Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1104
Revised: January 12, 2012
Accepted: February 8, 2012
Published online: March 14, 2012
AIM: To determine the efficacy of bevacizumab in patients with metastatic colorectal cancer (MCRC) who have failed prior chemotherapy without bevacizumab.
METHODS: Between March 2002 and June 2010, 40 patients in South Korea with MCRC who were treated with bevacizumab plus chemotherapy as a second or later-line treatment were analyzed retrospectively for their overall response rate (ORR), overall survival (OS), and progression-free survival (PFS). The tumor responses were assessed using the Response Evaluation Criteria in Solid Tumors guidelines.
RESULTS: All of the patients had progressed under prior chemotherapy without bevacizumab. Three patients (7.5%) exhibited an ORR, twenty one patients (52.5%) exhibited stable disease (SD), and fifteen patients (37.5%) exhibited disease progression. The median duration of the OS and PFS were 14.0 mo and 6.13 mo respectively. The median OSs were 16.60, 14.07 and 13.00 mo for second-line, third-line and fourth- or later-line treatments, respectively. The median PFSs were 7.23, 7.30 and 3.87 mo for the second-line, third-line and fourth- or later-line treatments, respectively.
CONCLUSION: In patients with MCRC, bevacizumab combined chemotherapy may be beneficial during second- or later-line treatment.
- Citation: Park LC, Lee HS, Shin SH, Park SJ, Park MI, Oh SY, Kwon HC, Baek JH, Choi YJ, Kang MJ, Kim YS. Bevacizumab as a second- or later-line of treatment for metastatic colorectal cancer. World J Gastroenterol 2012; 18(10): 1104-1109
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1104
Metastatic colorectal cancer (MCRC) is a common cancer, and significant advances have been made in the treatment of this disease. The integration of oxaliplatin or irinotecan chemotherapies in combination with 5-fluorouracil (5-FU) and leucovorin (LV) as a front-line therapy for MCRC is associated with significant improvements in the time-to-progression and overall survival (OS)[1-3].
The recent integration of bevacizumab in front-line therapy for MCRC has resulted in an additional positive impact on the outcome for colorectal cancer patients. Vascular endothelial growth factor (VEGF) is a diffusible, homodimeric glycoprotein this is produced by healthy and neoplastic cells and is a key promoter of angiogenesis under physiologic and pathologic conditions, including tumor progression[4]. The VEGF family includes six members [VEGF-A-E and placental growth factor (PIGF)]. The major mediator of tumor angiogenesis is VEGF-A[5]. Bevacizumab is a recombinant, humanized, monoclonal antibody directed against the VEGF ligand (VEGF-A); bevacizumab binds all isoforms of VEGF-A with a high affinity[6]. The inhibition of VEGF with bevacizumab has been shown to result in tumor reduction of colon cancer in xenograft models and acts in synergy with chemotherapy[7]. The addition of bevacizumab to 5-FU-based combination chemotherapy has been shown to result in a statistically significant and clinically meaningful improvement in survival among patients with MCRC[8-11]. The addition of bevacizumab to the FOLFOX chemotherapy regimen, consisting of 5-FU, LV, and oxaliplatin, provides a statistically significant and clinically meaningful improvement in OS, compared to FOLFOX alone, in patients with advanced or metastatic disease in whom the disease has progressed after adjuvant chemotherapy with FOLFIRI (5-FU, LV and irinotecan)[12,13]. However, based on the inconclusive results from multicenter studies, the role of bevacizumab in the treatment of patients with a disease that is refractory to 5-FU, irinotecan, and oxaliplatin is not yet known[14-16].
The present retrospective study was designed to determine the efficacy and safety of bevacizumab combined with chemotherapy in patients with MCRC who have failed prior chemotherapy without bevacizumab.
Between April 2005 and June 2010, 40 patients with MCRC were treated with bevacizumab plus chemotherapy as a second- or later-line treatment in Busan, South Korea. Patients who were eligible for the present study suffered from histologically-confirmed MCRC. Other inclusion criteria included an age of at least 20 years, and a life expectancy of > 3 mo. There were no limitations on the number of prior therapies or on the Eastern Cooperative Oncology Group (ECOG) performance status. However, adequate hematologic (an absolute neutrophil count > 1500/μL, hemoglobin > 9.0 g/dL, and a platelet count > 75 000/μL), hepatic (bilirubin < 2.0 mg/dL and transaminase levels < 3 times the upper normal limit), and renal functions (creatinine < 1.5 mg/dL and urinary excretion ≤ 500 mg of protein per day) were required.
The exclusion criteria included the presence of clinically significant cardiovascular disease, uncontrolled hypertension, central nervous system metastasis, major surgery within 6 wk, pregnancy or lactation, non-healing wounds, bleeding diatheses, the regular use of aspirin (> 325 mg/d) or other non-steroidal anti-inflammatory agents, pre-existing bleeding diatheses or coagulopathies, the need for full-dose anticoagulation or prior bevacizumab therapy.
Among the 40 patients in the present study, 12 patients received bevacizumab plus oxaliplatin-containing chemotherapy, 19 patients received bevacizumab plus irinotecan-containing chemotherapy, 8 patients received bevacizumab plus 5-FU and LV (FL), and 1 patient received bevacizumab alone. Bevacizumab plus FOLFOX or FOLFIRI chemotherapy was administered every 2 wk and consisted of the following: intravenous (IV) oxaliplatin (85 mg/m2) or irinotecan (150 mg/m2) over 2 h; IV LV (200 mg/m2) over 2 h, followed by a bolus of 5-FU (400 mg/m2); and infusional 5-FU (600 mg/m2) over 22 h, with FL repeated on day 1. The FL chemotherapy was administered every 4 wk [infusional 5-FU (375 mg/m2) over 24 h, and the IV LV (20 mg/m2)] was administered over 2 h for 5 d. Bevacizumab was administered intravenously at 5 mg/kg over 30 min to 90 min every 2 wk, prior to FOLFOX, FOLFIRI, or FL.
The objective of the present study was to evaluate the overall response rate (ORR), OS, progression-free survival (PFS), and toxicity of bevacizumab in patients who failed prior treatment. The tumor responses were assessed using Response Evaluation Criteria in Solid Tumors guidelines[17]. Progression was defined as a 20% increase at the time of disease progression.
The toxicities were graded using the NCI Common Terminology Criteria for Adverse Events (version 3.0)[18]. Radiographic assessments were performed at baseline (within 4 wk before starting chemotherapy) and every 6 wk to 8 wk. Radiologic evaluation consisted of a chest X-ray, bone scan, chest computed tomography (CT) scan, and abdominopelvic CT scan.
All of the patients who received at least three dose of therapy were included in the PFS and OS analyses. The OS was defined as the time elapsed between the initiation of the study therapy to the date of death from any cause. The PFS was defined as the time elapsed between the initiation of the study therapy to the date of the progressive disease (PD). Patients who died without a documented PD were considered to have had a PD at the time of death. Patients who were removed from therapy for toxicity without clinical or radiographic evidence of PD were censored. Patients who were lost to follow-up were censored at the last contact date. Survival curves were estimated using the Kaplan-Meier method.
The median age of the patients in the present study was 55.5 years. Forty patients received FOLFOX, FOLFIRI or FL, plus bevacizumab or bevacizumab alone, as a therapy. The metastatic sites were primarily located in the liver and/or lung (45.0%). Thirty one patients had previously undergone treatment with the FOLFOX regimen, twenty-five had undergone treatment with FOLFIRI, fifteen had undergone treatment with capcitabine alone and six had undergone treatment with FL. Seventeen patients (42.5%) were treated with bevacizumab combined chemotherapy as a second-line treatment, thirteen (32.5%) were treated as a third-line treatment, and ten (25.0%) were treated as a fourth- or later-line treatment. The majority of the patients were treated with Bevacizumab combined chemotherapy, including oxaliplatin (30.0%), irinotecan (47.5%), and fluoropyrimidine (20.0%). One patient (2.5%) was treated using bevacizumab alone, without the addition of other chemotherapy regimens. Additional patient demographics are summarized in Table 1.
| Characteristics | Value |
| n | 40 |
| Age (yr), median (range) | 55.50 (26-76) |
| Gender | |
| Male | 22 (55.0) |
| Female | 18 (45.0) |
| Primary tumor location | |
| Colon cancer | 10 (25.0) |
| Rectal cancer | 30 (75.0) |
| ECOG | |
| 0-1 | 31 (77.5) |
| ≥ 2 | 9 (22.5) |
| Metastatic sites | |
| Liver only | 6 (15.0) |
| Lung only | 4 (10.0) |
| Liver and lung only | 8 (20.0) |
| 2 sites, including liver or lung | 6 (15.0) |
| 2 sites, excluding liver and lung | 2 (5.0) |
| ≥ 3 sites | 9 (22.5) |
| 1 site, excluding liver and lung | 5 (12.5) |
| Number of metastatic sites | |
| 1 site | 15 (37.5) |
| ≥ 2 sites | 25 (62.5) |
| Histologic type | |
| Well | 9 (22.5) |
| Moderate | 9 (22.5) |
| Poor | 1 (2.5) |
| Unknown | 21 (52.5) |
| Previous chemotherapy | |
| Fluoropyrimidine + oxaliplatin | 31 |
| Fluoropyrimidine + irinotecan | 25 |
| Capecitabine combined | 15 |
| Fluoropyrimidine combined | 6 |
| Line number of bevacizumab | |
| 2nd line | 17 (42.5) |
| 3rd line | 13 (32.5) |
| 4th or later-line | 10 (25.0) |
| Chemotherapy associated with bevacizumab | |
| Oxaliplatin-combined | 12 (30.0) |
| Irinotecan- combined | 19 (47.5) |
| 5-fluorouracil-combined | 8 (20.0) |
| Bevacizumab alone | 1 (2.5) |
Three patients had partial responses, resulting in an ORR of 7.5%. Twenty-one patients exhibited a stable disease (SD), and fifteen patients exhibited a PD. The response rates of second-line, third-line, and fourth- or later-line treatments were noted in Table 2.
| Response | Patients | CR | PR | SD | PD | Unknown |
| Overall | 40 | 0 (0.0) | 3 (7.5) | 21 (52.5) | 15 (37.5) | 1 (2.5) |
| 2nd line | 17 | 0 (0.0) | 2 (13.3) | 11 (64.7) | 4 (23.5) | 0 (0.0) |
| 3rd line | 13 | 0 (0.0) | 1 (7.7) | 6 (46.2) | 5 (38.5) | 1 (7.7) |
| 4th or later-line | 10 | 0 (0.0) | 0 (0.0) | 4 (40.0) | 6 (60.0) | 0 (0.0) |
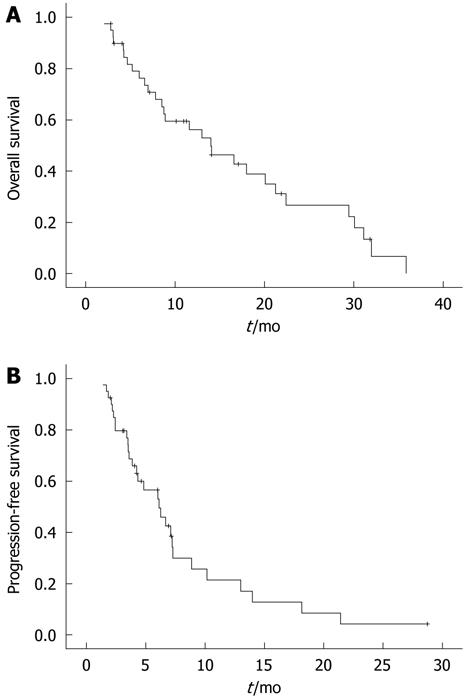
The median duration of the OS and PFS was 14.0 mo and 6.13 mo, respectively (Table 3 and Figure 1). The median OSs were 16.60 mo, 14.07 mo and 13.00 mo for the second-line, third-line and fourth- or later-line treatments, respectively. The median PFSs were 7.23, 7.30, and 3.87 mo for the second-line, third-line and fourth- or later-line treatments, respectively.
| End point | Median follow-up duration (range) (mo) | mOS (95% CI) (mo) | mPFS (95% CI) (mo) |
| Overall | 23.87 (6.13-77.83) | 14.00 (7.77-20.23) | 6.13 (3.94-8.33) |
| 2nd line | 22.37 (6.13-56.10) | 16.60 (3.22-29.98) | 7.23 (6.45-8.02) |
| 3rd line | 20.03 (13.87-57.77) | 14.07 (6.89-21.25) | 7.30 (3.84-10.77) |
| 4th and later-line | 51.12 (17.87-77.83) | 13.00 (4.48-21.52) | 3.87 (2.78-4.95) |
In colorectal cancer, the use of bevacizumab has been shown to result in an improvement in survival rates and response rates. Bevacizumab was investigated after a randomized phase II study in combination with FL as part of the first-line of treatment of MCRC and resulted in a considerable improvement in efficacy when compared with the FL control[9,10,14]. Based on these data, phase III studies were conducted. Compared with irinotecan plus 5-FU/LV (IFL) alone, IFL plus bevacizumab improved the PFS, the ORR and the OS[8]. In the TREE-2 trial, previously untreated patients with MCRC were randomly assigned to bevacizumab and one of the three oxaliplatin- and 5-FU-containing regimens used in the TREE 1 trial-(FOLFOX, oxaliplatin plus bolus 5-FU/LV, or capecitabine plus oxaliplatin). The bevacizumab-containing arms resulted in an improvement in the OS compared with the non-bevacizumab-containing groups in the TREE-1 study[19]. The administration of bevacizumab resulted in a superior response rate, PFS and OS in the treatment of MCRC in the second-line setting. Supporting evidence was presented by the ECOG 3200, a phase III study randomizing patients who progressed after first-line IFL to FOLFOX plus bevacizumab vs FOLFOX alone[12]. In the second-line setting, another study showed that bevacizumab plus irinotecan was an active and safe treatment option for patients failing oxaliplatin-based therapy[20]. The role of bevacizumab in combination with FL as a third-line treatment was studied in a phase II trial of patients who failed irinotecan- and oxlaiplain-based chemotherapy regimens. Based on previous study, the use of third-line FL plus bevacizumab in chemoresistant patients is considered an ineffective treatment[14]. However, additional reports presented different results than this previous report after bevacizumab combined chemotherapy as a third-lime treatment. Bevacizumab with FOLFIRI was reported to be well tolerated and to be a feasible treatment in patients with heavily treated advanced MCRC[21]. Two studies evaluated the efficacy and safety of bevacizumab plus FOLFIRI or FOLFOX in MCRC after failure with FOLFIRI and FOLFOX using a retrospective analysis. These studies concluded that bevacizumab plus FOLFIRI or FOLFOX as third-line or later treatment in patients with MCRC resulted in a modest activity and was relatively tolerable[16,22]. A summary of the data during bevacizumab-combined chemotherapy as a second- or later-line treatment in patients with MCRC is shown in Table 4.
| Ref. | Treatment line | Treatment regimen | n | ORR (%) | Median PFS (mo) | Median OS (mo) |
| Giantonio et al[12] | Second | BV + FOLFOX4 | 287 | 22.7 | 7.3 | 12.9 |
| FOLFOX4 | 285 | 8.6 | 4.7 | 10.8 | ||
| BV | 234 | 3.3 | 2.7 | 10.2 | ||
| Yildiz et al[20] | Second | BV + Irinotecan-based therapy | 40 | 20.0 | 6.0 | 14.0 |
| Chen et al[14] | Third | BV + FU/LV | 100 | 4.0 | 3.7 | 9.1 |
| Kwon et al[21] | Third | BV + FOLFIRI | 14 | 28.5 | 3.9 | 10.9 |
| Lièvre et al[22] | Second or later-line | BV + FOLFIRI or FOLFOX | 31 | 32.2 | 9.7 | 18.4 |
| Kang et al[16] | Third or later-line | BV + FOLFIRI or FOLFOX | 42 | 9.5 | 5.3 | 9.5 |
| Park et al | Second or later-line | BV + FOLFIRI or FOLFOX | 40 | 7.5 | 6.13 | 14.0 |
In the present study, the ORR was 7.5% for all of the patients, and the median duration of the OS and PFS was 14.0 mo and 6.13 mo, respectively. We suggest that bevacizumab combined chemotherapy as a second- or later-line treatment is an active and tolerable treatment in patients with MCRC after failure to response to previous chemotherapy.
The addition of bevacizumab to 5-fluorouracil (5-FU)-based combination chemotherapy as the first-line treatment results in a clinically meaningful improvement in the survival of patients with metastatic colorectal cancer (MCRC).
Based on the inconclusive results from multicenter studies, the role of bevacizumab in the treatment of patients with disease that is refractory to 5-FU, irinotecan, and oxaliplatin is unclear.
In previous studies, bevacizumab combined chemotherapy as a second- or later-line treatment was shown to have controversial results. In the present study, three patients exhibited partial responses, resulting in an overall response rate of 7.5%. The median duration of the overall survival (OS) and progression-free survival (PFS) was 14.0 mo and 6.13 mo, respectively. The median OSs were 16.60, 14.07 and 13.00 mo for the second-line, third-line and fourth- or later-line treatments, respectively. The median PFSs were 7.23 mo, 7.30 mo, and 3.87 mo the second-line, third-line and fourth- or later-line treatments, respectively.
The results of the present study suggest that bevacizumab combined chemotherapy as a second- or later-line treatment is an active and tolerable treatment option for patients with MCRC after failure to previous chemotherapy.
The present study is a good descriptive retrospective study that evaluated the efficacy of bevacizumab plus chemotherapy in patients with MCRC who have failed prior chemotherapy without bevacizumab.
Peer reviewer: Benjamin Perakath, Colorectal Surgery, Christian Medical College, Vellore, Department of Surgery Unit 2, Vellore 632004, India
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8283] [Cited by in F6Publishing: 8159] [Article Influence: 509.9] [Reference Citation Analysis (0)] |
| 2. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [PubMed] [Cited in This Article: ] |
| 3. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1700] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 4. | Dvorak HF. Discovery of vascular permeability factor (VPF). Exp Cell Res. 2006;312:522-526. [PubMed] [Cited in This Article: ] |
| 5. | Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368-4380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1170] [Cited by in F6Publishing: 1115] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 6. | Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851-856. [PubMed] [Cited in This Article: ] |
| 7. | Borgström P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203-4214. [PubMed] [Cited in This Article: ] |
| 8. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [PubMed] [Cited in This Article: ] |
| 9. | Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697-3705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 10. | Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1247] [Cited by in F6Publishing: 1177] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 11. | Bir A, Tan W, Wilding GE, Lombardo J, Fakih MG. 5-fluorouracil, leucovorin and oxaliplatin plus bevacizumab in the first-line treatment of metastatic colorectal cancer: a single-institute study. Oncology. 2007;72:4-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1709] [Cited by in F6Publishing: 1685] [Article Influence: 99.1] [Reference Citation Analysis (1)] |
| 13. | Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, Jaffe C, Rubinstein L, Zwiebel J, Kaplan RS. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24:3354-3360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Emmanouilides C, Pegram M, Robinson R, Hecht R, Kabbinavar F, Isacoff W. Anti-VEGF antibody bevacizumab (Avastin) with 5FU/LV as third line treatment for colorectal cancer. Tech Coloproctol. 2004;8 Suppl 1:s50-s52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kang BW, Kim TW, Lee JL, Ryu MH, Chang HM, Yu CS, Kim JC, Kim JH, Kang YK, Lee JS. Bevacizumab plus FOLFIRI or FOLFOX as third-line or later treatment in patients with metastatic colorectal cancer after failure of 5-fluorouracil, irinotecan, and oxaliplatin: a retrospective analysis. Med Oncol. 2009;26:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15860] [Cited by in F6Publishing: 18973] [Article Influence: 1264.9] [Reference Citation Analysis (1)] |
| 18. | Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1882] [Cited by in F6Publishing: 1974] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 19. | Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523-3529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 433] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 20. | Yildiz R, Buyukberber S, Uner A, Yamac D, Coskun U, Kaya AO, Ozturk B, Yaman E, Benekli M. Bevacizumab plus irinotecan-based therapy in metastatic colorectal cancer patients previously treated with oxaliplatin-based regimens. Cancer Invest. 2010;28:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kwon HC, Oh SY, Lee S, Kim SH, Kim HJ. Bevacizumab plus infusional 5-fluorouracil, leucovorin and irinotecan for advanced colorectal cancer that progressed after oxaliplatin and irinotecan chemotherapy: a pilot study. World J Gastroenterol. 2007;13:6231-6235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lièvre A, Samalin E, Mitry E, Assenat E, Boyer-Gestin C, Lepère C, Bachet JB, Portales F, Vaillant JN, Ychou M. Bevacizumab plus FOLFIRI or FOLFOX in chemotherapy-refractory patients with metastatic colorectal cancer: a retrospective study. BMC Cancer. 2009;9:347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |