested that specific MMP inhibitors decrease liver injury after normothermic ischemia associated with a concomitant reduction in inflammatory cytokine release[24,33] (Table 2). Other evidence has suggested that targeting MMP-9, using an anti-MMP neutralizing monoclonal antibody, leads to protection against damage after warm liver I/R; this approach appears to be more effective than using MMP inhibitors[23] (Table 2). Furthermore, experimental data suggest that MMP inhibition might be a promising approach in the context of pharmacological strategies designed to limit post-ischemic hepatic damage both in whole liver transplantation and in acute “small-for-size” liver graft injury[34].
That MMPs are secreted by Kupffer cells and hepatic stellate cells (HSCs) is a well-established fact[35]. MMP-9 are predominantly expressed in Kupffer cells, MMP-2 in HSC while MMP-3 and MMP-10 in hepatic stellate cells as well. Membrane type-1 MMP is found in significant amount in all liver cells.
However, another source of MMPs in the rat liver are sinusoidal the endothelial cells (SECs)[36]. In particular, the ability of HSCs to produce significant amounts of matrix degrading enzymes and their inhibitors has been demonstrated by Knittel et al[37]. In addition, hepatic MMPs, released from isolated rat SECs after preservation in cold Euro-Collins and UW solutions, increase as the length of time associated with cold preservation increases[27]. Hamada et al[23] have shown that MMP-9 expressed by leukocytes is also a key factor in cell transmigration and activation leading to liver injury. MMP-2 and MMP-9 are expressed not just in nonparenchymal liver cells but also in different subsets of leukocytes (T cells, neutrophils, monocytes, macrophages)[26].
Interestingly, recent data have demonstrated that the role of MMPs during I/R might be more complex than the mere destruction of the ECM or leucocyte recruitment to hepatic parenchyma[38]: the results have demonstrated that, although liver injury decreases in MMP-9-/- mice at 24 h after reperfusion, liver recovery after 72 h of reperfusion was significantly delayed in MMP-9-/- mice when compared with WT mice[38,39]. Thus, MMP-9 seems to play a dual role in liver I/R injury that varies with reperfusion times.
MMP expression and activity in liver I/R injury are topics under continuous development as are the factors involved in their activation. The mechanisms of I/R-induced liver injury include sequestration of inflammatory cells in the liver which causes oxygen radicals, nitric oxide (NO) and TNF-α to rise sharply[40]. In particular, research into the activation of Kupffer cells in I/R injury, which induces the production of proinflammatory cytokines including TNF-α and interleukin-1β (IL-1β), has led to an elucidation of the regulatory activity of cytokines on MMP expression and further suggested distinct roles for TNF-α and TGF-β1[41,42]; the early matrix degradation following liver damage may be enhanced by TNF-α, whereas the reduced matrix degradation observed during chronic tissue injury may be due to the TIMP-mediated action of TGF-β1 (Table 3). We recently demonstrated that the release of TNF-α, which occurs during the early stage of reperfusion after partial hepatic I/R injury, is related to an increase of MMP activity both in the ischemic region and in the non-ischemic lobe[43]. Furthermore, the increase in serum TNF-α after hepatic I/R is also correlated with MMP activation in the lung, a distant organ[44].
| Factors | MMPs Involved | I/R Model | Ref. |
| FN-α-β1 | MMP-9MT1-MMP/MMP-14 | Cold ischemia | Duarte et al[91], Am J Transpl 2012 |
| Coito[48], Curr Opin Organ Transplant 2011 | |||
| Moore et al[29], Am J Pathol 2007 | |||
| Fondevilla et al[95], Transplant Proc 2005 | |||
| Amersi et al[47], Am J Pathol 2003 | |||
| Tenascin-C | MMP-9 | Warm ischemia | Kuriyama et al[96], Hepatology 2011 |
| iNOS | MMP-9 | Warm ischemia | Hamada et al[46], Am J Pathol 2009 |
| TNF-α | MMP-2 and MMP-9 | Warm ischemia | Feng et al[39], J Surg Res 2013 |
| Palladini et al[43], Toxicol and Pathol 2012 | |||
| Khandoga et al[26], J Leukoc Biol 2006 | |||
| Chen et al[93], Transplant Proc 2005 | |||
| IL-1β | MMP-2 and MMP-9 | Warm ischemia | Shirahane et al[33], Surgery 2006 |
| IL-6 | MMP-9 | Warm ischemia | Hamada et al[46], Am J Pathol 2009 |
| IFNα-2a | MMP-2 and MMP-9 | Cholestasis | Bueno et al[54], J Hepatol 2000 |
| CD62 | MMP-2 and MMP-9 | Warm ischemia | Khandoga et al[26], J Leukoc Biol 2006 |
| Plasmin | MMP-9 | Cholestasis | Martínez-Rizo et al[97], Liver Int 2010 |
| TGF-β | MMP-9 | Warm ischemia | Feng et al[39], J Surg Res 2013 |
| MMP-13 | Cholestasis | Aldaba-Muruato et al[98], Can J Physiol Pharmacol 2012 | |
| IL-10 | MMP-2 and MMP-9 | Warm ischemia | Feng et al[99], Int Immunopharmacol 2012 |
Other evidence has also shown that MMP expression by HSCs is regulated in a cytokine-specific pattern. Since TNF-α causes a marked stimulation of MMPs, it may well be that TNF-α and HSC are involved in initial matrix breakdown after liver injury. This initial matrix breakdown may be essential for early tissue repair reactions triggered by tissue inflammation when acute hepatic damage occurs[42]. Moreover, other data suggest that inflammatory cytokines such as TNF-α have a role in ECM degradation after liver I/R injury and that hepatic TNF-α expression runs parallel to MMP induction[26].
Recently, using an orthotropic liver transplant model in Zucker-obese rats, the administration of the proteosomal inhibitor, bortezomib, was shown to inhibit MMP activation and reduce serum proinflammatory cytokines including TNF-α and IL-1β[45] (Table 2).
Significantly, some experiments have also been performed to test the role of inducible nitric oxide synthase (iNOS) expression on the modulation of MMP-9 activity in hepatic I/R injury. Using both mice lacking the gene encoding for iNOS and mice treated with a selective iNOS inhibitor, the authors concluded that MMP-9 activity was induced by iNOS-derived NO and that this also led to detachment of hepatocytes from the ECM and cell death, in addition to increasing leukocyte migration through ECM barriers[46] (Table 3).
Fibronectin (FN) is involved in leukocyte adhesion, migration and activation. Amersi et al[47] reported that blocking the interaction between FN and the integrin α4β1, the integrin receptors expressed on leukocytes, led to improved liver function in steatotic liver transplantation. Based on this evidence, they demonstrated that this is linked to a reduction in MMP-9 expression/activation on leukocytes of steatotic liver grafts[29]. MMP-9 expression during hepatic I/R was shown to be associated with massive leucocyte infiltration, extensive FN deposition and proinflammatory release, thus emerging as an important mediator of leukocyte traffic in liver I/R injury[48] (Table 3).
All this shows that numerous and rather complex mechanisms affect MMP modulation: for a list of endogenous compounds involved in MMP regulation see Table 3.
Biliary obstruction leads to a cholestatic inflammatory and fibrogenic process. Current evidence indicates that MMPs are of central importance for cholestatis-induced fibrosis but only limited evidence is currently available on their precise cellular origin and regulation within the damaged liver. Some authors have shown that marked alterations in the expression of MMPs and their inhibitors take place within the first week after BDL[49,50]. Specifically, they found that the proteolytic activities of MMP-2 and MMP-9 increased 2 d after BDL, peaked at day 10, and remained high throughout the study period[49]. The increase in gelatinase activities was accompanied by an increase in TIMP mRNA transcripts while no corresponding increase in TIMP protein activity was detected. This appears to arise from the formation of TIMP/MMP complexes. These findings suggest that complex changes in the local MMP/TIMP balance may underlie the pathological mechanisms of BDL fibrosis.
More recent publications support the view that analysis of the MMP activation not just 1-2 wk after BDL but even a few days after occlusion has a crucial role to play[51]. Ferrigno et al[50] have reported a marked alteration in gelatinase activity after BDL showing that this increase takes place in the first few days after BDL mainly in the right lobe. They also observed an increase in MMP-2 and MMP-9 that occurs significantly in the right lobe, more than in the median lobe and left lobe.
Although liver fibrosis has long been considered irreversible, recent studies suggest potential reversibility of liver fibrosis once the pathological trigger is removed[52]. Studies in patients with chronic hepatitis successfully treated with antivirals suggest recover even in cirrhotic patients[53]. In experimental models, reversibility of liver fibrosis depends on the degree of pre-established fibrosis. In an experimental model of cholestasis-induced fibrosis, MMP activity was upregulated in bile duct ligated rats treated with IFNα-2a. Bile duct ligation, itself, promoted MMP activity in both liver tissue and NPCs (non parenchymal cells) isolated from the same tissue[54].
In an elegant study, Popov et al[55] have shown that macrophages upregulate MMPs and become fibrolytic effector cells on apoptotic cholangiocyte engulfment in vitro, suggesting that phagocytosis-associated MMP induction in macrophages contributes significantly to biliary fibrosis reversal. A relevant finding of this study is the description of the subset of MMPs differentially regulated at the peak of matrix remodeling and degradation. In their study, the study of expression patterns during biliary fibrosis reversal in vivo suggested that MMPs, with the exception of MMP-2, that have a profibrogenic role[56], and MMP-13, that could be involved in removal of the fibrotic matrix.
During cholestasis a marked increase in liver and serum bile acid levels occurs, leading to acute liver toxicity, bile duct cells proliferation, and fibrosis progressing to cirrhosis[57-59]. However, the molecular mechanisms of liver injury induced by obstructive cholestasis remain unclear. Previous research has suggested a predominant hypothesis: inflammatory cell-mediated liver necrosis, and not bile acid-induced apoptosis, may be directly involved in cholestatic liver damage[60]. However, a recent study[61] indicates that bile acid composition between humans and rodents is different and that mechanisms of cholestasis in humans are different from rodent models.
In humans, during obstructive cholestasis, bile leaking back into the parenchyma can cause direct bile acid-induced necrosis, which, through release of damage-associated molecular patterns can initiate an inflammatory response.
Neutrophil accumulation has been directly implicated in the pathogenesis of early cholestatic liver injury[62,63]. After obstruction of the bile duct, an intense increase in biliary ductal pressure is produced[64] and this is quickly followed by ECM changes[65].
The accumulation of toxic bile acids induces hepatocyte injury, in part by activating death receptors[66]. This event triggers a secondary phase in which infiltration of inflammatory cells, activation of Kupffer cells and transformation of stellate cells to activated myofibroblasts occur, along with a MMPs-induced remodeling of the ECM. This structural hepatic changes further promotes liver injury and enhances hepatocyte apoptosis[67].
An increase in myeloperoxidase activity[68] and the formation of intracellular chlorotyrosine adduct in hepatocytes[62,63] are associated with neutrophil accumulation after bile duct ligation. The neutrophil-derived hypochlorous acid can induce liver injury by intracellular oxidative stress[69], prevented by inhibition of NADPH oxidase that protects against neutrophil cytotoxicity[70,71]. Furthermore, Nox1 and Nox2, hepatic NADPH oxidases respectively located in hepatic stellate cells and Kupffer cells, participate to BDL-induced fibrosis[72,73], though their role to the early liver injury has not yet been defined. Yang et al[74] suggest that the neutrophil-mediated liver injury is induced by MMP-induced cleavage of osteopontin (OPN), acting as an early pro-inflammatory signal after BDL in mice. In the cleavage of OPN into its pro-inflammatory form, MMP-3 and MMP-7 have a prominent role[75]. Yang et al[74] also reported that BDL induces MMP-3 early in the liver and, in addition, MMP-2, -3 and -9 activities increase in bile. Thus, probably, MMP-3 and other MMPs released into bile, activate OPN as potent chemoattractant for neutrophils. It is well known that MMPs are also involved in the modulation of cytokine and chemokine activity. MMPs can both generate chemotactic gradients by activating chemokines and cytokines, and inactivate these pro-inflammatory mediators[76]. The obstruction of the bile duct, induces an increase in biliary duct pressure, injuring the biliary epithelial cells. OPN and MMPs are released into bile and MMPs activates OPN, producing the factors attracting neutrophils. The high pressure in the biliary system occurring in BDL, provokes ruptures in the Canals of Hering. This process results in infiltration of bile into the parenchyma[77] and is facilitated by the expression on hepatocytes of intercellular adhesion molecule-1 (ICAM-1), induced by bile acids (BAs) into the parenchyma.
The development of biliary complications after liver transplantation is a major clinical problem, due to its relatively high frequency, complications, morbidity and even mortality. The formation of strictures in the liver bile ducts is accompanied by tissue remodeling in which MMP-2 and MMP-9 are considered to play a key role in connective tissue remodeling processes in the liver. The mechanisms by which ischemia/reperfusion (I/R) lead to liver injury are complex and multifactorial; these events also involve profound changes in MMP expression[24]. Based on the above considerations, further evaluation of a possible link between MMP-2 and 9 gene polymorphisms and non-anastomotic biliary strictures after liver transplantation might help explain MMP involvement[78]. Ten Hove et al[78] have shown that MMP-2 polymorphism is significantly associated with biliary strictures: genetically determined reduced MMP-2 tissue remodeling contributes to the development of biliary complications.
Reperfusion of liver grafts after cold preservation is associated with diminished bile production both in clinical liver transplantation and experimental models. Indeed, biliary complications represent a major surgical problem with an incidence of up to 30% after liver transplantation[79-81]. Cholangiocytes play a substantial role in the damage caused by preservation in hypothermic conditions: compared to hepatocytes and Kupffer cells, they are particularly susceptible to injury, and, in particular, to injury induced by cold hypoxia[82]. Hence, biliary strictures that occur after transplantation often require endoscopic, radiological and surgical procedures[83-85] designed to avoid graft dysfunction and/or re-transplantation.
Post-transplant biliary complications are usually classified into two types: (1) anastomotic strictures and (2) non-anastomotic strictures. Anastomotic strictures of the biliary tree are located where bile duct anastomosis occurred and are generally well treated by stent placement[86]. Their incidence is between 5% and 10%[82]. Non-anastomotic strictures may be either extrahepatic (Type I) or intrahepatic (Type II). Arising from hepatic artery thrombosis, stenosis or ischemic cholangiopathy, they account for 10%-25% of stricture complications after liver transplantation[82]. In addition, ischemic cholangiopathy seems to be associated with prolonged periods of cold ischemic storage, delayed arterization of the graft or transplant from a donor after cardiac death (DCD) indicating that I/R injuries play a key pathogenetic role[82].
Clinical evaluation of biliary complications after liver transplantation has shown that a storage time of over 10-12 h leads to biliary strictures and other complications in more than 25% of liver transplant recipients[87]: a retrospective review of liver transplant patients demonstrated that liver grafts procured from DCDs showed a higher re-transplantation rate due to ischemic tract biliary lesions combined with severe intrahepatic cholestasis[88]. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation published in 2014 confirmed and extended the finding that an increase in biliary complications, graft loss and mortality occurs with DCD liver transplantation[89]. Nevertheless the use of these organs needs to be balanced against the risk of recipients dying while on the waiting list[89].
Data from humans and experimental models supports the view that MMPs play a crucial role as modulators of tissue development, remodeling and repair in response to infection, disease of injury. Currently, it has been evaluating whether MMPs merely have a structural role in matrix remodeling, or they also have a role in regulating access to signaling molecules. One of the most important findings in MMP biology has been the realization that extracellular proteolysis is not only a mechanism that destroys structure or information. Instead, various studies have demonstrated that MMPs can release growth factors from the ECM and cell surfaces, activating latent proteins and generating new bioactive molecules through proteolysis.
Reperfusion damage is dependent on the degree of injury in previous phases and involves complex mechanisms and mediators that are not as yet completely understood.
Changes in extracellular MMP activities already occur in the early phases of reperfusion and are coupled with morphological changes to hepatic tissue, the biliary tree included. Significantly, as recent data have clarified, the multifactorial mechanisms of MMP modulation are associated to a possible dual role for MMPs during I/R injury; hence, only a detailed time-course evaluation of events occurring during reperfusion will provide specific indications for appropriate pharmacological treatments.
We thank Professor Anthony Baldry for revising the English and Mrs. Nicoletta Breda for editing assistance.
P- Reviewer: Goshayeshi L, Kamimura K, LoomisT, Marin JJG, Richter B S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1199] [Cited by in F6Publishing: 1220] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3381] [Cited by in F6Publishing: 3806] [Article Influence: 200.3] [Reference Citation Analysis (3)] |
| 3. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 385] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 4. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1567] [Cited by in F6Publishing: 1567] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 5. | Calabro SR, Maczurek AE, Morgan AJ, Tu T, Wen VW, Yee C, Mridha A, Lee M, d’Avigdor W, Locarnini SA. Hepatocyte produced matrix metalloproteinases are regulated by CD147 in liver fibrogenesis. PLoS One. 2014;9:e90571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443-446. [PubMed] [Cited in This Article: ] |
| 7. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] [Cited in This Article: ] |
| 8. | Zhen EY, Brittain IJ, Laska DA, Mitchell PG, Sumer EU, Karsdal MA, Duffin KL. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58:2420-2431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Muroski ME, Roycik MD, Newcomer RG, Van den Steen PE, Opdenakker G, Monroe HR, Sahab ZJ, Sang QX. Matrix metalloproteinase-9/gelatinase B is a putative therapeutic target of chronic obstructive pulmonary disease and multiple sclerosis. Curr Pharm Biotechnol. 2008;9:34-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Sasaki M, Kashima M, Ito T, Watanabe A, Izumiyama N, Sano M, Kagaya M, Shioya T, Miura M. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1beta and TNF-alpha. Mediators Inflamm. 2000;9:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Morgia G, Falsaperla M, Malaponte G, Madonia M, Indelicato M, Travali S, Mazzarino MC. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urol Res. 2005;33:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 13. | Consolo M, Amoroso A, Spandidos DA, Mazzarino MC. Matrix metalloproteinases and their inhibitors as markers of inflammation and fibrosis in chronic liver disease (Review). Int J Mol Med. 2009;24:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Iyer S, Wei S, Brew K, Acharya KR. Crystal structure of the catalytic domain of matrix metalloproteinase-1 in complex with the inhibitory domain of tissue inhibitor of metalloproteinase-1. J Biol Chem. 2007;282:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Hamze AB, Wei S, Bahudhanapati H, Kota S, Acharya KR, Brew K. Constraining specificity in the N-domain of tissue inhibitor of metalloproteinases-1; gelatinase-selective inhibitors. Protein Sci. 2007;16:1905-1913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Bernardo MM, Fridman R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem J. 2003;374:739-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Giraudi PJ, Becerra VJ, Marin V, Chavez-Tapia NC, Tiribelli C, Rosso N. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp Mol Pathol. 2015;98:85-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2182] [Article Influence: 121.2] [Reference Citation Analysis (1)] |
| 19. | Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Z Gastroenterol. 2007;45:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Osawa Y, Seki E, Adachi M, Taura K, Kodama Y, Siegmund SV, Schwabe RF, Brenner DA. Systemic mediators induce fibrogenic effects in normal liver after partial bile duct ligation. Liver Int. 2006;26:1138-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Viappiani S, Sariahmetoglu M, Schulz R. The role of matrix metalloproteinase inhibitors in ischemia-reperfusion injury in the liver. Curr Pharm Des. 2006;12:2923-2934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47:186-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Cursio R, Mari B, Louis K, Rostagno P, Saint-Paul MC, Giudicelli J, Bottero V, Anglard P, Yiotakis A, Dive V. Rat liver injury after normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. FASEB J. 2002;16:93-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Khandoga A, Kessler JS, Hanschen M, Khandoga AG, Burggraf D, Reichel C, Hamann GF, Enders G, Krombach F. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol. 2006;79:1295-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Upadhya GA, Strasberg SM. Glutathione, lactobionate, and histidine: cryptic inhibitors of matrix metalloproteinases contained in University of Wisconsin and histidine/tryptophan/ketoglutarate liver preservation solutions. Hepatology. 2000;31:1115-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Defamie V, Laurens M, Patrono D, Devel L, Brault A, Saint-Paul MC, Yiotakis A, Barbry P, Gugenheim J, Crenesse D. Matrix metalloproteinase inhibition protects rat livers from prolonged cold ischemia-warm reperfusion injury. Hepatology. 2008;47:177-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am J Pathol. 2007;170:567-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Kuyvenhoven JP, Ringers J, Verspaget HW, Lamers CB, van Hoek B. Serum matrix metalloproteinase MMP-2 and MMP-9 in the late phase of ischemia and reperfusion injury in human orthotopic liver transplantation. Transplant Proc. 2003;35:2967-2969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Kuyvenhoven JP, Verspaget HW, Gao Q, Ringers J, Smit VT, Lamers CB, van Hoek B. Assessment of serum matrix metalloproteinases MMP-2 and MMP-9 after human liver transplantation: increased serum MMP-9 level in acute rejection. Transplantation. 2004;77:1646-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Shirahane K, Yamaguchi K, Koga K, Watanabe M, Kuroki S, Tanaka M. Hepatic ischemia/reperfusion injury is prevented by a novel matrix metalloproteinase inhibitor, ONO-4817. Surgery. 2006;139:653-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Ma ZY, Qian JM, Rui XH, Wang FR, Wang QW, Cui YY, Peng ZH. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transplant. 2010;10:784-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 36. | Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, Ramadori G. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999;154:153-167. [PubMed] [Cited in This Article: ] |
| 38. | Ji J. Dual role of matrix metalloprotease 9 in liver ischemia and reperfusion injury. J Surg Res. 2013;185:545-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Feng M, Wang H, Wang Q, Guan W. Matrix metalloprotease 9 promotes liver recovery from ischemia and reperfusion injury. J Surg Res. 2013;180:156-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Chen CF, Wang D, Hwang CP, Liu HW, Wei J, Lee RP, Chen HI. The protective effect of niacinamide on ischemia-reperfusion-induced liver injury. J Biomed Sci. 2001;8:446-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Hato S, Urakami A, Yamano T, Uemura T, Ota T, Hirai R, Shimizu N. Attenuation of liver and lung injury after hepatic ischemia and reperfusion by a cytokine-suppressive agent, FR167653. Eur Surg Res. 2001;33:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443-453. [PubMed] [Cited in This Article: ] |
| 43. | Palladini G, Ferrigno A, Rizzo V, Boncompagni E, Richelmi P, Freitas I, Perlini S, Vairetti M. Lobe-specific heterogeneity and matrix metalloproteinase activation after ischemia/reperfusion injury in rat livers. Toxicol Pathol. 2012;40:722-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Palladini G, Ferrigno A, Rizzo V, Tarantola E, Bertone V, Freitas I, Perlini S, Richelmi P, Vairetti M. Lung matrix metalloproteinase activation following partial hepatic ischemia/reperfusion injury in rats. ScientificWorldJournal. 2014;2014:867548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Tiriveedhi V, Upadhya GA, Busch RA, Gunter KL, Dines JN, Knolhoff BL, Jia J, Sarma NJ, Ramachandran S, Anderson CD. Protective role of bortezomib in steatotic liver ischemia/reperfusion injury through abrogation of MMP activation and YKL-40 expression. Transpl Immunol. 2014;30:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Hamada T, Duarte S, Tsuchihashi S, Busuttil RW, Coito AJ. Inducible nitric oxide synthase deficiency impairs matrix metalloproteinase-9 activity and disrupts leukocyte migration in hepatic ischemia/reperfusion injury. Am J Pathol. 2009;174:2265-2277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Amersi F, Shen XD, Moore C, Melinek J, Busuttil RW, Kupiec-Weglinski JW, Coito AJ. Fibronectin-alpha 4 beta 1 integrin-mediated blockade protects genetically fat Zucker rat livers from ischemia/reperfusion injury. Am J Pathol. 2003;162:1229-1239. [PubMed] [Cited in This Article: ] |
| 48. | Coito AJ. Leukocyte transmigration across endothelial and extracellular matrix protein barriers in liver ischemia/reperfusion injury. Curr Opin Organ Transplant. 2011;16:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895-1902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Ferrigno A, Palladini G, Bianchi A, Rizzo V, Di Pasqua LG, Perlini S, Richelmi P, Vairetti M. Lobe-specific heterogeneity in asymmetric dimethylarginine and matrix metalloproteinase levels in a rat model of obstructive cholestasis. Biomed Res Int. 2014;2014:327537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Weerachayaphorn J, Luo Y, Mennone A, Soroka CJ, Harry K, Boyer JL. Deleterious effect of oltipraz on extrahepatic cholestasis in bile duct-ligated mice. J Hepatol. 2014;60:160-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Ramachandran P, Iredale JP. Liver fibrosis: a bidirectional model of fibrogenesis and resolution. QJM. 2012;105:813-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1428] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 54. | Bueno MR, Daneri A, Armendáriz-Borunda J. Cholestasis-induced fibrosis is reduced by interferon alpha-2a and is associated with elevated liver metalloprotease activity. J Hepatol. 2000;33:915-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Popov Y, Sverdlov DY, Bhaskar KR, Sharma AK, Millonig G, Patsenker E, Krahenbuhl S, Krahenbuhl L, Schuppan D. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G323-G334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 57. | Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 58. | Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 59. | Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 469] [Cited by in F6Publishing: 461] [Article Influence: 30.7] [Reference Citation Analysis (3)] |
| 60. | Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18:4985-4993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 148] [Cited by in F6Publishing: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 61. | Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, Kumer SC, Schmitt TM, Forster J, Fan F. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283:168-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 62. | Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 63. | Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499-G507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Lab Invest. 2004;84:153-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Häussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology. 2002;36:829-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | Kahraman A, Bronk SF, Cazanave S, Werneburg NW, Mott JL, Contreras PC, Gores GJ. Matrix metalloproteinase inhibitor, CTS-1027, attenuates liver injury and fibrosis in the bile duct-ligated mouse. Hepatol Res. 2009;39:805-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Demirbilek S, Tas E, Gurunluoglu K, Akin M, Aksoy RT, Emre MH, Aydin NE, Ay S, Ozatay N. Fluvastatin reduced liver injury in rat model of extrahepatic cholestasis. Pediatr Surg Int. 2007;23:155-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083-G1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 70. | Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760-G767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 71. | Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;287:G243-G252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, Kisseleva T, Brenner DA. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 73. | Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, Zhu K, Katsuyama M, Torok NJ, Yabe-Nishimura C. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011;54:949-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224:186-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem. 2001;276:28261-28267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 76. | Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 77. | Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Ten Hove WR, Korkmaz KS, op den Dries S, de Rooij BJ, van Hoek B, Porte RJ, van der Reijden JJ, Coenraad MJ, Dubbeld J, Hommes DW. Matrix metalloproteinase 2 genotype is associated with nonanastomotic biliary strictures after orthotopic liver transplantation. Liver Int. 2011;31:1110-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant. 2012;2012:164329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 81. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 82. | Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. 2014;20:6159-6169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 84] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 83. | Croome KP, McAlister V, Adams P, Marotta P, Wall W, Hernandez-Alejandro R. Endoscopic management of biliary complications following liver transplantation after donation from cardiac death donors. Can J Gastroenterol. 2012;26:607-610. [PubMed] [Cited in This Article: ] |
| 84. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Park JS, Kim MH, Lee SK, Seo DW, Lee SS, Han J, Min YI, Hwang S, Park KM, Lee YJ. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 86. | Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 87. | Ben-Ari Z, Pappo O, Mor E. Intrahepatic cholestasis after liver transplantation. Liver Transpl. 2003;9:1005-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Kaczmarek B, Manas MD, Jaques BC, Talbot D. Ischemic cholangiopathy after liver transplantation from controlled non-heart-beating donors-a single-center experience. Transplant Proc. 2007;39:2793-2795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | O’Neill S, Roebuck A, Khoo E, Wigmore SJ, Harrison EM. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transpl Int. 2014;27:1159-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 90. | Kuo KK, Wu BN, Chiu EY, Tseng CJ, Yeh JL, Liu CP, Chai CY, Chen IJ. NO donor KMUP-1 improves hepatic ischemia-reperfusion and hypoxic cell injury by inhibiting oxidative stress and pro-inflammatory signaling. Int J Immunopathol Pharmacol. 2013;26:93-106. [PubMed] [Cited in This Article: ] |
| 91. | Duarte S, Shen XD, Fondevila C, Busuttil RW, Coito AJ. Fibronectin-α4β1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am J Transplant. 2012;12:2689-2699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Fondevila C, Shen XD, Duarte S, Busuttil RW, Coito AJ. Cytoprotective effects of a cyclic RGD peptide in steatotic liver cold ischemia and reperfusion injury. Am J Transplant. 2009;9:2240-2250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Chen CF, Leu FJ, Chen HI, Wang D. Oxygen radicals and matrix metalloproteinases mediate reperfusion liver injury. Transplant Proc. 2005;37:4547-4549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Yang H, Majno P, Morel P, Toso C, Triponez F, Oberholzer J, Mentha G, Lou J. Prostaglandin E(1) protects human liver sinusoidal endothelial cell from apoptosis induced by hypoxia reoxygenation. Microvasc Res. 2002;64:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Fondevila C, Shen XD, Moore C, Busuttil RW, Coito AJ. Cyclic RGD peptides with high affinity for alpha5beta1 integrin protect genetically fat Zucker rat livers from cold ischemia/reperfusion injury. Transplant Proc. 2005;37:1679-1681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Kuriyama N, Duarte S, Hamada T, Busuttil RW, Coito AJ. Tenascin-C: a novel mediator of hepatic ischemia and reperfusion injury. Hepatology. 2011;54:2125-2136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Martínez-Rizo A, Bueno-Topete M, González-Cuevas J, Armendáriz-Borunda J. Plasmin plays a key role in the regulation of profibrogenic molecules in hepatic stellate cells. Liver Int. 2010;30:298-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Aldaba-Muruato LR, Moreno MG, Hernández-Mercado E, Shibayama M, Muriel P. Secondary biliary cirrhosis in the rat is prevented by decreasing NF-κB nuclear translocation and TGF-β expression using allopurinol, an inhibitor of xanthine oxidase. Can J Physiol Pharmacol. 2012;90:1469-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 99. | Feng M, Wang Q, Zhang F, Lu L. Ex vivo induced regulatory T cells regulate inflammatory response of Kupffer cells by TGF-beta and attenuate liver ischemia reperfusion injury. Int Immunopharmacol. 2012;12:189-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |