Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15837
Revised: May 26, 2014
Accepted: June 26, 2014
Published online: November 14, 2014
AIM: To investigate the effect of Lactobacillus-containing commercially available probiotic formulations in Germany during antibiotic treatment with an analysis of cost-efficiency.
METHODS: In an observational study, we analyzed the frequency of bowel movements from 258 patients with infections in a primary care hospital in western Germany; 107 of the patients were offered a probiotic drink containing at least 10 billion cultures of Lactobacillus casei DN 114001 b.i.d. The economic analysis was based on the costs of patient isolation vs preventive intake of probiotics. In a second pilot study, two commercially available probiotic drinks with different Lactobacillus casei strains were directly compared in 60 patients in a randomized controlled fashion.
RESULTS: In the first study, the incidence of antibiotic-associated diarrhea (AAD) was significantly reduced in the intervention group (6.5% vs 28.4%), and the duration of AAD in days was significantly shorter (1.7 ± 1.1 vs 3.1 ± 2.1). Higher age and creatinine and lower albumin were identified as risk factors for AAD. Ampicillin was the antibiotic with the highest rate of AAD (50%) and with the greatest AAD reduction in the probiotic group (4.2%, relative risk reduction 92%). The economic analysis showed a cost advantage of nearly 60000 €/year in a department of this size. The second study confirmed the preventive effect of the drink with Lactobacillus casei DN114001; however, there were no advantages found for the other tested probiotic drink containing Lactobacillus casei Shirota.
CONCLUSION: In contrast to a drink containing Lactobacillus casei Shirota, a commercially available probiotic drink containing Lactobacillus casei DN 114001 cost-efficiently reduces the prevalence of AAD during antibiotic treatment.
Core tip: The presented study used a large primary hospital cohort to test the effect of a commercially available probiotic drink in the prevention of antibiotic-associated diarrhea (AAD) in a decision-based manner. This is the first manufacturer-independent study showing a clear advantage of a probiotic drink containing Lactobacillus casei DN 114001 in AAD prevention. The study additionally contains a calculation of cost-effectiveness and identifies risk factors for AAD. In a second small pilot study, we compared for the first time two commercially available drinks in a head-to-head analysis.
-
Citation: Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing
Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J Gastroenterol 2014; 20(42): 15837-15844 - URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15837.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15837
Antibiotic-associated diarrhea (AAD) is a common problem in the treatment of infectious diseases. During treatment with antibiotic drugs, 5%-49% of patients, depending on the type of the antibiotic used, suffer from diarrhea[1,2]; typically, a pathogenic bacterium is not identified in the diarrhea. In approximately 10%-25% of cases, AAD is caused by Clostridium difficile (C. difficile), and the incidence of this bacterium is rising[3]. The presence of diarrhea requires the isolation of the patient until C. difficile-associated diarrhea (CDAD) has been ruled out by stool cultures. In primary hospitals without their own microbiology departments, this diagnostic procedure could take two or three days. During this period, the isolation of the patient leads to a lack of availability of the adjacent beds for other patients and to higher costs of patient care through the necessity of wearing coats, masks, caps and gloves to avoid transferring bacteria to other patients. These additional costs and the discomfort for patients, relatives and staff members could be avoided by a more rapid diagnostic procedure and by the prevention of AAD and CDAD[4]. Data from surveys and our own observations suggest that isolated patients are visited less frequently and consequently are treated worse than non-isolated patients[5-7].
A recent meta-analysis of 82 randomized controlled studies detected a statistically significant reduction in AAD when probiotics were used concomitantly during the administration of antibiotics. However, the studies[8-14] were extremely heterogeneous in terms of their patient population and use of probiotics and antibiotics. Although cultures of lactobacillus[15] were used in the majority of the studies, the bacterial strains were poorly defined and characterized. Clear evidence for a standardized adjuvant probiotic administration could not be formulated, although more than 11000 patients were included in these studies[16]. Another meta-analysis found a statistically significant advantage for lactobacillus strain probiotics; however, there was significant heterogeneity in the study design (study population, probiotic used, definition of AAD, dose and duration of probiotic administration)[17]. In a systematic review, four out of six studies of the highest quality supported an effect of probiotics against AAD[18].
A recent randomized, double-blind placebo-controlled study was not able to show the effectiveness of the administration of Saccharomyces boulardii in the reduction of AAD[19]; however, older studies and a meta-analysis detected an advantage for this strain[20].
The situation regarding probiotics is unclear, and specific recommendations are lacking[21].
Eser et al[1] recently summarized the available evidence and concluded that lactobacillus rhamnosus GG and casei-based probiotics and products using Saccharomyces boulardii and probiotic mixtures could be used in the prevention of AAD; these authors assigned a grade of evidence of 1a for the lactobacillus rhamnosus GG and casei-based probiotics and a grade of 2b for the Saccharomyces boulardii and probiotic mixtures[1]. Given the present data, it is difficult to share these recommendations.
We hypothesize that the prevention of AAD could be most effective if probiotics are used that are widely distributed and accepted by the patients and are not perceived as an additional drug/tablet (low threshold for intake resulting in good compliance). In Germany and in many other countries, several formulations of lactobacillus strains are sold as food products in supermarkets. Therefore, these formulations are easily available and could be used in the outpatient setting and during hospital stays. The majority of these products have a pleasant taste, and the distribution of these probiotic drinks is experienced in a very positive manner by the patients.
Only one study using a commercial product has been published[22]. This study showed a positive effect of the probiotic drink; however, the study was supported by the manufacturer and used an AAD definition that is unsuitable for use in hospitals (AAD defined as 3 or more loose stools in at least 3 consecutive days)[22]. However, in hospitals, diarrhea of one-day duration leads to the isolation of the patient.
The aim of our study was to test the efficacy of commercially available probiotic drinks (Actimel® and Yakult®) in the prevention of AAD in a decision-based manner regarding necessary isolation in a primary hospital (3 loose stools on one day) independently from the manufacturer. A calculation of the economic advantage of antibiotic-accompanied prophylactic treatment with probiotics was performed after finalizing the study. Data from the patients were used to identify risk factors for AAD to tailor preventive probiotic treatment to certain risk groups.
In a large Internal Medicine department in a primary hospital (Bethlehem hospital in Stolberg/Rhineland, Germany), we recorded the presence of AAD in three hospital wards (wards A5, A6 and A7). AAD was defined as at least 3 watery/fluid stools in a 24-h period. On ward A5, all of the patients treated with antibiotics were offered commercially available probiotic drinks [Actimel® (Danone GmbH, Munich, Germany)], 100 mL, each containing at least 1010 colony forming bacteria of Lactobacillus casei defensis DN-114001, bought via the canteen kitchen of the hospital) twice daily for the duration of antibiotic treatment over a period of 3 mo. On wards A6 and A7, all of the patients were treated as usual without any probiotics. The basic data of the patient groups are shown in Table 1. After this study period, all of the charts of the patients with informed consent on the three wards (n = 258) were analyzed retrospectively for information regarding diarrhea during treatment with antibiotics leading to the isolation of the patient. The results of ward A5 (n = 107) were compared with the two other wards (n = 151) of the same hospital, which served as the controls. Patients with sepsis, pancreatitis and oncologic diseases and patients on dialysis were excluded. These exclusion criteria followed the known safety profile of the probiotics and are consistent with recommendations from the literature[1,21,23,24].
| Variable | Actimel group (ward A5) | Controls (wards A6/A7) | P value |
| n | 107 | 151 | |
| Age (yr) | 70.8 ± 15.6 | 70.8 ± 16.5 | NS |
| Male gender | 48.6% | 52.3% | NS |
| PST 0 or 1 | 52.3% | 56.6% | NS |
| BMI | 25.7 ± 4.2 | 26.1 ± 4.8 | NS |
| Creatinine (mg/dL) | 1.2 ± 0.8 | 1.1 ± 0.6 | NS |
| Albumine (mg/dL) | 3.5 ± 0.4 | 3.5 ± 0.4 | NS |
| Diagnosis (site of infection/sepsis) | Pulmonary: 55.7% Genitourinary: 26.4% Biliary: 2.8% Sepsis: 5.7% Diverticulitis: 2.8% Dermal: 2.8% Misc.: 3.7% | Pulmonary: 50.0% Genitourinary: 22.0% Biliary: 8.0% Sepsis: 1.3% Diverticulitis: 2.7% Dermal: 4.0% Misc.: 12.0% | NS |
| Antibiotics | Ampicillin: 26.4% Cefuroxime: 23.5% Ceftriaxone: 12.2% Ciprofloxacin: 16.0% Clarithromycin: 12.2% Tazobactam: 3.8% Levofloxacin: 6.6% Moxifloxacin: 3.8% Metronidazole: 3.7% Clindamycin: 0.0% Cotrimoxazole: 0.9% | Ampicillin: 19.3% Cefuroxime: 18.7% Ceftriaxone: 10.7% Ciprofloxacin: 20.0% Clarithromycin: 6.7% Tazobactam: 2.7% Levofloxacin: 2.7% Moxifloxacin: 0.0% Metronidazole: 6.7% Clindamycin: 3.3% Cotrimoxazole: 4.9% | NS |
| Days of treatment | 7.3 ± 4.2 | 6.1 ± 3.6 | 0.008 |
After obtaining encouraging results in the first study, we designed a controlled randomized study (to our knowledge, the first head-to-head-comparison) using two different commercially available probiotic drinks, Actimel® (Danone, Lactobacillus casei defensis DN-114001) and Yakult® (Yakult Deutschland GmbH, Neuss, Germany, Lactobacillus casei Shirota). Both preparations are widely available as food products and claim to contain at least 1010 colony forming bacteria of Lactobacillus casei strains. The probiotic drinks were bought by the canteen kitchen of the hospital. This part of the study took place in the same Internal Medicine department and started only a short period after the end of the first study. Due to the sponsor-independent nature of the study, we were unable to design this study as double-blind. Consecutive patients on the three wards were asked to participate in this study by using probiotic drinks twice daily and were randomized in the sequence of admission. The basic data of the patient groups are shown in Table 2. After obtaining informed written consent, the patients were randomized and received open-label drinks. All of the patient charts were analyzed regarding diarrhea during the antibiotic treatment. The study was approved by the local ethics committee.
| Variable | Actimel group | Yakult group | P value |
| n | 30 | 30 | |
| Age (yr) | 69.7 ± 17.6 | 74.1 ± 17.5 | NS |
| Male gender | 63.3% | 60.0% | NS |
| PST 0 or 1 | 70.0% | 51.7% | NS |
| BMI | 25.9 ± 4.2 | 25.1 ± 4.1 | NS |
| Creatinine (mg/dL) | 1.2 ± 0.5 | 1.1 ± 0.3 | NS |
| Albumin (mg/dL) | 3.4 ± 0.3 | 3.3 ± 0.3 | NS |
| Diagnosis (aite of infection/aepsis) | Pulmonary: 30.0% Genitourinary: 50.0% Biliary: 3.3% Sepsis: 0.0% Diverticulitis: 0.0% Dermal: 13.3% Misc.: 3.3% | Pulmonary: 50.0% Genitourinary: 26.7% Biliary: 6.7% Sepsis: 3.3% Diverticulitis: 3.3% Dermal: 6.7% Misc.: 3.3% | NS |
| Antibiotics | Ampicillin: 20.0% Cefuroxime: 20.0% Ceftriaxone: 20.0% Ciprofloxacin: 0.0% Clarithromycin: 10.0% Tazobactam: 0.0% Levofloxacin: 20.0% Moxifloxacin: 0.0% Cotrimoxazole: 3.3% Penicillin: 13.3% | Ampicillin: 36.7% Cefuroxime: 10.0% Ceftriaxone: 10.0% Ciprofloxacin: 3.3% Clarithromycin:16.7% Tazobactam: 10.0% Levofloxacin: 20.0% Moxifloxacin: 3.3% Cotrimoxazole: 6.7% Penicillin: 0.0% | NS |
| Days of treatment | 6.0 ± 3.1 | 6.6 ± 3.7 | NS |
| Concurrent PPI treatment | 23.3% | 50.0% | 0.06 |
| Antibiotic treatment in history | 3.3% | 10.0% | NS |
The statistical analysis was performed using SPSS Version 20.0 (SPSS Inc., United States). All of the metric variables were analyzed regarding normal distribution with the Shapiro-Wilk test or the Kolmogorov-Smirnov test. Comparisons of independent normally distributed variables was performed using a t-test. The homogeneity of variances was tested with the Levene test. Non-normally distributed samples were compared using the Mann-Whitney-U test. The categorized data were analyzed with the chi-square test and Fisher’s exact test.
In all of the tests, two-sided significance was tested with the assumption of P < 0.05 as significant.
In the probiotics group, the incidence of AAD was much lower than that in the control group (6.5% vs 28.4%, P < 0.001). Probiotics reduced the duration of AAD from 3.1 ± 2.1 d in the control patients to 1.7 ± 1.1 d in the patients using these drinks (P = 0.015, for all data see Table 3). If analyzed according to the days of diarrhea relative to the entire duration of the hospital stay, the difference in AAD prevalence was higher (1.65% vs 14%). The OR for AAD in the probiotic group was 0.177; in the control group, the OR for AAD was 5.66.
| Parameter | Actimel group (ward A5) | Controls (wards A6/A7) | P value |
| Hospital stay (d) | 11.2 ± 6.8 | 12.2 ± 8.3 | NS |
| Diarrhea | 6.5% | 28.4% | < 0.001 |
| Duration of diarrhea (d) | 1.7 ± 1.1 | 3.1 ± 2.1 | 0.015 |
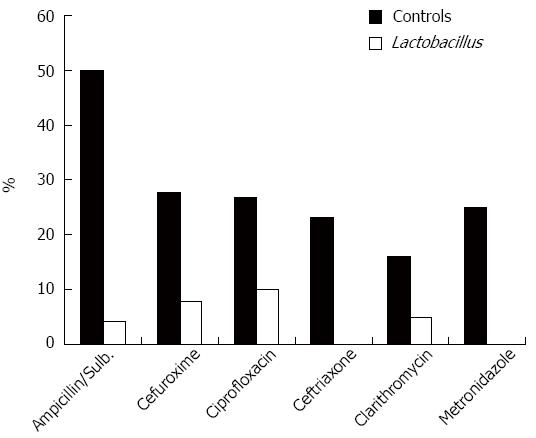
Figure 1 shows the incidence of AAD for each antibiotic. The majority of cases of AAD occurred with treatment using ampicillin/sulbactam. The greatest AAD reduction when using probiotic drinks was observed in treatment with ampicillin/sulbactam (4.2% vs 50%, relative risk reduction 92%), ceftriaxone and metronidazole (0% vs 23 and 25%, respectively, relative risk reduction in both cases 100%). We observed a very low incidence of AAD with piperacillin and clindamycin (data not shown). However, these antibiotics were seldom used on the standard care wards.
The length of the entire hospital stay was longer in the control patients with diarrhea (15.5 ± 10.6 d) than in the control patients without diarrhea (12.2 ± 8.3 d, P = 0.01) and in the patients with diarrhea using probiotics (12.75 ± 6.7 d, Table 4). However, the length of the entire hospital stay was not significantly different in the probiotic group compared with the control group (11.2 ± 6.8 vs 12.2 ± 8.3, P = 0.386, Table 3). The majority of episodes of AAD started on day 3 of treatment with antibiotics in both groups (data not shown).
| Parameter | Diarrhea | No diarrhea | P value |
| n | 49 | 206 | |
| Age (yr) | 74.9 ± 13.3 | 69.6 ± 16.5 | 0.028 |
| Gender | M: 20.0%F: 18.4% | M: 80.0%F: 81.6% | NS |
| Hospital stay (d) | 15.0 ± 10.1 | 11.1 ± 6.9 | 0.007 |
| Creatinine (mg/dL) | 1.5 ± 0.8 | 1.1 ± 0.6 | < 0.001 |
| Albumine (mg/dL) | 3.2 ± 0.2 | 3.4 ± 0.3 | 0.04 |
| BMI | 25.3 ± 4.7 | 26.1 ± 4.5 | NS |
Patients with diarrhea in both groups tended to be older, to have higher creatinine levels and to have lower albumin levels. There was no difference in diarrhea frequency regarding gender or body mass index (BMI) (Table 4).
In our hospital, we calculated the burden of patient isolation during AAD (requirement for protective masks, caps, gloves and coats) to be approximately 70 €/d. Based on the results from this first study and the number of patients in our department, we expect costs of approximately 77800 €/year for isolating patients without probiotic use and of approximately 14400 €/year for patients using probiotics. These calculations consider an isolation length of approximately 2 d as realistic until negative stool cultures are present. Even if the costs of the probiotic drinks (4586 €/year) are taken out of this sum, there is a clear advantage for the strategy of prophylactic probiotic use during antibiotic treatment (58800 €/year). Any additional costs for longer hospital stays of patients with diarrhea were not included in this calculation.
We observed significant differences in the frequency of diarrhea between the two groups. The diarrhea frequency in the Actimel group was similar to that of the first study (6.7%), whereas the presence of AAD was much higher in the Yakult group (33.3%), similar to the results of the control group from the first study (P = 0.021, Table 5). The duration of diarrhea was clearly shorter in the Actimel group (2.5 ± 0.7 vs 4.4 ± 2.5), although this difference did not reach significance. The only cases of C. difficile infection occurred in the Yakult group (3 cases, 10% of all patients in this group). The occurrence rates of AAD depending on the antibiotic used were similar to the first study (data not shown).
| Parameter | Actimel group | Yakult group | P value |
| Hospital stay (d) | 10.8 ± 7.7 | 11.8 ± 7.8 | NS |
| Diarrhea | 6.7% | 33.3% | 0.021 |
| Duration of diarrhea (d) | 2.5 ± 0.7 | 4.4 ± 2.5 | NS |
| CDAD | 0.0% | 10.0% | NS |
The patients with diarrhea tended to be older, to have lower albumin levels and to have higher creatinine levels; however, the creatinine levels did not reach statistical significance in the second study because of the limited number of patients (Table 6). We analyzed patients with and without diarrhea for the concurrent intake of PPIs and antibiotic treatment in the recent history and found significant differences (Table 6).
| Parameter | Diarrhea | No diarrhea | P value |
| n | 12 | 48 | |
| Age (yr) | 80.3 ± 13.4 | 69.8 ± 18 | 0.019 |
| Gender | M: 21.6%F:17.4% | M: 78.4%F: 82.6% | NS |
| Hospital stay (d) | 19.3 ± 10.9 | 9.3 ± 5.2 | < 0.001 |
| Creatinine (mg/dL) | 1.2 ± 0.4 | 1.1 ± 0.4 | NS |
| Albumine (mg/dL) | 3.3 ± 0.5 | 3.6 ± 0.4 | < 0.001 |
| BMI | 24.0 ± 4.0 | 25.9 ± 4.1 | NS |
| Concurrent PPI treat. | 75.0% | 27.1% | 0.005 |
| Antibiotic treatment in history | 33.3% | 0.0% | 0.001 |
Other analyzed factors (the presence of enteral nutrition, dementia, atrial fibrillation, chronic lung disease, congestive heart failure, diabetes mellitus, smoking, heavy alcohol consumption) were not significantly different between the study groups or between the patients with and without diarrhea (data not shown).
Our data from a large number of patients show for the first time in a manufacturer-independent manner that a commercially available probiotic drink (Actimel®) with a specific lactobacillus casei strain is able to significantly reduce the frequency and duration of AAD and that this reduction is cost-effective. In a large cohort in our department, the twice daily ingestion of this drink led to an AAD frequency of only 6.5%, whereas in the control group, AAD was approximately four times more frequent. The duration of AAD was significantly shorter in the Actimel patients. The patients and medical professionals benefited from the use of probiotic drinks because this protocol significantly reduced the necessity of isolating patients on the ward. Less isolation saves time for medical professionals and increases the quality of life for patients, relatives and medical staff. Additionally, in a cost analysis, we calculated the financial benefit for a department as large as ours to be more than 50000 € per year. Significant side effects were not recorded, suggesting that this probiotic drink is safe even for ill and old patients, taking into account the applied exclusion criteria.
Possible bias might result from the fact that the study was performed on three different wards and that the control group did not receive a placebo.
However, the second part of our study confirmed this result for Actimel in a ward-independent manner, although this part was performed with a smaller number of patients. Furthermore, we showed that another lactobacillus casei drink was not able to improve the AAD frequency. This result suggests a specific effect of the Lactobacillus casei strain used in Actimel or of the composition of the drink, including the other included bacterial strains (Lactobacillus bulgaricus and Streptococcus thermophilus). There might be some features of the study patients that caused Yakult to not be more successful than the control group of the first study. First, the patients in the Yakult group were older and had a worse performance status, although these differences did not reach significance. Additionally, the patients in the Yakult group had more pulmonary infections and more often received ampicillin as the antibiotic; ampicillin was associated with the highest AAD-frequency. This administration of ampicillin might be the reason for the three cases of CDAD in this group. In the Actimel group, no CDAD was observed, emphasizing the positive effect of the probiotic.
The second study confirmed the positive effects for one of the commercially available probiotic drinks containing the specific lactobacillus strain DN-114001; however, these results could not be transferred to all lactobacillus preparations because either the defined lactobacillus strain or the composition of the fermented milk drink might confer different effects on AAD.
In the analysis of patients with diarrhea vs the patients without diarrhea, several factors appeared to emerge as promoting AAD. In both study parts, the patients with diarrhea were older and had a lower albumin level and a higher creatinine level. The creatinine variable did not reach significance in the second study most likely because of the smaller patient number. These results, however, are in agreement with an earlier study[19], suggesting that these factors promote AAD. There could be pathophysiological explanations for these data. Doctors often forget to reduce the dose of the antibiotic according to the renal function in patients with only limited renal insufficiency. Additionally, the albumin level reflects the nutritional status and might influence the pharmacokinetics of antibiotic substances via blood protein binding. These findings and their possible explanations should lead to the careful dosing of antibiotics in older patients with mild to moderate renal insufficiency.
Treatment with antibiotics in the medical history has long been associated with CDAD[25]. Our data from the second study imply that prior antibiotic treatment might also be a risk factor for AAD. The role of concurrent PPI therapy is controversial[25,26]; however, our limited data support an association between concomitant PPI therapy and AAD. The number of patients in the second study is too low to obtain confident data regarding these factors, and future studies with higher power should examine these associations. These findings should further contribute to a reduction in the generous use of PPI.
Our data have implications for hospitals without their own microbiological departments. In such hospitals, ruling out specific infections, such as CDAD in the case of AAD, takes several days and necessitates the isolation of patients to avoid spreading the infection. In an era of increasing nosocomial infections and more severe CDAD infections, the avoidance of AAD is the best preventive measure; furthermore, the isolation of patients is a nuisance to the patients and the hospital staff and leads to worse medical care of these patients[5-7]. Additionally, cost-reduction is enormous and likely underreported because we did not include the cost of a longer hospital stay for AAD patients or for stool cultures.
The data from existing studies and our own data from the present study justify the use of probiotics for the prevention of AAD during antibiotic treatment, especially in geriatric patients with recent antibiotic use, renal insufficiency and lower albumin values. It is probable that even the generalized use of probiotics is cost-effective and safe. Our study implies that careful dose modifications in patients with lower albumin and higher creatinine levels might contribute to a lower prevalence of AAD; however, these issues should be investigated in separate studies.
Antibiotic-associated diarrhea (AAD) is an important problem that occurs during antibiotic treatment and has a significant economic burden. Probiotics have been shown to reduce AAD, especially when the probiotic contains Lactobacillus strains. Several probiotic formulations containing Lactobacillus casei are commercially available, have pleasant tastes and could contribute to AAD prevention.
The concept of using commercially available probiotic drinks for AAD prevention is attractive. These drinks are readily available, and the threshold for intake is low. The authors show in this study that one commercially available drink containing Lactobacillus casei DN 114001 was very effective in preventing AAD. Interestingly, a different commercial probiotic drink containing another Lactobacillus casei strain was not better in the prevention of AAD compared with the control group. Higher age, lower serum albumin and lower serum creatinine concentrations were identified as risk factors for AAD.
This is the first study without support from the manufacturer showing the effectiveness of a commercial probiotic drink in the prevention of AAD. Additionally, the authors conducted the first head-to-head study of two probiotic formulations in AAD prevention and showed that the specific Lactobacillus strain or the general drink composition is responsible for the AAD preventive effect. The application of the commercial probiotic drink containing Lactobacillus DN 114001 is highly cost-effective.
This study shows that the general use of probiotics during antibiotic treatment could reduce the occurrence of AAD and health costs. Additionally, risk groups of AAD are identified; antibiotic treatment should be supervised carefully in these groups.
AAD (antibiotic-associated diarrhea) denotes diarrhea occurring during antibiotic treatment. Some of these cases of diarrhea are related to Clostridium difficile. When experiencing episodes of diarrhea, patients must be isolated and can only be visited by individuals wearing protective coats, masks, gloves and caps. Probiotics are living microbes conferring a health benefit by modifying the intestinal microbiota of the host.
The article contains a few grammatical and formatting errors. The suggested corrections are listed below in more detail. As a general note, the authors should remove the deleted portions from their prior draft for the purpose of presentation.
P- Reviewer: Koch TR S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Eser A, Thalhammer F, Burghuber F, Högenauer C, Stockenhuber F, Wenisch C, Widhalm K, Reinisch W. [Probiotics for the prevention of antibiotic-induced diarrhea]. Z Gastroenterol. 2012;50:1089-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Bergogne-Bérézin E. Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents. 2000;16:521-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 472] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 4. | Avadhani A, Miley H. Probiotics for prevention of antibiotic-associated diarrhea and Clostridium difficile-associated disease in hospitalized adults--a meta-analysis. J Am Acad Nurse Pract. 2011;23:269-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31:354-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Vinski J, Bertin M, Sun Z, Gordon SM, Bokar D, Merlino J, Fraser TG. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33:513-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290:1899-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 402] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 8. | Koning CJ, Jonkers DM, Stobberingh EE, Mulder L, Rombouts FM, Stockbrügger RW. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am J Gastroenterol. 2008;103:178-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Merenstein DJ, Foster J, D’Amico F. A randomized clinical trial measuring the influence of kefir on antibiotic-associated diarrhea: the measuring the influence of Kefir (MILK) Study. Arch Pediatr Adolesc Med. 2009;163:750-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Cimperman L, Bayless G, Best K, Diligente A, Mordarski B, Oster M, Smith M, Vatakis F, Wiese D, Steiber A. A randomized, double-blind, placebo-controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol. 2011;45:785-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea - a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Song HJ, Kim JY, Jung SA, Kim SE, Park HS, Jeong Y, Hong SP, Cheon JH, Kim WH, Kim HJ. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic-associated diarrhea: a prospective, randomized, double-blind, multicenter study. J Korean Med Sci. 2010;25:1784-1791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wenus C, Goll R, Loken EB, Biong AS, Halvorsen DS, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008;62:299-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Kale-Pradhan PB, Jassal HK, Wilhelm SM. Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: a meta-analysis. Pharmacotherapy. 2010;30:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, Johnsen B, Shekelle PG. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 477] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 17. | Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de Wit N. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Pozzoni P, Riva A, Bellatorre AG, Amigoni M, Redaelli E, Ronchetti A, Stefani M, Tironi R, Molteni EE, Conte D. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: a single-center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2012;107:922-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202-2222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 325] [Cited by in F6Publishing: 300] [Article Influence: 21.4] [Reference Citation Analysis (8)] |
| 21. | Kotzampassi K, Giamarellos-Bourboulis EJ. Probiotics for infectious diseases: more drugs, less dietary supplementation. Int J Antimicrob Agents. 2012;40:288-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Hickson M, D’Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 343] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 23. | Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol. 2008;42 Suppl 2:S58-S63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Liong MT. Safety of probiotics: translocation and infection. Nutr Rev. 2008;66:192-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Naggie S, Miller BA, Zuzak KB, Pence BW, Mayo AJ, Nicholson BP, Kutty PK, McDonald LC, Woods CW. A case-control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med. 2011;124:276.e1-276.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |