Published online Mar 7, 2013. doi: 10.3748/wjg.v19.i9.1444
Revised: January 10, 2013
Accepted: January 18, 2013
Published online: March 7, 2013
AIM: To evaluate the effect of thienorphine on small intestinal transit in vivo and on guinea-pig ileum (GPI) contraction in vitro.
METHODS: The effects of thienorphine on intestinal transit were examined in mice and in isolated GPI. Buprenorphine and morphine served as controls. The distance traveled by the head of the charchol and the total length of the intestine were measured in vivo. Gastrointestinal transit was expressed as a percentage of the distance traveled by the head of the marker relative to the total length of the small intestine. The isolated GPI preparations were connected to an isotonic force transducer and equilibrated for at least 1 h before exposure to drugs. Acetylcholine was used for muscle stimulation.
RESULTS: Thienorphine (0.005-1.0 mg/kg, ig) or buprenorphine (0.005-1.0 mg/kg, sc) dose-dependently significantly inhibited gut transit compared with saline. Thienorphine inhibited gut transit less than buprenorphine. The maximum inhibition by thienorphine on the intestinal transit was 50%-60%, whereas the maximum inhibition by morphine on gut transit was about 100%. Thienorphine also exhibited less inhibition on acetylcholine-induced contraction of GPI, with a maximum inhibition of 65%, compared with 93% inhibition by buprenorphine and 100% inhibition by morphine. Thienorphine induced a concentration-dependent decrease in the basal tonus of spontaneous movement of the GPI, the effect of which was weaker than that with buprenorphine. The duration of the effect of thienorphine on the GPI was longer than that with buprenorphine.
CONCLUSION: Thienorphine had less influence, but a longer duration of action on GPI contraction and moderately inhibited intestinal transit.
- Citation: Zhou PL, Li YL, Yan LD, Yong Z, Yu G, Dong HJ, Yan H, Su RB, Gong ZH. Effect of thienorphine on intestinal transit and isolated guinea-pig ileum contraction. World J Gastroenterol 2013; 19(9): 1444-1450
- URL: https://www.wjgnet.com/1007-9327/full/v19/i9/1444.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i9.1444
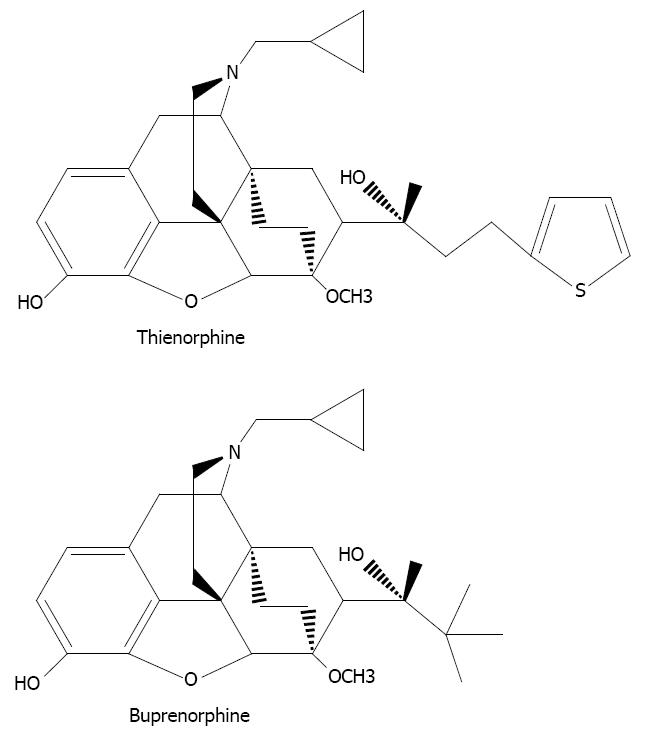
Opioids have a wide range of effects on the body, the most important of which is the treatment of moderate-to-severe pain. However, the utility of opioid agonists is limited by a number of well-known side effects, including tolerance, physical dependence, respiratory depression and gastrointestinal effects. In order to avoid addiction and other side effects, researchers have focused on the modification of oripavine. Buprenorphine, a semi-synthetic opioid derived from the opium alkaloid, thebaine, has been widely used for the treatment of opioid dependence. However, buprenorphine can only be administered sublingually in the clinic[1,2] due to its low oral bioavailability[3]. Thienorphine, N-Cyclopropylmethyl-7-[1-(R)-1-hydroxy-1-methyl-3-(thien-2-yl) propyl]-6, 14-endo-ethanotetrahydro-oripavine (Figure 1), is a new oripavine derivative designed by our institute through structural modification of buprenorphine[4]. Thienorphine exhibits higher oral bioavailability and has a stronger antinociceptive effect than buprenorphine, which is considered to be mediated by µ-opioid receptor agonism[5]. Furthermore, thienorphine has been proved to be a long-acting κ-opioid receptor agonist[6]. Although its efficacy in a rhesus monkey analgesic model was low, the antinociceptive effect of thienorphine (0.32 mg/kg) lasted for a week in monkeys[6] and the protective effect of thienorphine on morphine-induced lethality was as long as 15 d in mice[5]. Thienorphine also inhibited morphine-induced behavioral sensitization in mice[7] and has been used for the prevention of psychological dependence induced by morphine. Therefore, thienorphine, the new analog of buprenorphine, has several advantages over buprenorphine and may have wider application in the treatment of pain and opioid dependence.
Opiates can influence the autonomic outflow to the gut through their effect on the central nervous system[8] and have a direct effect on the bowel[9], and therefore induce changes in gastrointestinal motility and propulsion, leading to adverse effects on gastrointestinal function. To evaluate the effect of thienorphine on the intestinal tract, in vivo small intestinal transit in mice and in vitro guinea-pig ileum (GPI) assays were performed in the present study. Hopefully these assays may provide further evidence to understand the peripheral action and the possible adverse effects of thienorphine.
Male guinea-pigs (300-400 g) and male Kunming mice (18-22 g) were obtained from Beijing Animal Center (Beijing, China). Animals were housed in a temperature-controlled room (25 °C ± 1 °C) and maintained on a 12-h/12-h light/dark cycle. Animals had free access to food and water. Animal care and procedures were strictly in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and this study was approved by the Animal Care Committee of Beijing Institute of Pharmacology and Toxicology.
Thienorphine HCl and buprenorphine HCl were synthesized in our institute[4]. Naloxone and acetylcholine (Ach) were purchased from Sigma (St. Louis, MO, United States). NaCl, KCl, CaCl2, KH2PO4, NaHCO3, MgSO4, glucose and charcoal were provided by Beijing Chemical Plant. Morphine was produced by Qinghai Pharmaceutical Factory (Xining, China).
Mice were randomly divided into 29 groups with 10 animals in each group. The groups were treated with saline, thienorphine (0.005, 0.05, 0.5, 1.0, 2.5, 5.0, 10 and 20 mg/kg, ig), buprenorphine (0.005, 0.05, 0.5, 1.0, 2.5, 5.0, 10 and 20 mg/kg, sc), morphine (0.5, 1.0, 2.5, 5.0, 10 and 20 mg/kg, sc), and naloxone (0.1, 0.5, 1.0, 5.0, 10 and 20 mg/kg, sc), respectively. Charcoal, used as a marker, was administered orally to mice at 0.3 mL (5 g of charcoal in 100 mL of 0.5% methylcellulose). The mice were subcutaneously treated with saline (10 mL/kg), a single dose of buprenorphine, morphine or naloxone 15 min before administration of the marker. Thienorphine was administered intragastrically 30 min before the marker. At 15 min after charcoal administration, the mice were sacrificed by cervical dislocation, the abdomen was dissected and the intestine removed from the pyloric junction to the cecal end. The distance traveled by the head of the marker and the total length of the intestine were measured. Gastrointestinal transit was expressed as a percentage of the distance traveled by the head of the marker relative to the total length of the small intestine.
To determine the effects of an opioid receptor antagonist on the inhibition of intestinal transit by thienorphine (0.5 mg/kg), buprenorphine (0.5 mg/kg) and morphine (10 mg/kg), the animals were pretreated with the non-selective opioid antagonist, naloxone (10 mg/kg, sc), 15 min prior to the administration of these chemicals.
All experiments were performed on isolated ileum from male guinea-pigs weighing 300-400 g. The animals were stunned and decapitated, and the ileum was quickly isolated about 10 cm from the ileo-cecal junction. The myenteric plexus-longitudinal muscle (MPLM) was prepared using the method of Rang[10]. A glass rod was inserted into the lumen of an intestinal segment and the MPLM was removed by rubbing with a cotton swab soaked in Krebs’ solution. The preparations (2.0-2.5 cm length) were suspended under 1.0 g tension in a 10 mL organ bath containing Krebs’ solution (KCl 4.69 mmol/L, CaCl2 2.52 mmol/L, KH2PO4 1.18 mmol/L, MgSO4 1.22 mmol/L, NaHCO3 25.0 mmol/L, NaCl 118.06 mmol/L, Glucose 10.0 mmol/L, pH = 7.4), at 37 °C and bubbled with 95% O2 and 5% CO2. The preparations were connected to an isotonic force transducer linked to eight channel organ baths (Medlab6, Meiyi Ltd., Nanjing, China). All the tissues were stimulated by Ach[11,12]. Only the tissue preparations which responded to Ach (1 μmol/L) and produced contractions of more than 1.5 g tension were used. Preparations were equilibrated for at least 1 h with washes every 15 min before exposure to drugs. At the start of each experiment, a maximum response to Ach (1 μmol/L) was obtained in each tissue to confirm its suitability. After washing the preparation, the opioid agonists or antagonist were added to the organ bath for 10 min after which the second contraction with Ach was obtained. Maximal phasic responses were calculated as a percentage of the primary Ach-induced contraction, which was taken as 100% in each experiment. Each experiment was repeated with at least four separate tissue preparations obtained from different animals.
To determine the effects of naloxone, thienorphine or buprenorphine on the inhibition by morphine on MPLM preparations of GPI, the MPLM preparations were treated with morphine (2.4 mmol/L) for 10 min, and then washed twice with Krebs’ solution within 30 min. Before the second morphine (2.4 mmol/L) application, the preparations were treated with naloxone (0.5 mmol/L), thienorphine (32 μmol/L) or buprenorphine (32 μmol/L) for 10 min in the organ baths.
To study the influence of thienorphine on the recovery of GPI contraction, thienorphine (0.1 mmol/L), morphine (3.2 mmol/L), buprenorphine (0.1 mmol/L) or naloxone (1.0 mmol/L) were added to the organ baths containing the tissue preparations. After 10 min treatment, the preparations were washed with Krebs’ solution. The recovery of GPI contraction was evaluated based on stimulation with Ach (1 μmol/L) at different times during the prolonged washing process, and the recovery time-course curves were generated based on GPI contraction.
Statistical and curve-fitting analysis were performed using PRISM 5.0 (GraphPad Software Inc., La Jolla, CA, United States). The data were expressed as mean ± SE. Student’s t test was used to compare single treatment means with control means. Analysis of variance followed by Newman-Keuls post hoc test was used for analysis of multiple treatment means. P < 0.05 was considered statistically significant.
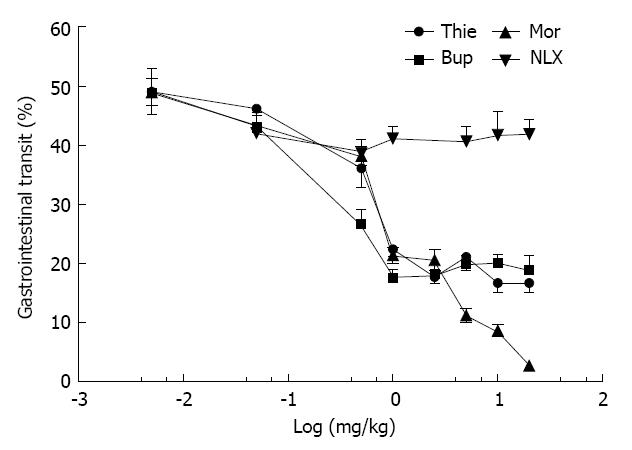
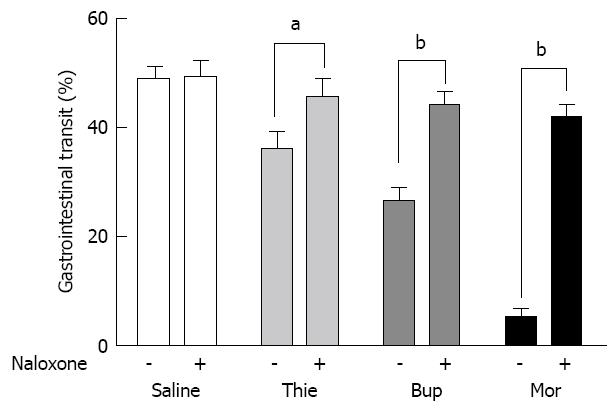
Charcoal (0.3 mL, charcoal in 100 mL 0.5% methylcellulose) did not induce diarrhea in the mice. Stools colored by the marker were of the same form as normal stools, but could easily be distinguished by their black color. Gastrointestinal transit over 15 min was approximately 50% in the saline treated mice. Morphine (0.05-20.0 mg/kg, sc) significantly inhibited gut transit (Figure 2). Thienorphine (0.005-1.0 mg/kg, ig) dose-dependently inhibited gut transit, however, the inhibitory effect was not as strong as that of buprenorphine (0.005-1.0 mg/kg, sc). At higher doses, both thienorphine (1.0-20.0 mg/kg, ig) and buprenorphine (1.0-20.0 mg/kg, sc) inhibited intestinal transit by about 50%-60%. Naloxone (0.5-20.0 mg/kg, sc) had no effect on gut transit compared with the saline treated group. However, naloxone (10.0 mg/kg, sc) antagonized the inhibitory effect of thienorphine (0.5 mg/kg, ig), buprenorphine (0.5 mg/kg, sc) and morphine (10.0 mg/kg, sc) on gastrointestinal transit when it was administered 15 min prior to these chemicals (Figure 3).
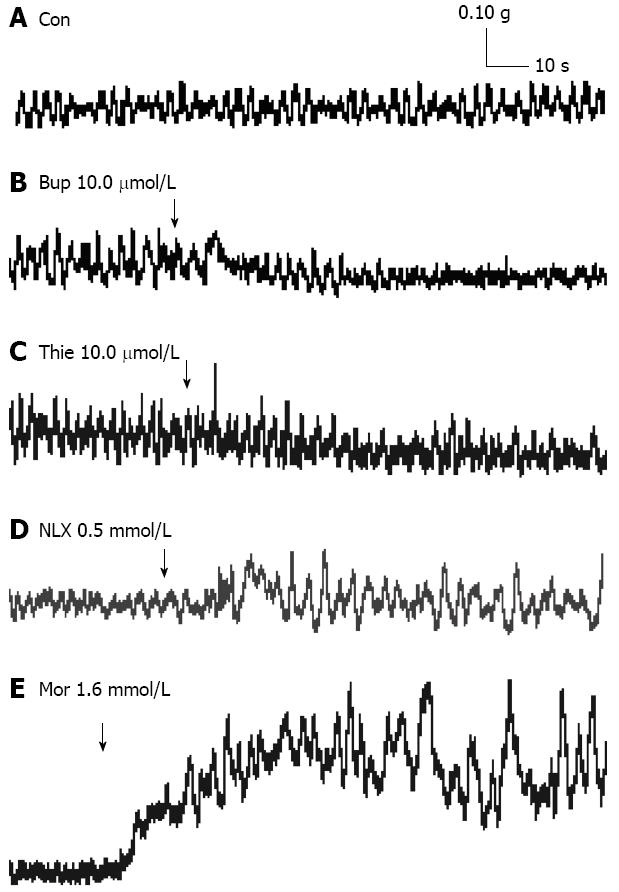
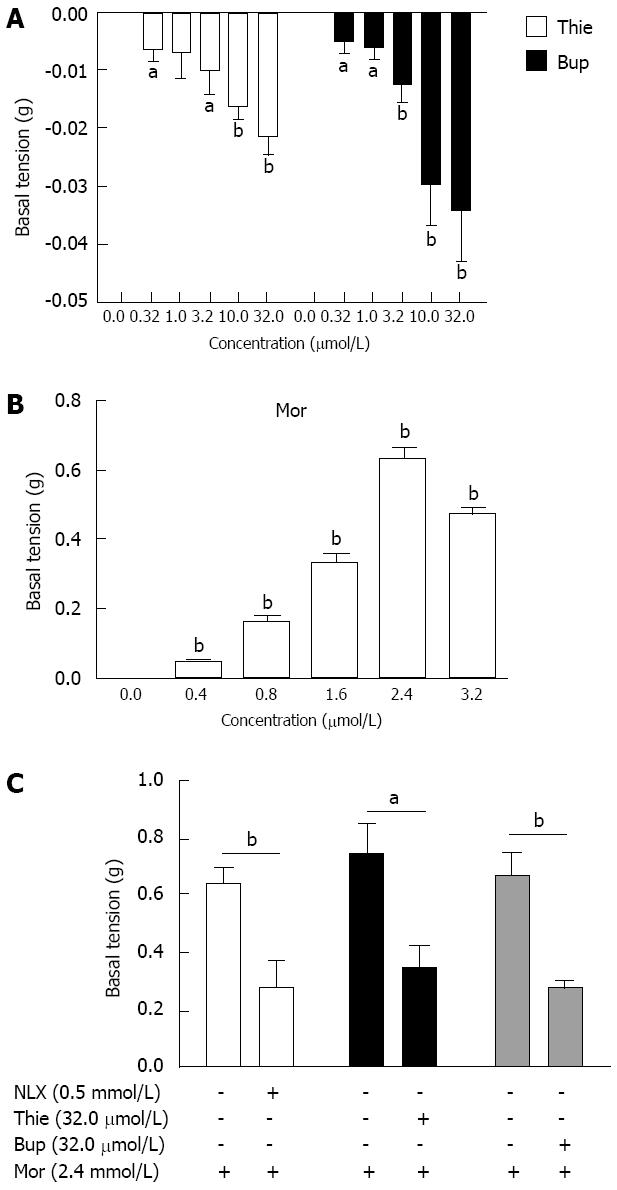
The GPI displayed regular spontaneous rhythmic contractions following the equilibration period of 45-60 min. A contraction lasted without fading for up to several hours in the control state (Figure 4A). Thienorphine (0.32-32.0 μmol/L) or buprenorphine (0.32-32.0 μmol/L) decreased the basal tonus of GPI contraction in a concentration-dependent manner. The basal tonus of GPI was decreased by 10.0 μmol/L of thienorphine or buprenorphine (Figure 4B and C). The maximum decrease in the basal tonus was 0.02 g by thienorphine (32.0 μmol/L) and 0.03 g by buprenorphine (32.0 μmol/L), with no difference between thienorphine and buprenorphine (Figure 5A). In comparison, morphine (0.4-3.2 mmol/L) concentration-dependently increased the basal tonus and spontaneous movement of GPI, and the maximum increase in basal tonus was about 0.65 g at 2.4 mmol/L (Figure 5B). The basal tonus and spontaneous movement of GPI was increased after the application of morphine 1.6 mmol/L (Figure 4E). Naloxone did not influence the basal contractile tonus, but increased the spontaneous movement of GPI at higher concentrations (Figure 4D).
Morphine (2.4 mmol/L) increased the basal tonus of GPI from 0.60-0.75 g (Figure 5C). After a 30 min resting period in Krebs’ solution, naloxone (0.5 mmol/L), thienorphine (32.0 μmol/L) or buprenorphine (32.0 μmol/L) was added to the organ baths, the increases in basal tonus following the second addition of morphine (2.4 mmol/L) were all significantly inhibited by about 50%-60% (Figure 5C). Therefore, thienorphine, as well as buprenorphine, showed a potent antagonizing effect against morphine.
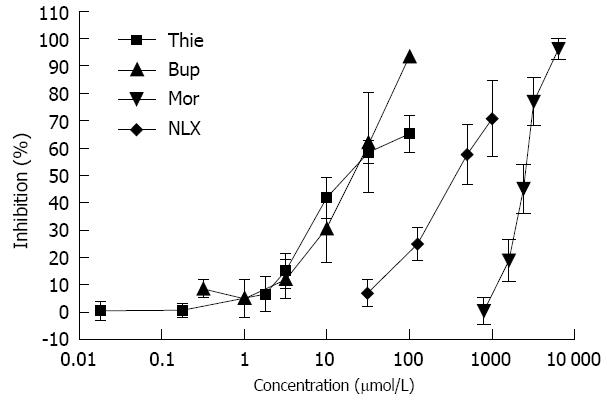
The effects of thienorphine, buprenorphine, morphine and naloxone on GPI contraction are shown in Figure 6. All three drugs inhibited ileal muscle contraction induced by Ach (1 μmol/L) in a concentration-dependent manner. Thienorphine exhibited a moderate inhibition of 65% (P < 0.01) at the highest concentration (100 μmol/L). However, buprenorphine exhibited a highly significant inhibition rate of 93% (P < 0.01) at 100 μmol/L. Morphine also showed a significant inhibition rate of 100% (P < 0.01) at the highest dose of 6.4 mmol/L. Naloxone exhibited an inhibition rate of 70% (P < 0.01) at the highest concentration of 1.0 mmol/L.
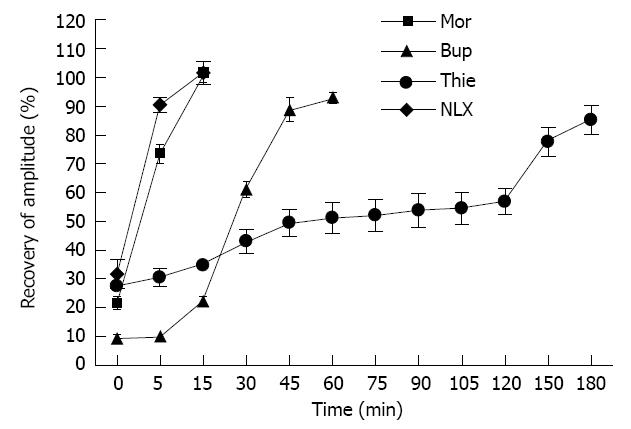
When GPI was treated with a single concentration of thienorphine 100 μmol/L, buprenorphine 100 μmol/L, morphine 3.2 mmol/L or naloxone 1.0 mmol/L, the contractile amplitude response to Ach (1 μmol/L) was 27.53% ± 4.21%, 9.60% ± 2.43%, 21.78% ± 4.78% and 31.75% ± 5.08% with respect to the amplitude before treatment, respectively. The ileal muscle strip was washed with Krebs’ solution. During the washing process, the ileal muscle contractile activity response to Ach (1 μmol/L) recovered more slowly in the thienorphine treated group than in the buprenorphine treated group. In the morphine and naloxone treated groups, the response of ileal muscle to Ach (1 μmol/L) recovered completely within 15 min. These results suggested that the effects of thienorphine on GPI lasted longer than those of the other opioids. At 60 min after the washout, the contractile amplitude of the ileal muscle after buprenorphine application recovered to 92.85% ± 3.89% with respect to the amplitude before treatment, whereas in the thienorphine treated preparation, the contractile amplitude remained at 51.25% ± 10.81%, which was significantly lower than that of the buprenorphine treated groups (P < 0.01). At 180 min after the washout, the response to Ach (1 μmol/L) of ileal muscle treated with thienorphine recovered to 85.25% ± 9.97% (Figure 7).
This study examined the effects of thienorphine on intestinal transit in mice and isolated GPI. The results indicated that thienorphine had a moderate inhibitory effect on intestinal transit in mice through the activation of opioid receptors. Compared with buprenorphine, thienorphine exhibited less inhibition, but the inhibitory effect on the contractility of GPI by direct or indirect activation of opioid receptors lasted longer. Therefore, thienorphine may have fewer adverse gastrointestinal effects when used for the treatment of opioid dependence.
The effects of opioids on gastrointestinal motility and transit have been attributed to the blockade of intestinal propulsion, resulting in slower elimination of the intestinal contents[13]. Similar to buprenorphine, thienorphine seemed to be a partial μ-opioid receptor agonist, and had weaker inhibition than morphine in the in vivo intestinal transit test. The present study showed 26.5% inhibition of gut transit by 0.5 mg/kg thienorphine and 6.1% inhibition by 0.05 mg/kg thienorphine. The Ki values of morphine for μ-, κ- and δ-opioid receptors were about 100-1000 times that of thienorphine. In the [35S]-GTPγS binding assay, the EC50 value for the stimulatory effects of thienorphine and morphine on μ-opioid receptors was 0.009 nmol/L and 18.24 nmol/L, and the maximum stimulatory values were 62.42% and 100%, respectively[5]. In the present study, the maximum inhibition by thienorphine on intestinal transit was 50%-60%, whereas the maximum inhibition by morphine on gut transit was approximately 100%. The in vivo results of thienorphine were in accordance with the [35S]-GTPγS binding assay, which indicated that the adverse gastrointestinal effects of thienorphine would not be as serious as those of morphine.
Furthermore, the inhibition by thienorphine or morphine on intestinal transit was antagonized by naloxone (10.0 mg/kg). The inhibitory effects of morphine on small intestinal transit in mice were associated with the contractile effects on circular muscle in the ileum through binding to μ-opioid receptors, resulting in the inhibition of descending peristalsis relaxation[14]. It was reported that few μ-opioid receptors were expressed in the mouse ileum, where δ- and κ-opioid receptors are dominant, and electrically induced contractions could be inhibited by δ- and κ-opioid receptor agonists, but not morphine[15]. Thienorphine displayed high affinities for both μ-, κ- and δ-opioid receptors and produced a maximum stimulation of 74.5% on κ-opioid receptors (in comparison with U69, 593), and 19.3% on μ-opioid receptors (in comparison with DAMGO)[6]. As a partial agonist of μ- and κ-opioid receptors, thienorphine also inhibited intestinal transit, but the effect was not as strong as that of morphine.
The MPLM from the GPI contains opioid peptide innervation, and enteric neurons modulate the release of enteric neurotransmitters[16], therefore, MPLM has been used to investigate the effects of opioids on intestinal motility. Stimulation of the ileum or the MPLM preparation caused rapid contractions which were abolished by atropine, suggesting the participation of cholinergic neurons. Morphine can decrease the spontaneous release of Ach from the GPI and inhibit the electric-evoked contraction of MPLM from the GPI[17]. Buprenorphine was approximately 100-fold more potent in inhibiting the electric-evoked contraction of MPLM than morphine, and the inhibition produced by buprenorphine was not eliminated by naloxone[18]. In addition, naloxone itself inhibited these contractions[18]. These results suggest that the effect of opioids on GPI contraction involves both a direct opioid receptor mechanism and indirect non-opioid receptor mechanisms. In the present study, MPLM preparations were stimulated by Ach instead of electrical stimulation. The inhibition of opioids on the release of Ach from the MPLM preparation could be ignored due to the application of Ach (1 μmol/L). Through activation of M2 and M3 acetylcholine receptors, Ach induced voltage-dependent and voltage-independent Ca2+ entry and intracellular Ca2+ release, leading to the contraction of intestinal smooth muscle[19]. Thienorphine and buprenorphine (0.01-100 μmol/L) induced the relaxation of contractile GPI under Ach stimulation in a concentration-dependent manner, which was similar to morphine and naloxone. Thienorphine was approximately 100-fold more potent than morphine, and the inhibition was not eliminated by naloxone. These results further proved the participation of indirect non-opioid receptors mechanisms. Activation of μ- opioid receptors led to an outward potassium conductance, and therefore produced membrane hyper-polarization and an increase in conductance, plus indirect inhibition of calcium entry[20]; voltage-clamp studies showed that a κ opioid receptor agonist directly reduced calcium currents in mouse DRG cells[21,22]; acute opioid exposure decreases calcium fluxes in neurons[23,24]; loperamide, a peripheral agonist of μ opioid receptors, was found to induce intestinal relaxation by opening KATP channels via the cAMP-PKA pathway[25] which further induced hyper-polarization of the cell membrane leading to relaxation of smooth muscle. Based on these studies, the inhibition by opioids on Ach-induced GPI contraction at higher concentrations further suggested the participation of other indirect non-opioid receptors or channels.
Thienorphine or buprenorphine induced a concentration-dependent decrease in the basal tonus and spontaneous movement of MPLM, whereas morphine concentration-dependently increased the basal tonus and spontaneous contraction of MPLM. The effect of thienorphine and buprenorphine was not antagonized by naloxone, while morphine-induced contraction was partially inhibited by naloxone or thienorphine. Similar results for morphine on the isolated circular muscle of mouse ileum have also been reported[14]. Therefore, the effect of theinorphine on the MPLM simulates neither naloxone nor morphine, the mechanisms of which still require further study.
In our previous study, the contractile activity of uterine strips after thienorphine treatment recovered more slowly than after buprenorphine treatment during the washing process. In the present study, the contractile activity of isolated guinea-pig ileum also recovered more slowly after thienorphine treatment compared with buprenorphine or morphine treatment. Therefore, in the in vitro isolated tissue assay, the effects of thienorphine lasted longer, which was consistent with its in vivo effects.
In this study, we demonstrated that thienorphine, a new derivative of buprenorphine, moderately inhibited intestinal transit, showed less inhibition on contractile amplitude and a longer duration of action on guinea-pig isolated ileum.
Thienorphine, the new analog of buprenorphine, had several advantages over buprenorphine and may have wider application in the treatment of pain and opioid dependence. However, the utility of opioids is limited by a number of well-known side effects, including tolerance, physical dependence, respiratory depression and gastrointestinal effects.
Opioids exhibit their effects through μ, κ and δ receptors both in the central and peripheral nervous system. Opiates can influence the autonomic outflow to the gut through their effects on the nervous system and have a direct effect on the bowel, and therefore induce changes in gastrointestinal motility and propulsion.
Thienorphine has several advantages over buprenorphine, such as stronger antinociceptive effects, potent rate-decreasing effects and better oral bioavailability than buprenorphine. Therefore, thienorphine, the new analog of buprenorphine, may have wider application in the treatment of pain and opioid dependence. This study reported on thienorphine, a new partial agonist of opioid receptors, which has fewer effects on intestinal transit and isolated guinea-pig ileum contraction.
Thienorphine has less influence and a longer duration of action on intestinal motility, and may be a new candidate for the treatment of pain and opioid dependence.
In vivo intestinal transit tests and isolated guinea-pig ileum assays are well reported methods used to study the effects of chemicals on smooth muscle contraction.
This is an interesting and important paper, which used well known tests for studying the effect of a new drug on motility. Thienorphine had moderate inhibition on intestinal transit, less inhibition on the contractile amplitude and longer duration of action on the guinea-pig isolated ileum. Thienorphine has less influence and longer duration on motility which may be a new useful candidate for the treatment of pain and opioid dependence.
P- Reviewer Rossignol JF S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Mendelson J, Upton RA, Everhart ET, Jacob P, Jones RT. Bioavailability of sublingual buprenorphine. J Clin Pharmacol. 1997;37:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Schuh KJ, Johanson CE. Pharmacokinetic comparison of the buprenorphine sublingual liquid and tablet. Drug Alcohol Depend. 1999;56:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Heel RC, Brogden RN, Speight TM, Avery GS. Buprenorphine: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1979;17:81-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 275] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Liu H, Zhong BH, Liu CH, Wu B, Gong ZH. Synthesis, crystal structure and pharmacological study of N-cyclopropylmethyl-7- [1-(R)-1-hydroxy-1-methyl-3-(thien-2-yl) propyl]-6,14-endo-ethanotetrahydro-oripavine. Acta Chim Slov. 2005;52:80-85. [Cited in This Article: ] |
| 5. | Yu G, Yue YJ, Cui MX, Gong ZH. Thienorphine is a potent long-acting partial opioid agonist: a comparative study with buprenorphine. J Pharmacol Exp Ther. 2006;318:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Li JX, Becker GL, Traynor JR, Gong ZH, France CP. Thienorphine: receptor binding and behavioral effects in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Zhao WL, Gong ZH, Liang JH. A new buprenorphine analogy, thenorphine, inhibits morphine-induced behavioral sensitization in mice. Acta Pharmacol Sin. 2004;25:1413-1418. [PubMed] [Cited in This Article: ] |
| 8. | Galligan JJ, Burks TF. Centrally mediated inhibition of small intestinal transit and motility by morphine in the rat. J Pharmacol Exp Ther. 1983;226:356-361. [PubMed] [Cited in This Article: ] |
| 9. | Burks TF. Mediation by 5-hydroxytryptamine of morphine stimulant actions in dog intestine. J Pharmacol Exp Ther. 1973;185:530-539. [PubMed] [Cited in This Article: ] |
| 10. | Rang HP. Stimulant actions of volatile anaesthetics on smooth muscle. Br J Pharmacol Chemother. 1964;22:356-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Ghosh MN. Fundamentals of experimental pharmacology. 3rd ed. Kolkata: Hilton and Company 2005; 110-120. [Cited in This Article: ] |
| 12. | Bhargava HN. Diversity of agents that modify opioid tolerance, physical dependence, abstinence syndrome, and self-administrative behavior. Pharmacol Rev. 1994;46:293-324. [PubMed] [Cited in This Article: ] |
| 13. | De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 203] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Iwata H, Tsuchiya S, Nakamura T, Yano S. Morphine leads to contraction of the ileal circular muscle via inhibition of the nitrergic pathway in mice. Eur J Pharmacol. 2007;574:66-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Smith CF, Waldron C, Brook NA. Opioid receptors in the mouse ileum. Arch Int Pharmacodyn Ther. 1988;291:122-131. [PubMed] [Cited in This Article: ] |
| 16. | Waterfield AA, Kosterlitz HW. Stereospecific increase by narcotic antagonists of evoked acetylcholine output in guinea-pig ileum. Life Sci. 1975;16:1787-1792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Szerb JC. Correlation between acetylcholine release and neuronal activity in the guinea-pig ileum myenteric plexus; effect of morphine. Neuroscience. 1982;7:327-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kajiwara M, Aoki K, Ishii K, Numata H, Matsumiya T, Oka T. Agonist and antagonist actions of buprenorphine on three types of opioid receptor in isolated preparations. Jpn J Pharmacol. 1986;40:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Suguro M, Matsuyama H, Tanahashi Y, Unno T, Kitazawa T, Yamada M, Komori S. Muscarinic receptor subtypes mediating Ca2+ sensitization of intestinal smooth muscle contraction: studies with receptor knockout mice. J Vet Med Sci. 2010;72:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | McFadzean I. The ionic mechanisms underlying opioid actions. Neuropeptides. 1988;11:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Werz MA, Macdonald RL. Dynorphin reduces voltage-dependent calcium conductance of mouse dorsal root ganglion neurons. Neuropeptides. 1984;5:253-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Macdonald RL, Werz MA. Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J Physiol. 1986;377:237-249. [PubMed] [Cited in This Article: ] |
| 23. | Bradford HF, Crowder JM, White EJ. Inhibitory actions of opioid compounds on calcium fluxes and neurotransmitter release from mammalian cerebral cortical slices. Br J Pharmacol. 1986;88:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Crowder JM, Norris DK, Bradford HF. Morphine inhibition of calcium fluxes, neurotransmitter release and protein and lipid phosphorylation in brain slices and synaptosomes. Biochem Pharmacol. 1986;35:2501-2507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Chen W, Chung HH, Cheng JT. Opiate-induced constipation related to activation of small intestine opioid μ2-receptors. World J Gastroenterol. 2012;18:1391-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |