Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.593
Peer-review started: April 24, 2014
First decision: August 6, 2014
Revised: August 21, 2014
Accepted: September 29, 2014
Article in press: September 29, 2014
Published online: January 14, 2015
AIM: To evaluate the new RetroView™ colonoscope and compare its ability to detect simulated polyps “hidden” behind colonic folds with that of a conventional colonoscope, utilizing anatomic colon models.
METHODS: Three anatomic colon models were prepared, with twelve simulated polyps “hidden” behind haustral folds and five placed in easily viewed locations in each model. Five blinded endoscopists examined two colon models in random order with the conventional or RetroView™ colonoscope, utilizing standard withdrawal technique. The third colon model was then examined with the RetroView™ colonoscope withdrawn initially in retroflexion and then in standard withdrawal. Polyp detection rates during standard and retroflexed withdrawal of the conventional and RetroView™ colonoscopes were determined. Polyp detection rates for combined standard and retroflexed withdrawal (combination withdrawal) with the RetroView™ colonoscope were also determined.
RESULTS: For hidden polyps, retroflexed withdrawal using the RetroView™ colonoscope detected more polyps than the conventional colonoscope in standard withdrawal (85% vs 12%, P = 0.0001). For hidden polyps, combination withdrawal with the RetroView™ colonoscope detected more polyps than the conventional colonoscope in standard withdrawal (93% vs 12%, P≤ 0.0001). The RetroView™ colonoscope in “combination withdrawal” was superior to other methods in detecting all (hidden + easily visible) polyps, with successful detection of 80 of 85 polyps (94%) compared to 28 (32%) polyps detected by the conventional colonoscope in standard withdrawal (P < 0.0001) and 67 (79%) polyps detected by the RetroView™ colonoscope in retroflexed withdrawal alone (P < 0.01). Continuous withdrawal of the colonoscope through the colon model while retroflexed was achieved by all endoscopists. In a post-test survey, four out of five colonoscopists reported that manipulation of the colonoscope was easy or very easy.
CONCLUSION: In simulated testing, the RetroView™ colonoscope increased detection of hidden polyps. Combining standard withdrawal with retroflexed withdrawal may become the new paradigm for “complete screening colonoscopy”.
Core tip: Polyps located on the proximal side of colon folds can be challenging to detect. The new RetroView™ colonoscope has a short turning radius that allows a retroflexed view of the colon during withdrawal. In this bench colon model study, the RetroView™ colonoscope detected more proximally-located, “hidden” polyps during retroflexed withdrawal, than a conventional colonoscope withdrawn in standard fashion. The highest polyp detection rate was achieved when the RetroView™ colonoscope was withdrawn in retroflexion followed by standard withdrawal. This combination of standard and retroflexed withdrawal holds promise for optimizing polyp detection in patients undergoing screening colonoscopy.
- Citation: McGill SK, Kothari S, Friedland S, Chen A, Park WG, Banerjee S. Short turn radius colonoscope in an anatomical model: Retroflexed withdrawal and detection of hidden polyps. World J Gastroenterol 2015; 21(2): 593-599
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/593.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.593
Colorectal cancer is the second most common cause of cancer related mortality in the United States with over 50000 deaths reported annually[1]. Colonoscopy is widely considered the optimal screening modality for colorectal cancer[2], and has been widely adopted for this purpose since Medicare coverage for screening colonoscopy was initiated in 2001. However, interval colorectal cancers following colonoscopy do occur, indicating that colonoscopy offers incomplete protection, particularly in the right colon[3-7]. Interval cancers may arise as a consequence of differential tumor biology, incompletely resected polyps or polyps that are entirely missed at colonoscopy. That polyps are missed at colonoscopy has long been evident. Three tandem colonoscopy studies performed over the last two decades have indicated that colonoscopy is associated with a significant polyp miss rate, with around 6%-27% of adenomas missed at colonoscopy, with the higher miss rates noted for smaller polyps[8-10].
Multiple factors may contribute to polyps being missed at colonoscopy, including suboptimal bowel preparation, inadequate colonic distension, unrecognized flat polyps, and inadequate endoscopy technique, particularly rapid colonoscope withdrawal. A significant additional factor is that polyps located on the proximal aspect of colonic haustral folds, flexures and valves may be missed due to the difficulties in visualizing these areas using conventional colonoscopes and standard withdrawal techniques[11,12]. When used in conjunction with standard withdrawal, retroflexion of a conventional colonoscope in the right colon with withdrawal in retroflexion up to the hepatic flexure (allowing visualization of the proximal aspect of colonic folds), was shown to detect additional polyps in 5.8% of patients undergoing screening or surveillance colonoscopy[13].
Colonoscope technology has evolved mainly on the optical front, with incorporation of high definition imaging, wide angled lenses and electronic chromoendoscopy such as narrow band imaging (NBI, Olympus America, Center Valley, PA), i-SCAN™ (PENTAX of America, Montvale, NJ) and Fuji Intelligent Chromo-Endoscopy (FICE™, Fujinon Endoscopy, Wayne, NJ). However, there has been no significant evolution of the mechanical ability of colonoscopes over the last two decades and visualizing the proximal aspects of folds, flexures and valves remains a challenge. Although use of a disposable retrograde viewing device advanced via the accessory channel of a standard colonoscope was shown to increase adenoma detection[14-17], this device has not been widely adopted due to technical, cost and payer issues.
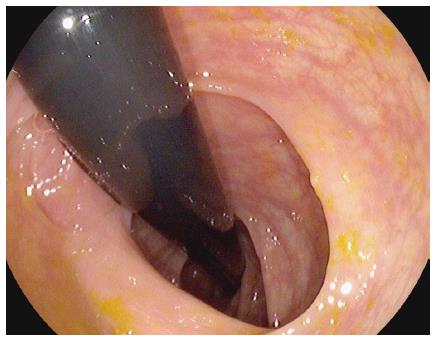
The PENTAX EC-3490TLi RetroView™ is a new slim colonoscope with a short turn radius (STR) at the colonoscope’s tip, allowing easy retroflexion in the right colon or transverse colon (Video 1). In addition, it has a relatively narrow retroflexed profile, which potentially allows complete withdrawal in full retroflexion from the cecum to the rectum in many patients (Figure 1). This may allow for detection of polyps hidden behind flexures, folds and valves, which may not be seen during a standard “forward viewing” withdrawal.
Our objective was to compare the ability of the PENTAX RetroView™ colonoscope with that of a conventional slim colonoscope in detecting simulated hidden polyps in an anatomic colonic model, particularly those situated behind flexures and folds. In this study, the polyp detection rate of the RetroView™ colonoscope on retroflexed withdrawal, standard withdrawal and “combination” withdrawal (retroflexed + standard withdrawal) was compared to that of the conventional colonoscope on standard withdrawal.
Three identical, realistic colon models constructed of silicone (DeLegge Medical, Mt. Pleasant, SC) incorporating anatomically correct haustral folds and flexures, with a colonic length of 127 cm were utilized for this study (Figure 2).
Simulated polyps comprised of beads of various colors measuring 4 mm wide and 3 mm high, held in place by metal pins. Seventeen polyps were placed in each of the three colon models; 12 (79%) were positioned on the proximal aspects of folds or flexures and 5 (21%) were positioned in “obvious” locations, where they would be expected to be seen on standard withdrawal. A “perfect score” would occur if the five endoscopists identified all 17 polyps in the colon model, for a total of 85 polyps. The location of bead placement, the order of colors and number of beads of each color were different for each model, to avoid a learning effect as the endoscopists evaluated the models sequentially.
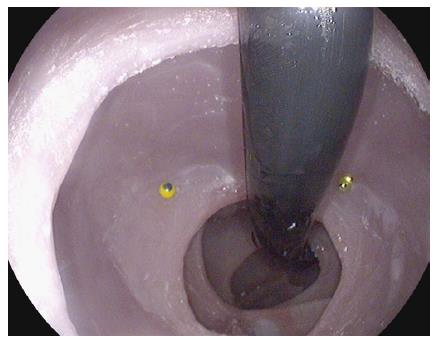
Two colonoscopes were used in this study: a conventional slim colonoscope (EC 3490K, PENTAX, Montvale, NJ) and the RetroView™ colonoscope (EC-3490TLi, PENTAX, Montvale, NJ). The RetroView™ colonoscope is visually identical to a conventional slim colonoscope. It is however unique in that it has a short turning radius and in the fully retroflexed position, the maximal width of the bending section (i.e., distance including main scope shaft and retroflexed shaft) is only 40 mm (Figure 3).
Five endoscopists with varying levels of experience participated in the study. A technical team prepared each model by advancing the selected colonoscopes to the cecum. The conventional colonoscope was advanced to the cecum in one model and the RetroView™ colonoscope was advanced to the cecum in two of the models. Each endoscopist first examined two models sequentially in random order with either the conventional colonoscope or the RetroView™ colonoscope utilizing standard withdrawal technique. They were blinded as to the type of colonoscope being used for these two colon models. They were asked to describe the unique color of each simulated polyp seen. Endoscopists were requested to limit their withdrawal time to 6 min.
The third model was then examined with the RetroView™ colonoscope initially in complete retroflexion by the endoscopist, and the number of simulated polyps detected was noted. This was followed by a standard withdrawal using the same colonoscope. Blinding to the colonoscope used for the third model was not possible, given the RetroView™ colonoscope’s unique ability to retroflex easily and need to be withdrawn in retroflexion for this study. The total number of polyps found by standard withdrawal and retroflexed withdrawal using the RetroView™ colonoscope were summed to determine the polyp detection rate with a “combination withdrawal”. The RetroView™ colonoscope’s ability to be withdrawn in complete retroflexion by each endoscopist all the way from the cecum to rectum in these models was noted.
Following the examination, endoscopists filled out a “post-test” questionnaire that asked about the overall ease of use of the RetroView™ colonoscope, the ease of manipulating and withdrawing the colonoscope in retroflexion and the ease of re-orientating to retroflexed views during colonoscope withdrawal. Optional responses to these questions were: very easy, easy, somewhat difficult, difficult or very difficult. At the time of this study, the RetroView™ colonoscope was not commercially available and the endoscopists were also were asked whether they would consider performing additional routine retroflexed withdrawal at colonoscopy when the colonoscope became commercially available.
Statistical comparisons were performed with use of the Cochran-Mantel-Haenszel test, stratifying the data by endoscopist.
The results were summed among the five endoscopists, and are shown in Table 1.
| A | B | C | D | P values | |
| Conventional colonoscope, Standard Withdrawal | RetroView™ colonoscope, Standard Withdrawal | RetroView™ colonoscope, Retroflexed Withdrawal | RetroView™ colonoscope, Combination Withdrawal (Standard + Retroflexed) | ||
| Hidden polyps | 7 (12) | 11 (18) | 51 (85) | 56 (93) | A vs B, P = 0.5 |
| (n = 60) | A vs C, P < 0.0001 | ||||
| A vs D, P < 0.0001 | |||||
| Obvious polyps | 21 (84) | 19 (76) | 16 (64) | 24 (96) | A vs B, P = 0.4 |
| (n =25) | A vs C, P = 0.7 | ||||
| A vs D, P < 0.0001 | |||||
| C vs D, P = 0.01 | |||||
| All polyps | 28 (32) | 30 (35) | 67 (79) | 80 (94) | A vs C, P < 0.001 |
| (n =85) | A vs D, P < 0.0001 | ||||
| C vs D, P < 0.01 |
The RetroView™ colonoscope on retroflexed withdrawal detected more hidden polyps located on the proximal aspects of folds compared to the conventional colonoscope on standard withdrawal, finding 51 of 60 (85%) such polyps, compared to just seven (12%) detected by the conventional colonoscope on standard withdrawal (P < 0.0001). Combination withdrawal yielded the highest detection rate, finding 56 (93%) of hidden polyps (P < 0.0001 vs conventional colonoscope). The RetroView™ colonoscope and conventional colonoscope detected similar numbers of polyps on standard withdrawal, 11 (18%) vs 7 (12%), (P = 0.5).
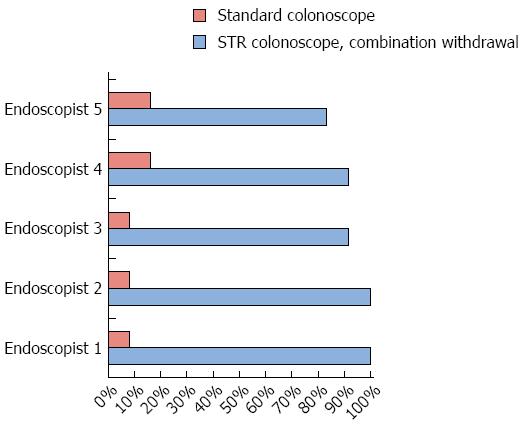
Endoscopists’ individual detection rates for hidden polyps ranged from 8%-17% using the conventional colonoscope, from 83%-92% using the RetroView™ colonoscope in retroflexion, and from 83%-100% for the RetroView™ colonoscope using combination withdrawal (Figure 4).
The RetroView™ colonoscope had a similar detection rate for obvious polyps in standard withdrawal as the conventional colonoscope in standard withdrawal, finding 19 polyps of the total 25 (76%) vs 21 polyps (84%) detected by the conventional colonoscope (P = 0.5). Combination withdrawal with the RetroView™ colonoscope found similar numbers of obvious polyps as the conventional colonoscope, finding 24 (96%) of such polyps (P = 0.36). The RetroView™ colonoscope had a lower detection rate for obvious polyps when retroflexed than when used in combination withdrawal-16 (64%) vs 24 (96%) (P = 0.01).
The RetroView™ colonoscope in “combination withdrawal” was superior to other methods in detecting all (hidden + easily visible) polyps, with successful detection of 80 of 85 polyps (94%) compared to 28 (32%) polyps detected by the conventional colonoscope in standard withdrawal (P < 0.0001) and 67 (79%) polyps detected by the RetroView™ colonoscope in retroflexed withdrawal alone (P < 0.01).
Ability to retroflex and withdrawal time: Complete retroflexed withdrawal with the RetroView™ colonoscope in the realistic anatomical colon model was achieved by all endosocopists. Average withdrawal time with the conventional colonoscope was 4 min 30 s. For the RetroView™ colonoscope, average withdrawal time was 4 min 24 s for standard withdrawal, 3 min 50 s for retroflexed withdrawal and 8 min and 8 s for combination withdrawal.
There was no individual polyp that was never detected by all of the examiners, indicating that misses were not the result of inadequate visualization.
Post-test questionnaire: On the post-test questionnaire, all participants indicated that overall, the RetroView™ colonoscope was either easy or very easy to use. Four endoscopists described manipulation of the RetroView™ colonoscope during retroflexed withdrawal as easy or very easy, while one described this as difficult. All endoscopists indicated that they would perform additional routine retroflexed withdrawal at colonoscopy when the RetroView™ colonoscope became commercially available.
Our study indicates that the RetroView™ colonoscope, in simulated testing using retroflexed or combination withdrawal, significantly improves the detection of “hidden” polyps located on the proximal aspect of colonic folds, compared to standard withdrawal using a conventional colonoscope. The highest detection rates for all polyps, both those that were hidden and placed in obvious locations, were achieved with combination withdrawal of the RetroView™ colonoscope.
Missed adenomas may lead to interval colon cancers and diminish the efficacy of colonoscopy[7], and prior studies suggest that the proximal aspects of colonic folds, flexures and valves are a common site for missed polyps[11,12]. An early study that appraised polyps that were identified by barium enema but missed at endoscopy demonstrated that missed lesions had a tendency to be located on the proximal aspects of haustral folds and valves[11]. More recently, Pickhardt et al[12] also demonstrated that 71.4% of non-rectal adenomas ≥ 6 mm in size missed at colonoscopy but detected at CT colonography, were located on the proximal side of colonic folds.
Thus visualization of the proximal aspects of colonic folds is desirable. Indeed, retroflexion of standard colonoscopes in the right colon was shown to increase the adenoma yield in a large study[13]. Potentially, this additional yield of “missed polyps” might be higher if the entire colon could be viewed in retroflexed withdrawal, in addition to the standard forward viewing withdrawal. However, with standard colonoscopes, retroflexed withdrawal is typically only possible in the right colon, due to their larger turn radius and width of the bending section when retroflexed. There has been no significant evolution in the mechanical ability of colonoscopes over the last two decades to address this issue. As a consequence, other techniques and technologies have emerged to address this unmet need, with variable results.
Colonoscopes incorporating a 170 degree wide angled lens rather than the standard 140 degree lens were introduced with the hope of improving polyp detection. However, clinical studies indicate that this colonoscope did not improve adenoma detection[18,19] or miss rates[20] in trials but only increased the discovery of small hyperplastic polyps[18]. Translucent caps have been fitted to the tip of colonoscopes to assist with depressing haustral folds to potentially improve colonic visualization and polyp detection. Again, studies evaluating adenoma detection rates with cap fitted colonoscopy have yielded mixed results[21-26], and it is unclear if this technique is beneficial. Similarly studies have indicated that high definition colonoscopes did not improve adenoma detection rates compared to older standard definition colonoscopes[27,28]. Finally, several studies comparing NBI and FICE with white light colonoscopy did not show any increase in adenoma detection rates[29-34].
The largest increase in the detection of additional polyps, over those detected by standard colonoscopy, have been reported with the Third Eye Retroscope (Avantis Medical Systems, Sunnyvale, CA), an auxiliary viewing device which allows retrograde views behind colonic folds and flexures. The device is advanced through the accessory channel of a standard colonoscope and when used in conjunction with the colonoscope, allows the endoscopist simultaneous forward and retrograde facing views of the colon[14,16,17].
The Third Eye Retroscope increased adenoma detection rates by 11%-25%[14-17], but has failed to be widely adopted due to several cost and technical issues. Utilizing this technology requires the purchase of a separate processor and of a new disposable device for each colonoscopy procedure, the cost of which is not reimbursed by most payers. The device occupies the working channel of the colonoscope which limits the ability to suction. This necessitates washing and suctioning of the colon during the colonoscope insertion phase, in cases of suboptimal bowel preparation. If a polyp is seen on the proximal aspect of a colonic fold, the viewing device has to be removed in order that a polypectomy device may be advanced. This may result in loss of visualization of the hidden polyp. The optics of the device are standard rather than high definition and are further impaired by the glare consequent upon the two light sources and lens systems, of the device and colonoscope, that face each other. Finally, the endoscopist has to get used to visualizing and processing two simultaneous video streams from the colonoscope and from the retroscope device.
In contrast, the RetroView™ colonoscope that we tested offers many advantages. It offers the ability to provide high definition views of the proximal aspects of colonic folds, flexures and valves with no additional equipment or device costs. The image is high definition and the colonoscope also incorporates zoom and electronic chromoendoscopy (i-SCAN) abilities. The suction/work channel of the colonoscope is unimpaired and available for use and detected polyps remain in view while polypectomy devices are advanced. Polypectomy can be performed with the colonoscope in retroflexion, without losing views of the polyp[35]. The main “cost” of using the colonoscope in both standard and retroflexed withdrawal, is the additional time necessary for colonoscope reinsertion and retroflexed withdrawal, which will result in a longer overall procedure time.
The results showed that the RetroView™ colonoscope in retroflexion detected fewer obvious polyps than combination withdrawal. As a small portion of the colon is obscured by the shaft of the colonoscope in retroflexion, full visualization of the colonic mucosa requires continuous back and forth torque during withdrawal. Not all of the study endoscopists performed this maneuver. The reduced detection rate of obvious polyps in retroflexion may have reflected this fact.
There are limitations to the current study. The anatomic colon model is stiff and its folds may not be “ironed out” with the colonoscope like the folds of a human colon, possibly making the detection of hidden polyps more difficult than in real life situations.
In conclusion, the RetroView™ colonoscope allowed withdrawal in complete retroflexion over the entire length of the anatomic colon model. It increased detection of polyps that were hidden behind folds and flexures. Combining standard withdrawal with retroflexed withdrawal promises to increase polyp detection rates and may become the new paradigm for “complete screening colonoscopy”. Studies are currently underway at our institution evaluating the RetroView™ colonoscope in human subjects.
Colonoscopy cancer is widely considered the optimal screening modality for colorectal cancer. However, polyps may be missed at colonoscopy, and these missed polyps can, in turn, potentially evolve into cancer. Polyps located on the proximal aspect of colonic folds, flexures and valves may be particularly difficult to visualize with conventional colonoscopes and using standard colonoscope withdrawal techniques.
Colonoscope and device innovations have emerged to address the issue of missed polyps. The Retroview™ colonoscope has a short turn radius that allows for easier retroflexion, to better visualize polyps located behind folds and flexures. This complements other advances in colonoscope and device technologies that improve polyp detection.
Compared to conventional colonoscopes, the tip of the RetroView™ colonoscope has a short turning radius, allowing for a narrow retroflexed profile and the potential to withdraw the colonoscope in retroflexion across most or all of the colon, in addition to a standard forward looking withdrawal. This combination of standard forward looking and retroflexed withdrawals allows visualization of both sides of colonic folds and flexures and may therefore improve polyp detection at colonoscopy. In the first known study to evaluate this new colonoscope, the authors describe its performance in a colon model.
The study suggests that the RetroView™ colonoscope can enhance polyp detection and thus may potentially improve colon cancer prevention by colonoscopy.
A “retroflexed view” is one in which the colonoscope tip is turned 180 degrees in order to look backwards. This allows excellent visualization of the proximal aspect of colonic folds.
This study is a first step in evaluating the RetroView™ colonoscope and its potential to improve colon polyp detection in humans. The authors evaluated the new colonoscope in finding “hidden” simulated colon polyps in a colon model, and found its performance to be excellent. Use of the RetroView™ colonoscope, utilizing a combination of standard forward looking and retroflexed withdrawals, holds the potential to improve polyp detection in patients undergoing screening colonoscopy.
P- Reviewer: De Palma R, Roy PK S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8406] [Cited by in F6Publishing: 8901] [Article Influence: 741.8] [Reference Citation Analysis (0)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3107] [Cited by in F6Publishing: 3009] [Article Influence: 97.1] [Reference Citation Analysis (1)] |
| 3. | Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 911] [Cited by in F6Publishing: 991] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 4. | Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 5. | Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, Shike M, Lanza E, Schatzkin A. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012;61:1576-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1089] [Cited by in F6Publishing: 1020] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 9. | Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst. 1990;82:1769-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 251] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 11. | Miller RE, Lehman G. Polypoid colonic lesions undetected by endoscopy. Radiology. 1978;129:295-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 341] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Waye JD, Heigh RI, Fleischer DE, Leighton JA, Gurudu S, Aldrich LB, Li J, Ramrakhiani S, Edmundowicz SA, Early DS. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos). Gastrointest Endosc. 2010;71:551-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | DeMarco DC, Odstrcil E, Lara LF, Bass D, Herdman C, Kinney T, Gupta K, Wolf L, Dewar T, Deas TM. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Triadafilopoulos G, Watts HD, Higgins J, Van Dam J. A novel retrograde-viewing auxiliary imaging device (Third Eye Retroscope) improves the detection of simulated polyps in anatomic models of the colon. Gastrointest Endosc. 2007;65:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Rex DK, Chadalawada V, Helper DJ. Wide angle colonoscopy with a prototype instrument: impact on miss rates and efficiency as determined by back-to-back colonoscopies. Am J Gastroenterol. 2003;98:2000-2005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Fatima H, Rex DK, Rothstein R, Rahmani E, Nehme O, Dewitt J, Helper D, Toor A, Bensen S. Cecal insertion and withdrawal times with wide-angle versus standard colonoscopes: a randomized controlled trial. Clin Gastroenterol Hepatol. 2008;6:109-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Deenadayalu VP, Chadalawada V, Rex DK. 170 degrees wide-angle colonoscope: effect on efficiency and miss rates. Am J Gastroenterol. 2004;99:2138-2142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Mathus-Vliegen EM, Dees J, Tytgat KM, van Leerdam ME, Fockens P, Kuipers EJ, Dekker E. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Horiuchi A, Nakayama Y, Kato N, Ichise Y, Kajiyama M, Tanaka N. Hood-assisted colonoscopy is more effective in detection of colorectal adenomas than narrow-band imaging. Clin Gastroenterol Hepatol. 2010;8:379-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Tada M, Inoue H, Yabata E, Okabe S, Endo M. Feasibility of the transparent cap-fitted colonoscope for screening and mucosal resection. Dis Colon Rectum. 1997;40:618-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Matsushita M, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Efficacy of total colonoscopy with a transparent cap in comparison with colonoscopy without the cap. Endoscopy. 1998;30:444-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Kondo S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, Ogura K, Okamoto M, Yoshida H, Kawabe T. A randomized controlled trial evaluating the usefulness of a transparent hood attached to the tip of the colonoscope. Am J Gastroenterol. 2007;102:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Shida T, Katsuura Y, Teramoto O, Kaiho M, Takano S, Yoshidome H, Miyazaki M. Transparent hood attached to the colonoscope: does it really work for all types of colonoscopes? Surg Endosc. 2008;22:2654-2658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Tribonias G, Theodoropoulou A, Konstantinidis K, Vardas E, Karmiris K, Chroniaris N, Chlouverakis G, Paspatis GA. Comparison of standard vs high-definition, wide-angle colonoscopy for polyp detection: a randomized controlled trial. Colorectal Dis. 2010;12:e260-e266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Pellisé M, Fernández-Esparrach G, Cárdenas A, Sendino O, Ricart E, Vaquero E, Gimeno-García AZ, de Miguel CR, Zabalza M, Ginès A. Impact of wide-angle, high-definition endoscopy in the diagnosis of colorectal neoplasia: a randomized controlled trial. Gastroenterology. 2008;135:1062-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, Schröder A, Scheel M, Wiedenmann B, Rösch T. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology. 2009;136:410-416.e1; quiz 715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Aminalai A, Rösch T, Aschenbeck J, Mayr M, Drossel R, Schröder A, Scheel M, Treytnar D, Gauger U, Stange G. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J Gastroenterol. 2010;105:2383-2388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Chung SJ, Kim D, Song JH, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Rex DK, Khashab M. Colonoscopic polypectomy in retroflexion. Gastrointest Endosc. 2006;63:144-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |