Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9513
Revised: March 19, 2014
Accepted: April 21, 2014
Published online: July 28, 2014
AIM: To investigate the role of caveolin-3 (CAV3) and cholecystokinin A receptor (CCKAR) in cholesterol gallstone disease (CGD).
METHODS: To establish a mouse model of CGD, male C57BL/6 mice were fed with a lithogenic diet containing 1.0% cholic acid, 1.25% cholesterol and 15% fat; a similar control group was given a normal diet. The fresh liver weights and liver-to-body weight ratio were compared between the two groups after one month. Serum lipid profile and bile composition were determined with an autoanalyzer. The Cav3 and Cckar mRNA and CAV3 and CCKAR protein levels in the liver and gallbladder were determined via real-time polymerase chain reaction and Western blot, respectively.
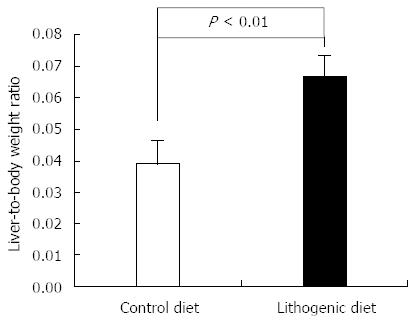
RESULTS: Establishment of the mouse CGD model was verified by the presence of cholesterol gallstones in mice fed the lithogenic diet. Compared with mice maintained on a normal diet, those fed the lithogenic diet had significantly higher mean liver-to-body weight ratio (0.067 ± 0.007 vs 0.039 ± 0.007, P < 0.01), serum total cholesterol (4.22 ± 0.46 mmol/L vs 2.21 ± 0.11 mmol/L, P < 0.001), bile total cholesterol (1.33 ± 0.33 mmol/L vs 0.21 ± 0.11 mmol/L, P < 0.001), and bile phospholipid concentrations (3.55 ± 1.40 mmol/L vs 1.55 ± 0.63 mmol/L, P = 0.04), but lower total bile acid concentrations (726.48 ± 51.83 μmol/L vs 839.83 ± 23.74 μmol/L, P = 0.007). The lithogenic diet was also associated with significantly lower CAV3 in the liver and lower CAV3 and CCKAR in the gallbladder compared with the control mice (all P < 0.05).
CONCLUSION: CAV3 and CCKAR may be involved in cholesterol gallstone disease.
Core tip: Cholesterol gallstone disease (CGD) is one of the most common digestive diseases worldwide, while the mechanisms of this disease are not fully understood. In this study, we established a mouse model of CGD and observed that the formation of gallstones was accompanied by an increase in serum and bile total cholesterol concentrations, while by a decrease in total bile acid concentration in bile. The formation of gallstones was also accompanied by downregulation of hepatic caveolin-3 (CAV3) expression, and downregulation of CAV3 and cholecystokinin A receptor (CCKAR) expression in the gallbladder. Our results suggest that CAV3 and CCKAR may be involved in CGD.
- Citation: Xu GQ, Xu CF, Chen HT, Liu S, Teng XD, Xu GY, Yu CH. Association of caveolin-3 and cholecystokinin A receptor with cholesterol gallstone disease in mice. World J Gastroenterol 2014; 20(28): 9513-9518
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9513.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9513
Cholesterol gallstone disease (CGD) accounts for 80%-90% of the gallstones found at cholecystectomy, and is the most common digestive disease requiring hospital admission[1,2]. Epidemiological studies conducted in the United States showed that 10%-15% of adults have CGD, and more than one million people are newly diagnosed annually[3,4]. The prevalence of CGD has increased in developing countries possibly due to the western lifestyle adopted[5,6].
CGD results from bile lipids and bile salts imbalance in the gallbladder[7]. The development of CGD is associated with supersaturation of bile with cholesterol, rapid precipitation of cholesterol crystals in the gallbladder, increased bile salt hydrophobicity, and inflammation of the gallbladder[8]. Of these events, precipitation of excess cholesterol in the bile is a prerequisite for gallstone formation[9]. In general, cholesterol is solubilized in mixed micelles together with bile salts and phospholipids in bile. However, cholesterol precipitation and subsequent cholesterol gallstone formation may occur as a result of cholesterol supersaturation in bile[10,11].
Multifactorial factors are involved in the mechanism of CGD[12]. Impaired gallbladder emptying, which provides sufficient time for cholesterol crystal nucleation, contributes significantly to gallstone formation[13]. Caveolins are a family of small integral membrane proteins consisting of caveolin-1, caveolin-2, and caveolin-3 (CAV3)[14]. Caveolin-1 and caveolin-2 are the major structural proteins of caveolae, and are abundantly coexpressed in endothelial and adipose cells[15], while CAV3 is mainly found in muscle cells[16]. Caveolins are postulated to be mainly involved in modulation of cholesterol movement and storage[14,17]. A recent in vitro study observed that CAV3 was involved in the regulation of gallbladder muscle hypomotility[18]. Knockdown of CAV3, mediated via small interfering RNA, decreased contraction of gallbladder muscle cells isolated from guinea pigs, and increased cholecystokinin A receptor (CCKAR) in the caveolae[18]. CCKAR is a major physiologic mediator of smooth muscle contraction of the gallbladder. Lack of CCKAR may deteriorate gallbladder contraction and enhance gallstone formation[19]. Polymorphism of Cckar gene was associated with gallstone formation in humans[20,21].
To date, the in vivo association between CAV3 and CGD has not been fully elucidated, and whether CCKAR is differentially expressed during the process of cholesterol gallstone formation remains uncertain. In this study, we established a mouse model of CGD induced by a lithogenic diet, and investigated the association of CAV3 and CCKAR with CGD.
Eight-week-old male C57BL/6 mice were purchased from Shanghai SLAC Laboratory Animals (Shanghai, China) and maintained in a standard facility. After acclimatized to laboratory conditions for two weeks, the mice were randomly divided into two groups (n = 8, each), and fed for one month ad libitum either a standard laboratory chow diet (control) or a lithogenic diet containing 1.0% cholic acid, 1.25% cholesterol and 15% fat. All experiments were carried out in accordance with current institutional guidelines for the care and use of experimental animals.
The mice were sacrificed after one month of experimental feeding. Blood, liver, bile, and gallbladder samples were harvested. Serum triglyceride, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured in a Hitachi autoanalyzer 7600 (Hitachi, Tokyo, Japan). The bile samples were diluted 6-fold with deionized water, and bile composition was also analyzed using the Hitachi autoanalyzer 7600 in accordance with standard procedures.
To evaluate the expression levels of Cav3 and Cckar mRNA in the livers and gallbladders, real-time polymerase chain reaction (PCR) was performed using an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) with a TaKaRa real-time PCR kit in accordance with the manufacturer’s instructions. All primers used in this study (Table 1) were designed with Primer Premier 5.0 software (Premier, Canada) and synthesized at Invitrogen (Invitrogen Biotechnology, Shanghai, China). PCR products were quantified by measuring the calculated cycle thresholds for individual targets and β-actin mRNA. The comparative 2-ΔΔCT method was used for quantification and statistical analysis, and results were expressed as fold changes relative to controls.
| Gene | Forward primers | Reverse primers |
| Cav3 | 5'-TGAGGACATTGTGAAGGTAGA-3' | 5'-TACTTGGAGACGGTGAACG-3' |
| Cckar | 5'-CTTCCTGTTGCCAAGTGA-3' | 5'-TTAGCCTCTTCTCTTTAGCA-3' |
| β-actin | 5'-GAAGATCAAGATCATTGCTCCT-3' | 5'-TGGAAGGTGGACAGTGAG-3' |
Proteins extracted from mouse livers and gallbladders were run on 12% SDS-PAGE and transferred onto nitrocellulose membranes. The transferred membranes were incubated for 2 h at room temperature with blocking buffer TBST (20 mmol/L Tris-HCl, 140 mmol/L NaCl, 0.05% Tween-20, pH 7.5) containing 5% skim milk, and then incubated overnight at 4 °C with anti-CAV3 (1:3000; BD transduction) and anti-CCKAR (1:500; Santa Cruz) antibodies. After five 3-min washes in TBST, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (1:1000, Beijing Zhongshan Biotechnology, Beijing, China). The antigens were detected using an EZ-ECL kit (Biological Industries, Israel). The intensities of the separate bands were analyzed using QuantityOne software (Bio-Rad), and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology).
Statistical analyses were performed using SPSS software version 13.0 (SPSS, Chicago, IL). Data are expressed as mean ± SD and were compared using Student’s t test. P < 0.05 (2-sided tests) was considered statistically significant.
Mice fed with lithogenic diet containing 1.0% cholic acid, 1.25% cholesterol and 15% fat for one month developed gallstones. In addition, the mice fed the lithogenic diet had significantly higher mean liver weight and liver-to-body weight ratio compared with the control mice fed a normal diet (Figure 1). Histological analysis showed significant fatty infiltration of the liver in mice fed a lithogenic diet, while the control mice exhibited normal liver architecture.
Mice fed a lithogenic diet had significantly higher levels of serum total cholesterol, HDL-C, and LDL-C compared with the control mice (Table 2). Bile total cholesterol and phospholipid concentrations were also significantly higher in mice fed a lithogenic diet, while total bile acid levels in bile were significantly lower (Table 2).
| Control diet | Lithogenic diet | P value | ||
| Serum lipid profile | Triglyceride, mmol/L | 0.83 ± 0.15 | 0.75 ± 0.04 | 0.355 |
| Total cholesterol, mmol/L | 2.21 ± 0.11 | 4.22 ± 0.46 | < 0.001 | |
| HDL-C, mmol/L | 1.35 ± 0.11 | 1.86 ±0.10 | < 0.001 | |
| LDL-C, mmol/L | 0.58 ± 0.12 | 2.19 ± 0.43 | < 0.001 | |
| Bile composition | Total cholesterol, mmol/L | 0.21 ± 0.11 | 1.33 ± 0.33 | < 0.001 |
| Phospholipids, mmol/L | 1.55 ± 0.63 | 3.55 ± 1.40 | 0.040 | |
| Total bile acids, μmol/L | 839.83 ± 23.74 | 726.48 ± 51.83 | 0.007 |
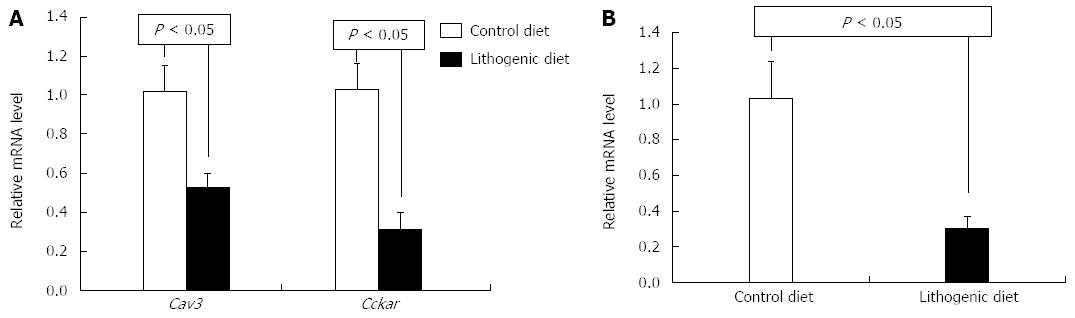
To investigate the associations between CAV3 and CCKAR and CGD, the mRNA levels of Cav3 and Cckar in the livers and gallbladders were analyzed. Real-time PCR showed that mRNA levels of Cav3 and Cckar in the gallbladder of mice fed a lithogenic diet were significantly lower than those of the control mice (Figure 2A). Cav3 mRNA levels in the liver of mice fed a lithogenic diet was also significantly lower than those of the controls (Figure 2B). Cckar mRNA was not detected in the liver.
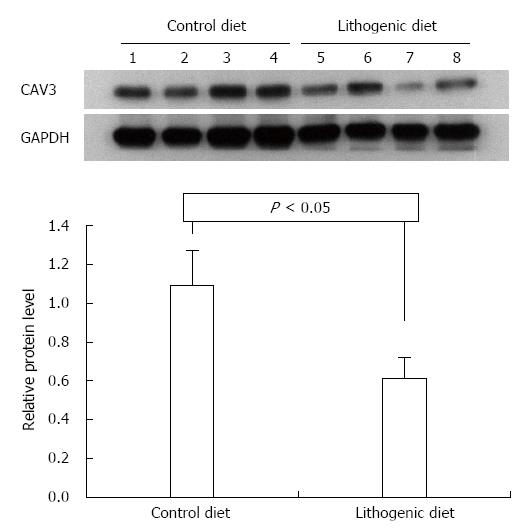
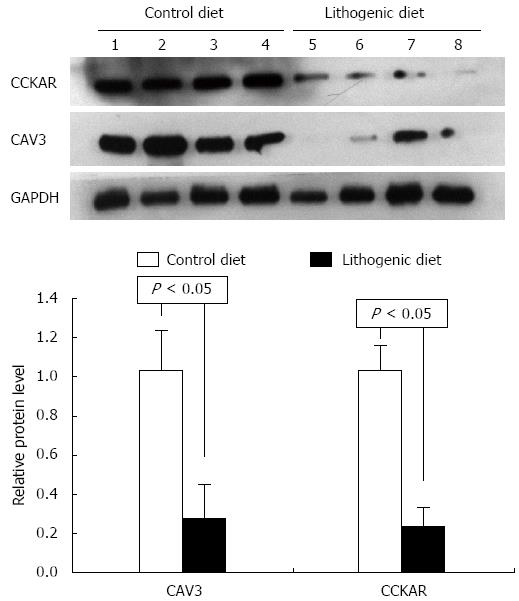
The proteins CAV3 and CCKAR were analyzed in the livers and gallbladders by Western blot. According to the mRNA expression results, the protein levels for CAV3 and CCKAR in the gallbladder of mice fed a lithogenic diet were significantly lower compared with the controls (Figure 3). The protein levels of CAV3 in the liver of mice fed a lithogenic diet were also significantly lower than in the controls (Figure 4). These results suggested that the development of CGD was accompanied by downregulation of CAV3 expression in the liver, and downregulation of CAV3 and CCKAR expression in the gallbladder, implying that CAV3 and CCKAR may be involved in CGD.
In this study, we established a mouse model of CGD and observed that the formation of gallstones was accompanied by increases in serum and bile total cholesterol concentrations, while total bile acid concentrations in bile decreased. Lower levels of hepatic CAV3 expression, and CAV3 and CCKAR in the gallbladder were also found in the mouse model compared with the control mice.
The mechanisms underlying the pathogenesis of CGD are incompletely understood[22]. Epidemiologic studies imply the involvement of multiple environmental factors and genetic elements in cholesterol gallstone formation[23,24]. The former includes diet as a risk factor for cholesterol gallstone formation[25]. In the present study, mice developed cholesterol gallstones fed for one month with a lithogenic diet containing 1.0% cholic acid, 1.25% cholesterol and 15% fat. Significant increases in serum total cholesterol and LDL-C levels were observed in mice fed the lithogenic diet. This change mimics the alterations in serum lipid profiles observed in CGD patients[26,27].
Hypersecretion of biliary cholesterol and cholesterol supersaturation of the bile are the most important prerequisites for gallstone formation[11]. Bile acids are the major components of bile, and are involved in cholesterol elimination. Decreases in total bile acid levels may be associated with reduced cholesterol elimination in the bile and subsequent cholesterol supersaturation. In the present study, we observed that bile total cholesterol and phospholipid levels were significantly higher in the mouse CGD model, while total bile acid concentrations in bile were lower than in the control mice.
Decreased gallbladder motility is crucial to the formation of cholesterol crystals in bile[13]. The mechanisms associated with decreased gallbladder motility remain unclear. Caveolins are scaffolding proteins that have important roles in cholesterol homeostasis[14,17]. Besides its role in cholesterol metabolism, CAV3 was also found to be involved in regulation of gallbladder muscle hypomotility in vitro[18]. However, the in vivo association between CAV3 and CGD is not fully understood. In this study, we observed that CAV3 expression was lower in the liver and gallbladder of the mouse model of CGD, compared with the normal controls.
CCKAR is a major mediator of smooth muscle contraction of the gallbladder. One-third of CCKAR (-/-) mice spontaneously develop gallstone disease at 12 and 24 mo of age[19]. Polymorphism of Cckar gene in patients is also an independent risk factor for gallstone disease[21]. However, whether CCKAR is differentially expressed during the process of CGD formation remains unclear. Here, we observed that the expression of CCKAR was significantly lower in the gallbladder of CGD mice than in the control group. These observations suggest the involvement of CAV3 and CCKAR in cholesterol gallstones. However, the precise mechanism remains to be determined. Further study is also needed to clarify whether our findings are relevant to humans, and the possibility of therapeutic intervention for the disease.
In conclusion, our results showed that both mRNA and protein of CAV3 and CCKAR were differentially expressed in the liver and gallbladder of a mouse model of CGD compared with control mice. Further investigation may enhance our understanding of the pathogenesis of CGD, and enable exploration of novel therapeutic targets.
Cholesterol gallstone disease is common, which can cause a high disease burden. The pathogenesis of cholesterol gallstones remains unclear, and strategies for prevention and efficient nonsurgical therapies for the disease are lacking.
The precise pathogenesis of cholesterol gallstones disease has been extensively investigated in recent years.
This study found that the formation of gallstones in mice was accompanied by increase in serum and bile total cholesterol concentrations, while by decrease in total bile acid concentration in bile. The formation of gallstones was also accompanied by downregulation of hepatic caveolin-3 expression, and downregulation of caveolin-3 and cholecystokinin A receptor expression in the gallbladder.
The results showed that both mRNA and caveolin-3 protein and cholecystokinin A receptor were differentially expressed in the liver and gallbladder of a mouse model of cholesterol gallstone disease (CGD) compared with control mice. Further investigation may enhance our understanding of the pathogenesis of CGD, and enable exploration of novel therapeutic targets.
Caveolins are a family of small integral membrane proteins consisting of caveolin-1, caveolin-2, and caveolin-3. Caveolins are postulated to be mainly involved in modulation of cholesterol movement and storage. Recently, caveolin-3 was observed to be involved in the pathogenesis of cholesterol-induced gallbladder muscle hypomotility. Cholecystokinin A receptor is a major physiologic mediator of smooth muscle contraction of the gallbladder.
This is an interesting and well written manuscript addressing some known and some postulated causes for the production of cholesterol gallstones in an animal model. This is an interesting piece of work but the authors wait to see whether this is relevant to humans and secondly whether there is any therapeutic intervention that can prevent these effects.
P- Reviewer: Bramhall S, Li YY S- Editor: Gou SX L- Editor: Ma JY E- Editor: Ma S
| 1. | Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20:1-19. [PubMed] [Cited in This Article: ] |
| 2. | Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [PubMed] [Cited in This Article: ] |
| 4. | Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157-69, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 424] [Article Influence: 23.6] [Reference Citation Analysis (5)] |
| 6. | Yoo EH, Lee SY. The prevalence and risk factors for gallstone disease. Clin Chem Lab Med. 2009;47:795-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Méndez-Sánchez N, Cárdenas-Vázquez R, Ponciano-Rodríguez G, Uribe M. Pathophysiology of cholesterol gallstone disease. Arch Med Res. 1996;27:433-441. [PubMed] [Cited in This Article: ] |
| 8. | Hay DW, Carey MC. Pathophysiology and pathogenesis of cholesterol gallstone formation. Semin Liver Dis. 1990;10:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Admirand WH, Small DM. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968;47:1043-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 816] [Cited by in F6Publishing: 811] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 428] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 11. | Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50 Suppl:S406-S411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Van Erpecum KJ. Pathogenesis of cholesterol and pigment gallstones: an update. Clin Res Hepatol Gastroenterol. 2011;35:281-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Pauletzki JG, Xu QW, Shaffer EA. Inhibition of gallbladder emptying decreases cholesterol saturation in bile in the Richardson ground squirrel. Hepatology. 1995;22:325-331. [PubMed] [Cited in This Article: ] |
| 14. | Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 645] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 15. | Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419-5422. [PubMed] [Cited in This Article: ] |
| 16. | Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160-15165. [PubMed] [Cited in This Article: ] |
| 17. | Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 871] [Cited by in F6Publishing: 867] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 18. | Cong P, Pricolo V, Biancani P, Behar J. Effects of cholesterol on CCK-1 receptors and caveolin-3 proteins recycling in human gallbladder muscle. Am J Physiol Gastrointest Liver Physiol. 2010;299:G742-G750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Sato N, Miyasaka K, Suzuki S, Kanai S, Ohta M, Kawanami T, Yoshida Y, Takiguchi S, Noda T, Takata Y. Lack of cholecystokinin-A receptor enhanced gallstone formation: a study in CCK-A receptor gene knockout mice. Dig Dis Sci. 2003;48:1944-1947. [PubMed] [Cited in This Article: ] |
| 20. | Miyasaka K, Takata Y, Funakoshi A. Association of cholecystokinin A receptor gene polymorphism with cholelithiasis and the molecular mechanisms of this polymorphism. J Gastroenterol. 2002;37 Suppl 14:102-106. [PubMed] [Cited in This Article: ] |
| 21. | Srivastava A, Pandey SN, Dixit M, Choudhuri G, Mittal B. Cholecystokinin receptor A gene polymorphism in gallstone disease and gallbladder cancer. J Gastroenterol Hepatol. 2008;23:970-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Macronutrients and insulin resistance in cholesterol gallstone disease. Am J Gastroenterol. 2008;103:2932-2939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Di Ciaula A, Wang DQ, Bonfrate L, Portincasa P. Current views on genetics and epigenetics of cholesterol gallstone disease. Cholesterol. 2013;2013:298421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | von Kampen O, Buch S, Nothnagel M, Azocar L, Molina H, Brosch M, Erhart W, von Schönfels W, Egberts J, Seeger M. Genetic and functional identification of the likely causative variant for cholesterol gallstone disease at the ABCG5/8 lithogenic locus. Hepatology. 2013;57:2407-2417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Méndez-Sánchez N, Zamora-Valdés D, Chávez-Tapia NC, Uribe M. Role of diet in cholesterol gallstone formation. Clin Chim Acta. 2007;376:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Kurtul N, Pençe S, Kocoglu H, Aksoy H, Capan Y. Serum lipid and lipoproteins in gallstone patients. Acta Medica (Hradec Kralove). 2002;45:79-81. [PubMed] [Cited in This Article: ] |
| 27. | Andreotti G, Chen J, Gao YT, Rashid A, Chang SC, Shen MC, Wang BS, Han TQ, Zhang BH, Danforth KN. Serum lipid levels and the risk of biliary tract cancers and biliary stones: A population-based study in China. Int J Cancer. 2008;122:2322-2329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |