Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.988
Revised: November 29, 2013
Accepted: January 6, 2014
Published online: January 28, 2014
Radiofrequency ablation (RFA) causes focal coagulation necrosis in tissue. Its first clinical application was reported in 2000, and RFA has since been commonly used in both primary and metastatic lung cancer. The procedure is typically performed using computed tomography guidance, and the techniques for introducing the electrode to the tumor are simple and resemble those used in percutaneous lung biopsy. The most common complication is pneumothorax, which occurs in up to 50% of procedures; chest tube placement for pneumothorax is required in up to 25% of procedures. Other severe complications, such as pleural effusion requiring chest tube placement, infection, and nerve injury, are rare. The local efficacy depends on tumor size, and local progression after RFA is not rare, occurring in 10% or more of patients. The local progression rate is particularly high for tumors > 3 cm. Repeat RFA may be used to treat local progression. Short- to mid-term survival after RFA appears promising and is approximately 85%-95% at 1 year and 45%-55% at 3 years. Long-term survival data are sparse. Better survival may be expected for patients with small metastasis, low carcinoembryonic antigen levels, and/or no extrapulmonary metastasis. The notable advantages of RFA are that it is simple and minimally invasive; preserves pulmonary function; can be repeated; and is applicable regardless of previous treatments. Its most substantial limitation is limited local efficacy. Although surgery is still the method of choice for treatment with curative intent, the ultimate application of RFA may be to replace metastasectomy for small metastases. Randomized trials comparing RFA with surgery are needed.
Core tip: Radiofrequency ablation (RFA) for pulmonary metastasis of colorectal cancer is technically simple. The procedure rarely results in death. The most common complication is pneumothorax, which occurs in up to 50% of patients. Severe complications are rare. Local progression after RFA is not rare and occurs in 10% or more of cases. The short- to mid-term survival after RFA appears promising and is approximately 85%-95% at 1 year and 45%-55% at 3 years. Long-term survival data are sparse. Better survival may be expected for patients with small metastasis, low carcinoembryonic antigen levels, and/or no extrapulmonary metastasis.
- Citation: Hiraki T, Gobara H, Iguchi T, Fujiwara H, Matsui Y, Kanazawa S. Radiofrequency ablation as treatment for pulmonary metastasis of colorectal cancer. World J Gastroenterol 2014; 20(4): 988-996
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/988.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.988
Colon cancer is the third most common cancer and the second most common cause of cancer-related mortality in the United States, and 10%-30% of patients with colon cancer have pulmonary metastasis at presentation[1,2]. Even if metastasis is not initially present, the cancer may recur in the lungs after curative resection of the primary cancer. Kobayashi et al[3] surveyed 5230 patients who underwent curative resection for colorectal cancer and found that 906 patients (17%) developed recurrence at a median of 1.4 years after surgery. The first recurrence site in 250 patients (5%) was the lungs, which was the second most common site of recurrence after the liver (373 patients, 7%). Although lung recurrence is usually accompanied by recurrence at other sites, recurrence was confined to the lungs in 2%-10% of patients who develop distant metastases[4,5].
A meta-analysis demonstrated that patients with untreated locally advanced or metastatic colorectal cancer had a median survival of 8 mo[6]. The International Registry of Lung Metastases[7] revealed that the 5-year survival rate for patients who underwent complete resection of lung metastasis was 36%, compared to 13% for patients who did not undergo complete resection. A large-scale, multicenter retrospective study in Japan[3] also reported significantly better survival in patients who underwent resection for pulmonary recurrence. Thus, surgery is considered the treatment of choice for curative intent. However, that study[3] also indicated that less than half (38%) of the patients with pulmonary recurrence underwent surgical resection. Mitry et al[2] reported that only 4% of patients with synchronous pulmonary metastases and 14% of patients with metachronous pulmonary metastases were curatively resected. These data indicate that many patients with pulmonary metastases are not considered suitable for surgery. Therefore, the development of less invasive local therapies, such as radiofrequency ablation (RFA), may be attractive.
RFA causes focal coagulation necrosis in tissue via the delivery of energy in the form of an alternating electrical current with a frequency of 460-500 kHz in the radio wave range. The alternating electrical current causes the agitation of ionic dipolar molecules in surrounding tissue and fluids, resulting in frictional heating. The exposure of cells to temperatures of 50-52 °C for 4-6 min may induce cytotoxicity[8]. Between 60 °C and 100 °C, there is a near instantaneous induction of protein coagulation, which irreversibly damages key cytosolic and mitochondrial enzymes, as well as nucleic acid-histone protein complexes[8]. Thus, the aim of the RFA procedure is to generate temperatures > 50 °C in cancer cells.
Since Dupuy et al[9] reported the first clinical use of RFA to treat lung cancer in 2000, RFA has been commonly used as a treatment for both primary and metastatic lung cancer. The thermal and electrical conductivity of air are low, and thus the effects of RFA on the lungs may be tissue-specific. Accordingly, it has been demonstrated that a given quantity of RF current produces a larger volume of ablation of tumors in the lungs than in subcutaneous tissues or the kidneys[10]. Conversely, alveolar air and ventilation may limit the ablation zone in the surrounding parenchyma, as saline infusion into the lung parenchyma to reduce alveolar air and bronchial balloon occlusion enlarged the ablation zone in animal experiments[11,12]. This difficulty in ablating the marginal parenchyma may account for the relatively high frequency of local progression after RFA of lung cancer.
RFA is indicated in patients who are considered nonsurgical candidates and for whom the treatment of lung cancer is expected to contribute to prolonged survival. The procedure is not indicated in patients with poor performance status (e.g., PS ≥ 3), leucocyte count < 3000 cells/μL, uncorrected coagulopathy (e.g., a platelet count < 50000/μL or a prothrombin time-international ratio > 1.5), poor pulmonary function (e.g., predicted forced respiratory volume in 1 sec ≤ 1000 mL), poor cardiac function (e.g., New York Heart Association Class ≥ III), uncorrected diabetes (e.g., HbA1c ≥ 7), and uncontrollable extrapulmonary cancer. The procedure is feasible, but patients with tumors in contact with the heart and aorta are at a higher risk of local progression[13].
The electrode used for lung RFA is usually either a multitined expandable electrode or an internally cooled electrode[14]. The multitined expandable electrode, which is more commonly used for lung RFA, consists of an array of multiple electrode tines that expand from a single, centrally positioned large needle cannula. The internally cooled electrode consists of dual-lumen needles with non-insulated active tips, in which internal cooling is achieved by continuous perfusion with chilled saline.
We suggest that the procedure should be performed by physicians who are familiar with both computed tomography (CT)-guided intervention and RFA. The procedure is usually conducted under local anesthesia, but epidural or general anesthesia may also be used. CT is the only image-guidance modality that can be used for lung RFA. CT fluoroscopy permits a near real-time image display, thereby facilitating the procedure. The techniques used to introduce the electrode into the tumor under CT guidance are simple and similar to those used in percutaneous lung biopsy. A prospective multicenter clinical trial showed that treatment was successfully completed in 99% (105/106) of patients[15]. Multiplanar reconstruction of CT images is useful for confirming proper positioning of the electrode. After introducing the electrode into the tumor, a given RF energy is applied for a variable duration. In our institution, an ablation algorithm based on electrode type is used; this algorithm has been described in the literature[16]. The procedure should aim to obtain an ablative margin of at least 0.5 cm around the tumor to treat the microscopic extension of cancer cells around the macroscopic mass and thereby decrease the risk of local progression. To obtain an adequate ablative margin, repositioning of the electrode followed by application of RF energy (so-called “multiple overlapping ablations”) may be performed.
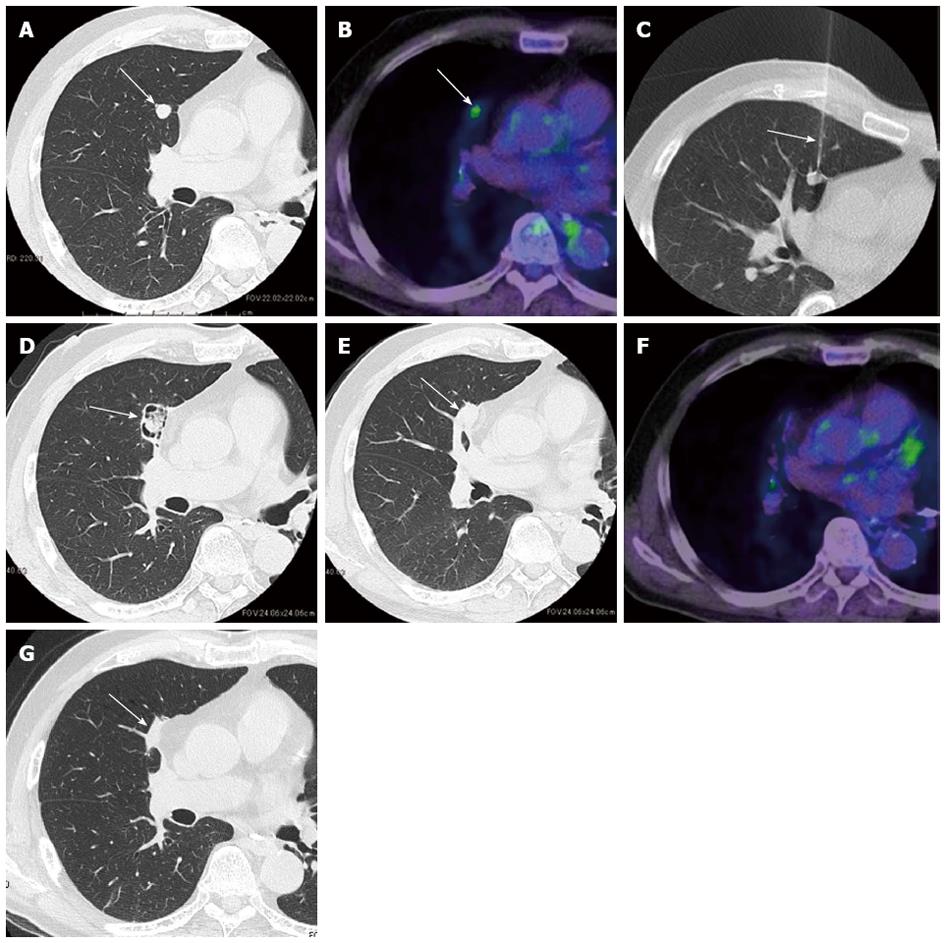
Figure 1 shows radiological images of a pulmonary metastasis from a colorectal cancer patient treated with RFA. Local efficacy is evaluated primarily by sequential follow-up CT scans. During the first 6 mo after RFA, the size of the ablated lesion may exceed the tumor size before ablation because the lesion includes the ablated marginal parenchyma surrounding the tumor[17-19]. Thus, at a given time point during this period, local efficacy cannot be evaluated by comparing the tumor size with the pretreatment tumor size. Consequently, CT images are first obtained in the early period (e.g., 1 mo) after RFA as a point of reference. Thereafter, it is possible to evaluate local efficacy by comparing the size and geometry of the ablation zone with the previous CT images. When the tumor is completely ablated, the ablation zone gradually decreases in size[20] and typically becomes scar-like tissue. Local tumor progression is considered to occur when the ablation zone increases in size[20]. In our experience, in most cases of local tumor progression, a nodule appears in the periphery of the ablation zone that always enlarges if untreated. Such a nodule generally exhibits some degree of contrast enhancement that distinguishes it from the unenhanced necrotic tumor tissue[20]. Thus, contrast-enhanced CT images can be helpful in confirming the diagnosis of local progression. However, in our experience, local progression is diagnosed by careful observation of the size and geometry of the ablation zones. Therefore, we are of the opinion that contrast-enhanced CT is preferable but not essential for diagnosing local progression.
Positron emission tomography may also be used to evaluate local efficacy. Focal areas of increased fluorodeoxyglucose uptake at the ablated zone are suggestive of local tumor progression. However, attention should be paid to possible false-positive results during the first 3 mo[17,21] or even at 24 mo[22] after RFA, due to inflammation induced by RFA.
A review of the literature was conducted by searching the PubMed database. The results were limited to studies published in English, and the search was performed with the keywords “colorectal”, “lung”, and “radiofrequency ablation”. The citations of all electronically identified articles were further manually searched for potentially relevant studies. Human clinical studies on the efficacy of RFA of pulmonary metastases from colorectal cancer were selected, while animal experiments, case reports, and reviews were excluded. All relevant articles were subsequently evaluated.
Table 1 summarizes the results for the use of RFA to treat pulmonary metastases in patients with colorectal cancer. A group at St. George Hospital in Australia published several reports on the use of RFA to treat pulmonary metastases in patients with colorectal cancer[18,23-28]. In 2003, Steinke et al[18] published their preliminary study, which mainly focused on morbidity. In total, 20 nonsurgical candidates with 41 pulmonary metastases from colorectal cancer were treated with RFA. The procedure resulted in technical failure for 1 tumor. A total of 10 (50%) patients developed pneumothorax, and 5 patients (25%) required chest tube placement. Intrapulmonary hemorrhage occurred in 3 (7.5%) of the 40 tumors, but all cases were self-limiting. In 2007, Yan et al[26] reported the mid-term outcomes of 55 nonsurgical candidates, including morbidities, local efficacy, and survival. No hospital mortality was reported. The periprocedural morbidity rate was 42%, which included intrapulmonary bleeding (9%), pneumothorax (29%), pleural effusion (7%), and persistent pleuritic chest pain for more than 1 wk (4%). Some patients experienced more than 1 adverse event. In total, 9 patients had pneumothorax that required chest tube placement (16%). The median duration of hospital stay was 1 d, and the median follow-up period was 24 mo. The proportion of local progression at the time of the study was 38%. The 1-, 2-, and 3-year overall survival rates were 85%, 64%, and 46%, respectively, and the median overall survival was 33 mo. The 1- and 2-year local progression-free survival rates were 74% and 56%, respectively. The 1- and 2-year local progression-free survival rates were 88% and 69%, respectively, for the patients in which the largest lung metastasis was ≤ 3 cm, and 27% and 18%, respectively, for the patients in which the largest lung metastasis was > 3 cm. The 1- and 2-year overall progression-free survival rates were 61% and 34%, respectively. The median overall progression-free survival was 15 mo. Univariate analyses identified the following factors as significant for local progression-free survival: the size of the largest lung metastasis, the location of the lung metastases, and post-RFA carcinoembryonic antigen (CEA) levels at 1 and 3 mo. According to multivariate analysis, a largest lung metastasis of > 3 cm (HR = 8.3) and post-RFA CEA level of > 5 ng/mL at 1 mo (HR = 3.5) were independently associated with reduced local progression-free survival. Two factors were found to be significant for overall progression-free survival: sex and size of the largest lung metastasis. In multivariate analysis, only a largest lung metastasis of > 3 cm (HR = 5.1) was independently associated with reduced overall progression-free survival. Yan et al[27] also reported a learning curve for RFA in which morbidity was reduced. The same group[28] reported the outcomes of an open-label prospective trial of RFA for 148 nonsurgical candidates with lung metastases from several primary cancers; 73% of these patients had primary colorectal cancer. Although the data for the colorectal cancer patient subgroup was limited, the median overall survival for patients with colorectal cancer was found to be 60 mo.
| Ref. | Center | Year | n | Patient age (yr) | No. of tumors per patient | Tumor size (cm) | Follow-up period (mo) | Mortality and morbidity | Local efficacy | Survival | Prognostic factors |
| Steinke et al[18] | St. George Hospital | 2003 | 20 | 62 (mean) | 2.1 | 1.4 (mean) | 14 (median) | Mortality: 0%, Overall PTX: 50%, PTX requiring chest tube placement: 25%, Self-limiting intrapulmonary hemorrhage: 7.5% | NA | NA | NA |
| Yan et al[26] | St. George Hospital | 2007 | 55 | 62 (mean) | NA | 2.1 (mean) | 24 (median) | Mortality: 0%, Overall morbidity rate: 42%, PTX: 29%, Pleural effusion: 7%, PTX requiring chest tube placement: 16%, Self-limiting intrapulmonary hemorrhage: 9% | Proportion of local tumor progression: 38% | 1-/2-/3-year OS rate: 85%/64%/46%, respectively, Median OS: 33 mo, 1-/2-year local PFS rate: 74%/56%, respectively, 1-/2-year overall PFS rate: 61%/34%, respectively, Median overall PFS: 15 mo | Tumor size and CEA level at 1 month after RFA for local PFS by multivariate analyses, Tumor size for overall PFS by multivariate analyses |
| Lencioni et al[15] | Multicenter in the United States, United kingdom, Italy, Germany, and Australia | 2008 | 53 | 63 (mean) | 2.2 | 1.4 (mean) | NA | Mortality: 0% | Proportion of local tumor progression: 9% | OS 1-/2-year: 89%/66%, Cancer-specific survival 1-/2-year: 91%/68% | NA |
| Hiraki et al[30] | Okayama University | 2007 | 27 | 62 (mean) | 1.8 | 1.5 (mean) | 20 (median) | Mortality: 0%, Overall PTX: 49%, PTX requiring chest tube placement: 7.3%, Pleural effusion: 15%, | Primary and secondary proportion of local tumor progression: 31% and 20%, respectively1-/2-/3-year primary and secondary local control rate: 72% and 85%/56% and 62%/56% and 62%, respectively | 1-/2-/3-year OS rate: 96%/54%/48%, respectively, Mean OS: 33 mo | Extrapulmonary metastasis for OS by univariate analyses |
| Yamakado et al[31] | Multicenter in Japan | 2007 | 71 | 64 (mean) | 2.2 | 2.4 (mean) | 19 (mean) | Mortality: 0%, Overall PTX: 37%, PTX requiring chest tube placement: 20%, Pleural effusion: 14%, > 38 °C fever: 20%, Empyema requiring chest tube placement: 1.4% | Proportion of local tumor progression: 17% | 1-/2-/3-year OS rate: 84%/62%/46%, respectively, Median OS: 31 mo | Extrapulmonary metastasis and tumor size for OS by multivariate analyses |
| Yamakado et al[32] | Mie University | 2009 | 78 | 66 (mean) | 2.5 | 2.0 (mean) | 25 (mean) | Mortality: 0%, Overall PTX: 22%, PTX requiring chest tube placement: 13%, Pleural effusion requiring chest tube placement: 1.4% | Proportion of local tumor progression: 14%, 1-/3-/5-year local control rate: 90%/79%/79% | 1-/3-/5-year OS rate: 84%/56%/35%, respectively, Median OS: 38 mo | Extrapulmonary metastasis and CEA level for OS by multivariate analyses |
| Petre et al[33] | Memorial Sloan-Kettering Cancer Center | 2013 | 45 | 63 (mean) | 1.5 | 0.4–3.5 | 18 (median) | Mortality: 0%, Overall PTX: 33%, PTX requiring chest tube placement: 19%, Overall pleural effusion: 5%, Pleural effusion requiring chest tube placement: 1.7%, Pneumonia: 1.7% | Primary and secondary proportion of local tumor progression: 13% and 7.2%, respectively | 1-/2-/3-year OS rate: 95%/72%/50%, respectively, Median OS: 46 mo, 1-/2-/3-year primary and secondary local PFS rate: 92% and 95%/77% and 89%/77% and 89%, respectively | Number of pulmonary metastasis for OS by univariate analyses |
| Gillams et al[34] | University College London Medical School | 2013 | 122 | 68 (median) | 3.3 | 1.7 (mean) | NA | Mortality: 0%, Late procedure-related death: 0.4%, Major complication: 3.9%, PTX requiring chest tube placement: 15%, Pleural effusion requiring chest tube placement: 1.2%, Infection: 2.0%, Nerve injury: 0.8% | Proportion of local tumor progression: 19% | OS 3-year rate: 57%, Median OS: 41 mo | None |
Simon et al[29] reported a mixed population comprising 153 nonsurgical candidates with 189 lung cancers, including 18 patients with pulmonary metastasis from colorectal cancer. Although the data from the colorectal cancer subgroup were scarce, the 1-, 2-, 3-, and 5-year survival rates for those patients were 87%, 78%, 57%, and 57%, respectively. Lencioni et al[15] performed a prospective, multicenter clinical trial of RFA using a mixed population comprising 106 nonsurgical candidates with primary lung cancer and pulmonary metastasis from various primary cancers. In total, 53 patients had metastases from colorectal cancer. No procedure-related deaths occurred. Complete treatment was confirmed for ≥ 1 year in 91% of the patients with pulmonary metastases from colorectal cancer. The 1- and 2-year overall survival rates for the patients with colorectal metastases were 89% and 66%, respectively. The cancer-specific 1- and 2-year survival rates were 91% and 68%, respectively.
Hiraki et al[30] assessed the outcomes of 27 nonsurgical candidates with pulmonary metastases from colorectal cancer; these patients comprised a total of 41 RFA sessions. There was no mortality or sequela. Pneumothorax occurred after 49% of the sessions, and chest tube placement was required after 7.3% of the sessions. Pleural effusion was encountered after 15% of the sessions. Local progression after RFA was observed in 31% (15/49) of the tumors; 5 of these locally progressing tumors were completely treated by repeating the procedure. Thus, local progression was observed in 20% of the tumors at the time of study. The primary local control rates were 72% at 1 year, 56% at 2 years, and 56% at 3 years. By repeating the procedure for local progression, the local control rates were improved to 85% at 1 year, 62% at 2 years, and 62% at 3 years. The 1-, 2-, and 3-year survival rates were 96%, 54%, and 48%, respectively. The mean survival time was 33 mo. Univariate analysis revealed that the presence of extrapulmonary metastasis at the time of RFA was the only significant factor associated with survival.
Yamakado et al[31] reported the results of a multicenter study in Japan comprising 71 nonsurgical candidates. No mortality was observed. Fever (> 38 °C) developed in 14 patients (20%), and asymptomatic pleural effusion was observed in 10 (14%) patients. Pneumothorax developed in 26 (37%) patients, 14 (20%) of whom required a chest tube. Empyema developed in 1 (1.4%) patient. Local tumor progression was observed in 12 (17%) of the 71 patients during the mean follow-up period of 19 mo. The proportion of patients with local tumor progression was 11% (7/61) in those with tumors ≤ 3 cm and 50% (5/10) in those with tumors > 3 cm. This difference was statistically significant. The 1-, 2-, and 3-year overall survival rates were 84%, 62%, and 46%, respectively. The median survival time was 31 mo. Univariate analyses revealed that extrapulmonary metastasis, tumor size, and CEA level were significant prognostic factors. The first 2 factors were also significant according to multivariate analysis. Subsequently, Yamakado et al[32] reported a single-center study involving 78 patients with pulmonary metastases from colorectal cancer. The mean follow-up period was 24.6 mo. Pneumothorax developed in 22% (31/140) of the sessions, and pneumothorax and pleural effusion requiring chest tube placement occurred in 13% (18/140) and 1.4% (2/140) of the sessions, respectively. Local tumor progression was observed in 11 patients (14%). The 1-, 3-, and 5-year local tumor progression rates were 10%, 21%, and 21%, respectively. The 1-, 3-, and 5-year local tumor progression rates were 5%, 14%, and 14% in patients with tumors ≤ 3 cm and 53%, 69%, and 69% in patients with tumors > 3 cm. This difference was statistically significant. The 1-, 3-, and 5-year survival rates were 84%, 56%, and 35%, respectively, and the median survival time was 38 mo. Univariate analyses identified maximum tumor diameter of ≤ 3 cm, single-lung metastasis, absence of extrapulmonary metastasis, and normal CEA levels as prognostic factors. Multivariate analysis also indicated that the latter 2 variables were significantly independent prognostic factors. The 1-, 3-, and 5-year survival rates were 98%, 83%, and 57%, respectively, in the 54 patients with no extrapulmonary metastases and 97%, 86%, and 63%, respectively, in the 33 patients with negative CEA levels.
Petre et al[33] studied 45 nonsurgical candidates with 69 pulmonary metastases (< 3.5 cm) from colorectal cancer. The median hospital stay was 1 d. There was no periprocedural mortality. Pneumothorax occurred in 33% of the sessions, with 12 (19%) patients requiring a percutaneous chest tube. There were 3 cases of pleural effusion, one of which required catheter drainage. One patient developed bacterial pneumonia. The median follow-up period was 18 mo after RFA. Of the 69 lesions, local tumor progression occurred in 9 lesions (13%) at a median of 11.1 mo after RFA. Lesions > 1.5 cm had a tendency toward a higher risk of local progression compared with lesions ≤ 1.5 cm (HR = 7.03). Among the lesions that progressed, 4 were re-treated with RFA, and the secondary (after repeat ablations) effectiveness rate was 93% (64/69 lesions). The primary and secondary local tumor progression-free survival rates were 92% and 95%, respectively, at 1 year, 77% and 89%, respectively, at 2 years, and 77% and 89%, respectively, at 3 years. The median overall survival time after the RFA procedure was 46 mo. The 1-, 2-, and 3-year overall survival rates from the time of RFA were 95%, 72%, and 50%, respectively. Univariate analyses using various variables revealed that the only significant prognostic factor was the number of pulmonary metastasis at the time of RFA.
Gillams et al[34] performed 256 RFA procedures in 122 patients with a total of 398 metastases. The major complication rate was 3.9%. There were no cases of prolonged air leak. The 30-d mortality rate was 0%. There were 10 major complications (3.9%): 3 pleural effusions requiring drain insertion; 5 infections, including 1 delayed infection that resulted in fatal hemoptysis; and 2 nerve injuries (1 recurrent laryngeal nerve injury and 1 brachial plexus injury). Pneumothorax requiring drainage occurred in 39 (15%) of the procedures. The local progression analysis included 268 tumors with > 6 mo of imaging follow-up data available for review. On a tumor-by-tumor basis, 52 (19%) of 268 tumors progressed locally. The mean and median times to local progression were 9 and 8 mo (range 2-27 mo), respectively. The median overall survival and 3-year survival rate were 41 mo and 57%, respectively. No significant prognostic factors were identified, although survival tended to be better in patients with smaller tumors.
In summary, RFA for colorectal pulmonary metastasis is a safe procedure that rarely results in death. The most common complication is pneumothorax, which occurs in up to 50% of procedures. Chest tube placement for pneumothorax is required after up to 25% of procedures. Other severe complications, such as pleural effusion requiring chest tube placement, infection, and nerve injury, are rare. Local tumor progression after RFA is not rare (10% or more) and is particularly common for tumors > 3 cm. Short- to mid-term survival after RFA appears promising, with survival rates of approximately 85%-95% at 1 year and 45%-55% at 3 years. Long-term (5 years or more) survival data are sparse. Significant prognostic factors include number and size of pulmonary metastases, CEA levels, and extrapulmonary metastasis.
The ultimate application of RFA may be to replace metastasectomy. Accordingly, survival data after surgical resection of pulmonary metastases from colorectal cancer should be assessed. Pfannschmidt et al[35] systematically reviewed 20 published series of surgical resection of pulmonary metastases from colorectal cancer. The postoperative mortality ranged from 0% to 2.4%, and approximately 40% of patients remained alive 5 years after resection. Fiorentino et al[36] also performed a systematic review of 51 articles on pulmonary metastasectomy in colorectal cancer. Most pulmonary metastasectomies were performed for a single metastasis. The 5-year survival rate after single metastasectomy was approximately 50%, whereas the rate after multiple metastasectomy was 30%. Recently, Gonzalez et al[37] performed a systematic review of 25 studies involving a total of 2925 patients and found that the median 5-year survival rate was 43.5%. At present, data for long-term survival after RFA are too sparse to compare with surgical data, although the short- to mid-term survival data are promising. In our opinion, given the high local progression rate for tumors > 3 cm after RFA, patients with such tumors should undergo surgery whenever operable. As a therapy for small tumors, RFA may be competitive with metastasectomy, which must be validated in future trials.
RFA has various notable advantages. The procedure is simple and can be performed percutaneously using local anesthesia. The procedure is also safe and minimally invasive. Thus, RFA may enable long-term survival or even a cure for patients with pulmonary metastases who are considered nonsurgical candidates because of comorbidities and/or refusal to undergo surgery. Given that pulmonary metastases are usually of a multifocal nature and, consequently, pose a high risk of intrapulmonary de novo recurrence after therapy, the treatment for pulmonary metastases must be repeatable and should preserve as much of the parenchyma as possible to preserve pulmonary function. The repeatability of the procedure may also be a great advantage of RFA. Repeat procedures may also be used to effectively treat local tumor progression[38]. The influence of RFA on pulmonary function was found to be minimal[39-41], and RFA may be applied regardless of previous treatments. Consequently, this method can be used as a second salvage treatment for recurrence after surgery, radiation therapy, or chemotherapy and in combination with other treatments to eradicate multiple cancers.
There are also disadvantages of the use of RFA. CT is used for the procedure, which is associated with radiation exposure to both the patient and the physician. Thus, the use of CT fluoroscopy, although useful, should be minimized. The procedure is also accompanied by a high risk of pneumothorax. The most substantial disadvantage of RFA may be its limited local efficacy.
RFA for pulmonary metastasis of colorectal cancer is safe and minimally invasive. The most common complication, which occurs in up to 50% of cases, is pneumothorax. However, in most cases, this can be treated conservatively. The local efficacy of RFA depends on the tumor size, and local progression after RFA is not rare, occurring in 10% or more of cases. The local progression rate is particularly high for tumors > 3 cm. The short- to mid-term survival data after RFA are promising, with 1- and 3-year survival rates of approximately 85%-95% and 45%-55%, respectively. Long-term survival data remain sparse. Better survival may be expected for patients with small metastasis, low carcinoembryonic antigen levels, and/or no extrapulmonary metastasis. The ultimate application of RFA may be to replace metastasectomy for small metastases. Future studies should include randomized trials comparing RFA with surgery.
P- Reviewers: Bloomston PM, Borzio M, Ewertsen C S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4370] [Cited by in F6Publishing: 4241] [Article Influence: 235.6] [Reference Citation Analysis (0)] |
| 2. | Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59:1383-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 248] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 3. | Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141:67-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | McCormack PM, Ginsberg RJ. Current management of colorectal metastases to lung. Chest Surg Clin N Am. 1998;8:119-126. [PubMed] [Cited in This Article: ] |
| 5. | Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 363] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg. 1997;113:37-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1072] [Cited by in F6Publishing: 1019] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 8. | Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities--part I. J Vasc Interv Radiol. 2001;12:1021-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 280] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Ahmed M, Liu Z, Afzal KS, Weeks D, Lobo SM, Kruskal JB, Lenkinski RE, Goldberg SN. Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;230:761-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Iishi T, Hiraki T, Mimura H, Gobara H, Kurose T, Fujiwara H, Sakurai J, Yanai H, Yoshino T, Kanazawa S. Infusion of hypertonic saline into the lung parenchyma during radiofrequency ablation of the lungs with multitined expandable electrodes: results using a porcine model. Acta Med Okayama. 2009;63:137-144. [PubMed] [Cited in This Article: ] |
| 12. | Anai H, Uchida BT, Pavcnik D, Seong CK, Baker P, Correa LO, Corless CL, Geyik S, Yavuz K, Sakaguchi H. Effects of blood flow and/or ventilation restriction on radiofrequency coagulation size in the lung: an experimental study in swine. Cardiovasc Intervent Radiol. 2006;29:838-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Iguchi T, Hiraki T, Gobara H, Mimura H, Fujiwara H, Tajiri N, Sakurai J, Yasui K, Date H, Kanazawa S. Percutaneous radiofrequency ablation of lung tumors close to the heart or aorta: evaluation of safety and effectiveness. J Vasc Interv Radiol. 2007;18:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Rose SC, Thistlethwaite PA, Sewell PE, Vance RB. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17:927-51; quiz 951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, Helmberger T, Gillams AR, Frilling A, Ambrogi M. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Hiraki T, Gobara H, Mimura H, Toyooka S, Fujiwara H, Yasui K, Sano Y, Iguchi T, Sakurai J, Tajiri N. Radiofrequency ablation of lung cancer at Okayama University Hospital: a review of 10 years of experience. Acta Med Okayama. 2011;65:287-297. [PubMed] [Cited in This Article: ] |
| 17. | Alafate A, Shinya T, Okumura Y, Sato S, Hiraki T, Ishii H, Gobara H, Kato K, Fujiwara T, Miyoshi S. The Maximum standardized uptake value is more reliable than size measurement in early follow-up to evaluate potential pulmonary malignancies following radiofrequency ablation. Acta Med Okayama. 2013;67:105-112. [PubMed] [Cited in This Article: ] |
| 18. | Steinke K, King J, Glenn D, Morris DL. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr. 2003;27:750-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Bojarski JD, Dupuy DE, Mayo-Smith WW. CT imaging findings of pulmonary neoplasms after treatment with radiofrequency ablation: results in 32 tumors. AJR Am J Roentgenol. 2005;185:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics. 2012;32:947-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Deandreis D, Leboulleux S, Dromain C, Auperin A, Coulot J, Lumbroso J, Deschamps F, Rao P, Schlumberger M, de Baère T. Role of FDG PET/CT and chest CT in the follow-up of lung lesions treated with radiofrequency ablation. Radiology. 2011;258:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Sharma A, Lanuti M, He W, Palmer EL, Shepard JA, Digumarthy SR. Increase in fluorodeoxyglucose positron emission tomography activity following complete radiofrequency ablation of lung tumors. J Comput Assist Tomogr. 2013;37:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | King J, Glenn D, Clark W, Zhao J, Steinke K, Clingan P, Morris DL. Percutaneous radiofrequency ablation of pulmonary metastases in patients with colorectal cancer. Br J Surg. 2004;91:217-223. [PubMed] [Cited in This Article: ] |
| 24. | Steinke K, Glenn D, King J, Clark W, Zhao J, Clingan P, Morris DL. Percutaneous imaging-guided radiofrequency ablation in patients with colorectal pulmonary metastases: 1-year follow-up. Ann Surg Oncol. 2004;11:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Yan TD, King J, Sjarif A, Glenn D, Steinke K, Morris DL. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13:1529-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Yan TD, King J, Sjarif A, Glenn D, Steinke K, Al-Kindy A, Morris DL. Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:1718-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Yan TD, King J, Sjarif A, Glenn D, Steinke K, Morris DL. Learning curve for percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: a prospective study of 70 consecutive cases. Ann Surg Oncol. 2006;13:1588-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Chua TC, Sarkar A, Saxena A, Glenn D, Zhao J, Morris DL. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol. 2010;21:2017-2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, Mayo-Smith WW. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 394] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 30. | Hiraki T, Gobara H, Iishi T, Sano Y, Iguchi T, Fujiwara H, Tajiri N, Sakurai J, Date H, Mimura H. Percutaneous radiofrequency ablation for pulmonary metastases from colorectal cancer: midterm results in 27 patients. J Vasc Interv Radiol. 2007;18:1264-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Yamakado K, Hase S, Matsuoka T, Tanigawa N, Nakatsuka A, Takaki H, Takao M, Inoue Y, Kanazawa S, Inoue Y. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicenter study in Japan. J Vasc Interv Radiol. 2007;18:393-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Yamakado K, Inoue Y, Takao M, Takaki H, Nakatsuka A, Uraki J, Kashima M, Kusunoki M, Shimpo H, Takeda K. Long-term results of radiofrequency ablation in colorectal lung metastases: single center experience. Oncol Rep. 2009;22:885-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Petre EN, Jia X, Thornton RH, Sofocleous CT, Alago W, Kemeny NE, Solomon SB. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer. 2013;12:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Gillams A, Khan Z, Osborn P, Lees W. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol. 2013;36:724-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 390] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 36. | Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med. 2010;103:60-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:572-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 38. | Hiraki T, Mimura H, Gobara H, Sano Y, Fujiwara H, Date H, Kanazawa S. Repeat radiofrequency ablation for local progression of lung tumors: does it have a role in local tumor control? J Vasc Interv Radiol. 2008;19:706-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Ambrogi MC, Lucchi M, Dini P, Melfi F, Fontanini G, Faviana P, Fanucchi O, Mussi A. Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardiothorac Surg. 2006;30:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Tada A, Hiraki T, Iguchi T, Gobara H, Mimura H, Toyooka S, Kiura K, Tsuda T, Mitsuhashi T, Kanazawa S. Influence of radiofrequency ablation of lung cancer on pulmonary function. Cardiovasc Intervent Radiol. 2012;35:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | de Baère T, Palussière J, Aupérin A, Hakime A, Abdel-Rehim M, Kind M, Dromain C, Ravaud A, Tebboune N, Boige V. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240:587-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 259] [Article Influence: 14.4] [Reference Citation Analysis (1)] |