Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7104
Revised: August 25, 2012
Accepted: September 19, 2012
Published online: December 21, 2012
We present a case with hepatic myelopathy (HM) due to a surgical splenorenal shunt that was successfully treated by endovascular interventional techniques. A 39-year-old man presented with progressive spastic paraparesis of his lower limbs 14 mo after a splenorenal shunt. A portal venogram identified a widened patent splenorenal shunt. We used an occlusion balloon catheter initially to occlude the shunt. Further monitoring of the patient revealed a decrease in his serum ammonia level and an improvement in leg strength. We then used an Amplatzer vascular plug (AVP) to enable closure of the shunt. During the follow up period of 7 mo, the patient experienced significant clinical improvement and normalization of blood ammonia, without any complications. Occlusion of a surgically created splenorenal shunt with AVP represents an alternative therapy to surgery or coil embolization that can help to relieve shunt-induced HM symptoms.
- Citation: Wang MQ, Liu FY, Duan F. Management of surgical splenorenal shunt-related hepatic myelopathy with endovascular interventional techniques. World J Gastroenterol 2012; 18(47): 7104-7108
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7104
Hepatic myelopathy (HM) is a rarely reported disorder characterized by progressive spastic paraparesis of the lower extremities due to impaired corticospinal tract function in the setting of cirrhosis or portosystemic shunting[1-3]. Over 85% of reported HM cases have been associated with surgical, angiographic, or spontaneous portosystemic shunting. Both HM and hepatic encephalopathy (HE) after a surgical portosystemic shunt are thought to be secondary to the increased shunting of portal venous toxins to the systemic circulation and from hypoperfusion and ischemia of the hepatocytes. However, unlike HE, conservative treatment of HM is usually considered inefficient.
Recently, outcomes for a small number of patients who have undergone liver transplantation (LT) for HM suggest a potential neurological benefit, especially with earlier transplantation[4-6]. However, for patients with normal liver function or Child-Pugh A grade cirrhosis, the choice of LT vs other treatments (i.e., narrowing or occlusion of the shunt) is debatable[3,4]. In addition, the limited donor organ supply remains a major issue. In the present study, we report a case with HM due to a surgical splenorenal shunt that was successfully occluded by endovascular interventional techniques, which resulted in significant clinical improvement and normalization of blood ammonia.
A 39-year-old man presented in April 2010 with an 11 mo history of slowly progressive spastic paraparesis of his lower extremities. The patient in question had a history of hepatitis B disease. In March 2008, he underwent a surgical splenorenal shunt and splenectomy due to recurrent esophagogastric variceal bleeding and thrombocytopenia. In May 2009, fourteen months after his surgical splenorenal shunt, gait impairment was first noticed. An episode of HE was not seen during this period. The patient received conservative medical management, including protein restriction, non-absorbable antibiotics, lactulose and physical therapy, after the onset of gait impairment. However, these measures did not prevent the progressive decline in his mobility and this necessitated the use of a cane because of marked leg weakness, gait imbalance, and instability.
Physical examination revealed normal mental status and cranial nerve function, and the absence of asterixis. Kayser-Fleischer rings were absent, upper extremity strength and tendon reflexes were normal, and Hoffman’s sign was absent. In the lower extremities, the legs were spastic, the tone was more noticeably increased with brisk tendon reflexes and there was clonus at the ankles. Plantar reflexes were extensor and the patient was unable to move his legs. There was no atrophy or any fasciculations, nor any evidence of ascites or peripheral edema.
Laboratory investigations showed normal serum levels of electrolytes, glucose, vitamin B12, and creatinine. He had mild anemia (hemoglobin level of 112 g/L; normal range, 120-160 g/L) and thrombocytopenia (85 × 103/mm3; normal range, 100 × 103-300 × 103/mm3). Prothrombin time was 16 s (normal range, 11-14.5 s). Other values were as follows: 28 μmol/L total bilirubin (normal range, 3-20 μmol/L); 15 μmol/L conjugated bilirubin (normal range, 0-7 μmol/L); 156 μmol/L ammonia (normal range, 18-45 μmol/L); 56 U/L alanine aminotransferase (normal range, 5-40 U/L); 65 U/L aspartate aminotransferase (normal range, 15-45 U/L); 126 U/L alkaline phosphatase (normal range, 30-115 U/L); 36 g/L albumin (normal range, 35-50 g/L); 45 g/L globulin (normal range, 21-40 g/L); international normalized ratio 1.3 (normal range, 0.9-1.2). His liver was graded as Child-Pugh class A.
Serum examinations for hepatitis B surface antigen and hepatitis B e-antigen were positive, but a test for hepatitis B core antigen was negative. Antibodies to hepatitis C virus and human immunodeficiency virus were negative, syphilis serology was negative, and an analysis of the cerebrospinal fluid was normal.
Magnetic resonance imaging of the brain and spine was normal, and an abdominal computed tomography (CT) revealed a cirrhotic liver. An abdominal ultrasound examination showed features of cirrhosis of liver and a Doppler study showed a widely-open splenorenal shunt. Electro-encephalography revealed no definite abnormality and an endoscopic examination revealed no esophagogastric varices.
Given the aforementioned extensive neurological evaluation, which did not identify any alternative explanation for the patient’s spastic paraparesis of the lower extremities, the diagnosis of HM secondary to the surgical splenorenal shunting was made. After admission, with protein restriction and medication with lactulose, neomycin, B-vitamins, physical therapy, and antispastic agents for 3 wk, the patient showed no improvement in his spastic paraparesis, and his hyperammonemia remained within the range of 140-180 μmol/L.
After discussion with hepatologists, gastroenterologists, and vascular surgeons at the authors’ hospital, we decided to occlude the surgical splenorenal shunt to prevent further neurologic deterioration. Informed consent was obtained from the patient.
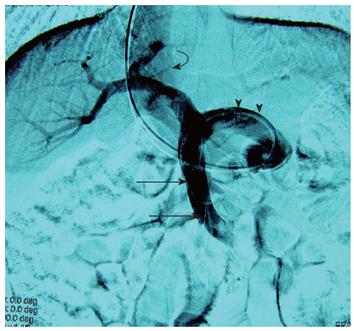
Under local anesthesia (2% lidocaine) and fluoroscopic guidance, venous access was obtained through the right internal jugular vein, and a 9 Fr with an angled-tip 80-cm long introducer sheath (Cook, Bloomington, Indiana) was advanced into the inferior vena cava. The splenorenal shunt was then catheterized with a 5 Fr Cobra catheter (Terumo Corporation, Tokyo, Japan) and an angled-tip 0.035-inch hydrophilic guidewire (Terumo). Initial portography and pressure measurements were performed with a 5 Fr multiple-side-hole catheter (Cook) positioned in the superior mesenteric vein. At the portal venogram, a widened patent shunt was seen (Figure 1), with a portacaval pressure gradient of 8.0 mmHg. The portal vein pressure was 12.5 mmHg.
We initially placed an occlusion balloon catheter into the splenorenal shunt tract to test if the patient could be tolerable to the full embolization procedure. Following the angiographic procedure, over a 0.035-inch Amplatz wire (Cook), the 9 Fr introducer sheath advanced into the splenorenal shunt. A 7 Fr latex balloon catheter, with a maximum outer diameter of 16 mm (Boston Scientific, Watertown, Mass) was then inserted into the splenorenal shunt through the sheath. Under the inflated balloon, injection of contrast material through the sheath showed complete occlusion of the surgical splenorenal shunt. After the balloon occlusion, heparin infusion was given via a peripheral vein, at a dosage of 120 mg/24 h, to avoid catheter-related thrombosis.
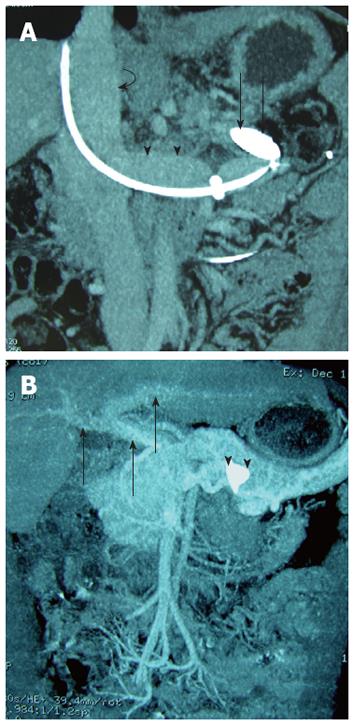
Following the balloon occlusion procedure, the patient’s plasma ammonia level decreased to 110 μmol/L, 70 μmol/L, 60 μmol/L and 50 μmol/L, on post-procedural days 2, 3, 5 and 6, respectively. He reported a mild improvement in his leg strength and balance, while the other HE-related symptoms remained unchanged. Follow-up CT on post-procedural day 5 showed the balloon positioned appropriately (Figure 2A). Repeat liver function tests were normal, and repeat endoscopic examination confirmed no induction of esophagogastric varices. There was no evidence of ascites. Six days after the balloon occlusion procedure, we decide to occlusion the splenorenal shunt permanently with endovascular embolization.
Given the relatively larger diameter of the splenorenal shunt, we decided to use an Amplatzer vascular plug (AVP) instead of conventional embolization materials (i.e., coils, glue, and particles).
The diameter of the surgical splenorenal shunt was approximately 13 mm in diameter, calculated on the angiography workstation. Given the diameter of the shunt, we used an 18-mm AVP (AGA Medical Corp., Golden Valley, MN, United States) with the 30%-50% oversizing recommended by the manufacturer, to prevent plug migration. For deploying the AVP, the 9 Fr introducer sheath advanced over a 0.035-inch Amplatz wire (Cook) into the most distal extreme of the splenorenal shunt. The AVP device was then advanced through the 9 Fr sheath and initially deployed by retracting the sheath, but without detaching the device. A control injection was performed, to ensure satisfactory positioning of the device and to enable repositioning. When in a satisfactory position, the AVP was detached by anticlockwise rotation of the AVP guide wire to unscrew it from the AVP device proper. Within minutes of deployment, the shunt had completely stopped. Injection of contrast material through the sheath showed complete occlusion of the surgical shunt.
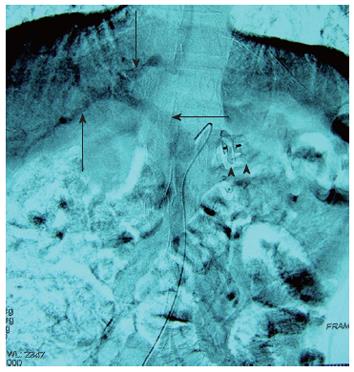
Following AVP embolization, an indirect digital subtraction portography, carried out during the same procedure and obtained with a 4 Fr catheter (Terumo) inserted into the superior mesenteric artery (SMA) via the right femoral artery approach, was used to evaluate portal vein system. Digital subtraction SMA angiogram at the portal venous phase demonstrated a marked improvement in the opacification of the portal vein in comparison with that of the portography pre-deployment of the AVP (Figure 3), indicating an increase in antegrade intrahepatic portal vein perfusion. No contrast material passed through the surgical splenorenal shunt.
No complication was noted during and after the procedure. Five days after the AVP embolization, the patient’s serum ammonia level had normalized to 40 μmol/L.
The patient was discharged 6 d post-AVP embolization. Doppler US confirmed complete occlusion of the splenorenal shunt. In June 2010, one month after the embolization, the patient reported a gradual improvement in strength; he was still moderately weak but was able to walk short distances (50-100 m) with crutches.
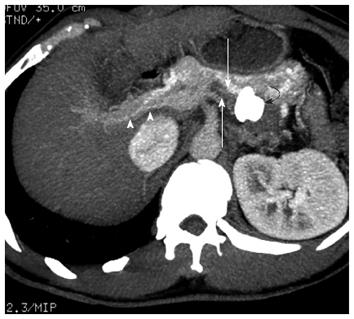
By 3 mo after the procedure, he was able to walk about 300-500 m with crutches. He still complained of stiffness and spasms in his legs, but improved compared to 3 mo ago. A follow-up contrast enhanced CT scan at the venous phase confirmed that the AVP position was correct, that there was opacification of the intrahepatic portal veins, that the splenorenal shunt tract was thrombosed, and that there was no opacification of the surgical shunt or varices (Figures 2B and 4).
The patient continued to show improvement in his HM symptoms over the next few months. In December 2010, 7 mo after the splenorenal shunt embolization, his strength improved significantly, he was able to walk 1 to 2 km aided by crutches, and had only mild stiffness in both legs. Esophagogastroscopy showed no evidence of esophagogastric varices.
HM responds poorly to conservative medical therapy and it has a poor prognosis because of its progressive and irreversible nature[2,3]. Currently, treatment options in HM include surgical ligation, liver transplantation, shunt reduction, or occlusion by interventional procedures. Surgical ligation has been reported to be effective, but is used only occasionally[1]. LT remains a potentially definitive treatment for HM in patients with decompensated cirrhosis of Child-Pugh B and C grade[4-6]. However, for patients with normal function liver or Child-Pugh A grade cirrhosis, the choice of LT vs other treatments (i.e., narrowing or occlusion of the shunt) is debatable[1,2]. Our patient had no history of HE, and his laboratory studies showed no liver dysfunction, with the exception of an increase in his serum ammonia level. Thus, occlusion of the surgical splenorenal shunt may be an alternative therapeutic option.
Interventional endovascular shunt occlusion has been used previously to treat post-surgical shunt HE and post-transjugular intrahepatic portosystemic shunt (TIPS) HE[2,7]; however, the usefulness of the technique for post-surgical shunt HM has not yet been determined. In the present case, the patient’s gait impairment was noticed 14 mo after his surgical splenorenal shunt, with a progressive decline in his mobility afterward. We successfully occluded the large surgical splenorenal shunt using an AVP. Following AVP embolization, the patient reported a gradual improvement in leg strength and balance. Seven months later the patient was able to walk 1 to 2 km aided by crutches, with only mild residual spasticity of his lower extremities. To our knowledge, reversal of HM by occlusion of a surgical splenorenal shunt with AVP has not been reported.
Possible embolizing materials for the embolization of the portosystemic shunt are coils, a detachable balloon, and an AVP. We chose AVP implantation for our patient due to the relatively large size of the surgical splenorenal shunt. In this situation, a number of coils would be needed to achieve adequate vessel closure. In addition, coil migration may occur when used in short shunt tracts[7-10]. AVPs has recently been shown to be effective in the occlusion of internal iliac arteries[9], the treatment of pulmonary arteriovenous malformations[10], and as an occlusion system for a splenorenal shunt arising after TIPS[8,11]. The advantage of the AVP, compared to coils, is that it can be more precisely placed within the vessel and that it can be repositioned or removed, if necessary.
There is some doubt as to whether portal pressure could rise after portosystemic shunt embolization. This would constitute a critical complication for the patient, resulting in the aggravation of esophageal varices or even the development of new varices[2,12,13]. One study concluded that, to avoid the consequences of a sudden increase in portal pressure, embolization should be indicated only in patients with absent or mild esophageal varices and with no signs of hepatic failure, such as ascites or jaundice[14]. In addition, routine periprocedural endoscopy is recommended in this setting to minimize the incidence of embolization-related complications. In this report, we used an occlusion balloon catheter initially to occlude the surgical shunt. Further monitoring of the patient over the next few days revealed no evidence of induction varices or ascites; thus we decided to use an AVP to enable closure of the shunt.
Our case raises several unique points not previously noted in the literature. Firstly, to our knowledge, this is the first report of a surgical shunt relate-HM successfully embolized with AVP resulting in an immediate improvement in intrahepatic portal perfusion, normalization of blood ammonia, and a gradual improvement of HM-related symptoms. Secondly, since we were able to document a temporary balloon occlusion of the surgical shunt prior to permanent embolization, it may be possible to predict clinical and laboratory improvement. Finally, in patients with normal function liver or Child-Pugh A grade cirrhosis, shunt occlusion may represent a suitable alternative therapy to LT that can help to relieve shunt-induced HM symptoms.
In conclusion, for the present case, we successfully occluded a large surgical splenorenal shunt using an AVP, which resulted in significant clinical improvement of the shunt-induced HM symptoms. This technique represents a viable alternative to surgery or coil embolization, although further study is necessary. In addition, trial balloon occlusion of the shunt prior to complete permanent embolization can be used to predict the clinical and laboratory improvement.
Peer reviewer: Dr. Andres Cardenas, Institut de Malalties, Digestives i Metaboliques Hospital Clinic, Villaroel 170, Esc 7-4, 08036 Barcelona, Spain
S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Utku U, Asil T, Balci K, Uzunca I, Celik Y. Hepatic myelopathy with spastic paraparesis. Clin Neurol Neurosurg. 2005;107:514-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Conn HO, Rössle M, Levy L, Glocker FX. Portosystemic myelopathy: spastic paraparesis after portosystemic shunting. Scand J Gastroenterol. 2006;41:619-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | O'Brien J, Staples C, Florin T. Trouble with a shunt: alcohol and spastic paraparesis. Hepatic myelopathy. Gastroenterology. 2010;139:1099, 1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Weissenborn K, Tietge UJ, Bokemeyer M, Mohammadi B, Bode U, Manns MP, Caselitz M. Liver transplantation improves hepatic myelopathy: evidence by three cases. Gastroenterology. 2003;124:346-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Caldwell C, Werdiger N, Jakab S, Schilsky M, Arvelakis A, Kulkarni S, Emre S. Use of model for end-stage liver disease exception points for early liver transplantation and successful reversal of hepatic myelopathy with a review of the literature. Liver Transpl. 2010;16:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Baccarani U, Zola E, Adani GL, Cavalletti M, Schiff S, Cagnin A, Poci C, Merkel C, Amodio P, Montagnese S. Reversal of hepatic myelopathy after liver transplantation: fifteen plus one. Liver Transpl. 2010;16:1336-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Tuite DJ, Kessel DO, Nicholson AA, Patel JV, McPherson SJ, Shaw DR. Initial clinical experience using the Amplatzer Vascular Plug. Cardiovasc Intervent Radiol. 2007;30:650-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Kessler J, Trerotola SO. Use of the Amplatzer Vascular Plug for embolization of a large retroperitoneal shunt during transjugular intrahepatic portosystemic shunt creation for gastric variceal bleeding. J Vasc Interv Radiol. 2006;17:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ha CD, Calcagno D. Amplatzer Vascular Plug to occlude the internal iliac arteries in patients undergoing aortoiliac aneurysm repair. J Vasc Surg. 2005;42:1058-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Ferro C, Rossi UG, Bovio G, Seitun S, Rossi GA. Percutaneous transcatheter embolization of a large pulmonary arteriovenous fistula with an Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2007;30:328-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | White HA, Travis SJ. The Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2008;31:448-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Pattynama PM, Wils A, van der Linden E, van Dijk LC. Embolization with the Amplatzer Vascular Plug in TIPS patients. Cardiovasc Intervent Radiol. 2007;30:1218-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Boixadera H, Tomasello A, Quiroga S, Cordoba J, Perez M, Segarra A. Successful embolization of a spontaneous mesocaval shunt using the Amplatzer Vascular Plug II. Cardiovasc Intervent Radiol. 2010;33:1044-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 2007;20:120-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |