Published online Jan 7, 2012. doi: 10.3748/wjg.v18.i1.64
Revised: July 7, 2011
Accepted: July 14, 2011
Published online: January 7, 2012
AIM: To demonstrate the oncologic outcomes of low rectal cancer and to clarify the risk factors for survival, focusing particularly on the type of surgery performed.
METHODS: Data from patients with low rectal carcinomas who underwent surgery, either sphincter-preserving surgery (SPS) or abdominoperineal resection (APR), at The First Affiliated Hospital of Sun Yat-sen University in China from August 1994 to December 2005 were retrospectively analyzed.
RESULTS: Of 331 patients with low rectal cancer, 159 (48.0%) were treated with SPS. A higher incidence of positive resection margins and a higher 5-year cumulative local recurrence rate (14.7% vs 6.8%, P = 0.041) were observed in patients after APR compared to SPS. The five-year overall survival (OS) was 54.6% after APR and 66.8% after SPS (P = 0.018), and the 5-year disease-free survival (DFS) was 52.9% after APR and 65.5% after SPS (P = 0.013). In multivariate analysis, poor OS and DFS were significantly related to positive resection margins, pT3-4, and pTNM III-IV but not to the type of surgery.
CONCLUSION: Despite a higher rate of positive resection margins after APR, the type of surgery was not identified as an independent risk factor for survival.
- Citation: Chen ZH, Song XM, Chen SC, Li MZ, Li XX, Zhan WH, He YL. Risk factors for adverse outcome in low rectal cancer. World J Gastroenterol 2012; 18(1): 64-69
- URL: https://www.wjgnet.com/1007-9327/full/v18/i1/64.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i1.64
Colorectal cancer is the fourth most prevalent malignancy in the world. In 2010, it was estimated that there were approximately 39 670 new rectal cancers in the United States[1]. In China, approximately 70% of rectal cancers are located below the peritoneal reflection[2], an obvious difference from the typical location of rectal cancers in patients in Western countries.
Treatment outcomes for rectal cancer have been dramatically improved by applying the total mesorectum excision (TME) principle[3], the double-stapling technique[4], and the concept of shorter distal margins over the past few decades[5]. More patients with rectal tumors are being managed with various types of sphincter-preserving surgery (SPS). However, for low rectal cancer, challenges remain for some patients who require abdominoperineal resection (APR) to achieve a safe distal margin. Improvements in survival and control of local tumor recurrence for patients with mid and upper rectal cancers have not been as difficult to achieve as in those patients with lower-third rectal tumors. One of the possible reasons for this discrepancy may be the higher rate of circumferential margin (CRM) and inadvertent bowel perforations in APR[6]. However, whether the type of surgery is a risk factor for oncologic outcomes in patients with low rectal cancers is still controversial[7-10].
Herein, we collected data from a single institute in China and performed a retrospective, consecutive cohort study attempting to demonstrate the oncologic outcomes of low rectal cancer and to clarify risk factors for survival, especially focusing on the type of surgery.
From August 1994 to December 2005, a total of 353 patients with primary low rectal carcinomas (0 to 7 cm from the anal verge) underwent open surgery at The First Affiliated Hospital of Sun Yat-sen University in China. Patients who underwent local excision (n = 3), the Hartmann procedure (n = 2), palliative colostomy (n = 7) or who were lost follow-up (n = 10) were excluded, resulting in 331 patients enrolled into the final study.
The clinicopathologic features, including gender, age, tumor size (maximum tumor diameter), distance of the tumor from the anal verge, operative procedure, resection margins, histopathologic grade, pathologic stage, mucin production[11], adjuvant therapy, and oncologic outcome, were fully reviewed.
All patients underwent surgery according to the principles of TME[3]. APR was performed in cases where the tumor was too close (usually ≤ 2 cm) to the dentate line or where the differentiation of the tumor was poor (necessitating a longer distal resection margin). Low anterior resection (LAR) was adopted as often as possible, especially for tumors with a distance < 2 cm from the dentate line. To observe the effects of LAR with a colonic J-pouch, we performed a prospective clinical trial from 1998 to 2002 in which 16 out of 331 patients had a J-pouch created. In this study, LAR (with or without the creation of a J-pouch) and Bacon and Parks procedure were combined as SPS.
The Bacon pull-through procedure was performed for some patients with ultra low rectal cancers. In brief, in this procedure the mobilization of the sigmoid colon and the rectum is identical to that in LAR and APR. The anal sphincter is dilated to four to six finger breadths, and then the submucosa is infiltrated with 1:300 000 epinephrine. A circular incision is made in the mucosa at the anoderm at a point 5 mm from the dentate line. Isolation is performed upward beneath the rectal mucosa until reaching the upper limit of the internal sphincter; then the rectum is cut off and moved away from the abdomen. A 2-cm soft plastic pipe is inserted into the proximal colon. The colon is pulled through from the anus, and several sutures are made between the sigmoid colon and the anal canal. The second stage of the procedure is performed 14 to 21 days later. Excess bowel is amputated, and the anastomosis is completed. The Parks procedure is similar to the Bacon pull-through procedure, the difference being that the anastomosis between the colon and the anal canal is accomplished directly in the Parks procedure without the need to pull through the colon.
The CRM was not used at our institute during the study period. Instead, “resection margins” were adopted to record the status of the proximal, distal, and circumferential resection margins. Neoadjuvant chemoradiation therapy (CRT) was not extensively performed at our institute during the study period. Only 5 patients in the SPS group and 12 in the APR group received neoadjuvant therapy.
All of the patients were followed up every three to six months for the first two years, then every six months for the next three years, and then once a year thereafter. Digital palpation, abdominal and pelvic computed tomography (CT) scan, chest X-ray, total colonoscopy and carcinoembryonic antigen (CEA) were routinely measured to exclude local recurrence or metastasis.
Statistic analysis was performed using the SPSS 17.0 statistical package (SPSS, Inc., Chicago, IL, United States). The Student t-test and Chi-square test were used to analyze continuous and categorical variables, respectively. Overall survival, cancer-related survival and local recurrence rates were calculated using the Kaplan-Meier method. Potential prognostic factors were investigated using the log-rank test first, and then covariates with P-values < 0.05 were selected for backward multivariate Cox regression analysis. All P-values less than 0.05 with two sides were considered significant.
Patient characteristics and treatment details are summarized in Table 1. There were 211 male and 120 female patients with a median age of 56 years (range, 17 years-91 years). The median tumor size was 4.0 cm (range, 0.5 cm-15.0 cm). The median distance between the anal verge and the tumor was 4.0 cm (range, 1.0 cm-7.0 cm), and tumors in the APR group were significantly closer to the anus than those in the SPS group (median, 3.0 cm vs 5.0 cm, P < 0.001).
| Characteristic | APR (%)n = 172 | SPS (%)n = 159 | Pvalue |
| Median age (yr) | 56.0 | 58.0 | 0.334 |
| Median tumor size (cm) | 4.0 | 4.0 | 0.301 |
| Median distance of tumor from the anal verge (cm) | 3.0 | 5.0 | < 0.001 |
| Gender (male) | 115 (66.9) | 96 (60.4) | 0.220 |
| Positive resection margins | 23 (13.4) | 10 (6.3) | 0.032 |
| Histopathologic grade | 0.238 | ||
| Well | 34 (19.8) | 27 (17.0) | |
| Moderate | 102 (59.3) | 108 (67.9) | |
| Poor | 36 (20.9) | 24 (15.1) | |
| pTNM stage | 0.445 | ||
| I | 20 (11.6) | 22 (13.8) | |
| II | 63 (36.6) | 69 (43.4) | |
| III | 73 (42.4) | 56 (35.2) | |
| IV | 16 (9.4) | 12 (7.6) | |
| Mucin production | 14 (8.1) | 10 (6.3) | 0.517 |
| Adjuvant chemotherapy | 34 (19.8) | 29 (18.2) | |
| Year of surgery | 0.001 | ||
| 1994-2001 | 101 (58.7) | 65 (40.9) | |
| 2002-2005 | 71 (41.3) | 94 (59.1) |
Of the 331 patients with low rectal cancer, 172 patients received the APR procedure and 159 underwent SPS, including 130 APR, 13 Bacon or Parks procedures, and 16 APR with a colonic J-pouch. Before 2001, only 40.9% (65 out of 166) of patients underwent SPS, while after 2001 more (59.1%, 94 out of 165) patients were able to undergo anus-sparing surgery (65/166 vs 94/165, P = 0.001). Combined resection of organs was performed for 35 patients, including 10 patients who underwent resection of solitary liver metastasis. Only 4 (2.5%) patients had a protective stoma after SPS.
Resection margins were microscopically positive in 33 (10%) patients. More patients in the APR group were diagnosed with “positive resection margins” than in the SPS group (13.4% vs 6.3%, P = 0.032). Data on tumor stage and tumor differentiation are presented in Table 1.
There was no mortality. Anastomotic leakage occurred in 6 (3.8%, 6 out of 159 SPS) patients, all of whom did not have a protective stoma before, and these patients were treated conservatively. In total, 63 (19.0%) patients received adjuvant chemotherapy with 5-fluorouracil plus leucovorin or FOLFOX (oxaliplatin, 5-fluorouracil, and folinic acid).
Patients were followed up with a median follow-up time of 61 mo (range, 1 mo-194 mo). At the last follow-up in November 2010, 177 patients (53.5%) were alive, and 154 (46.7%) were dead.
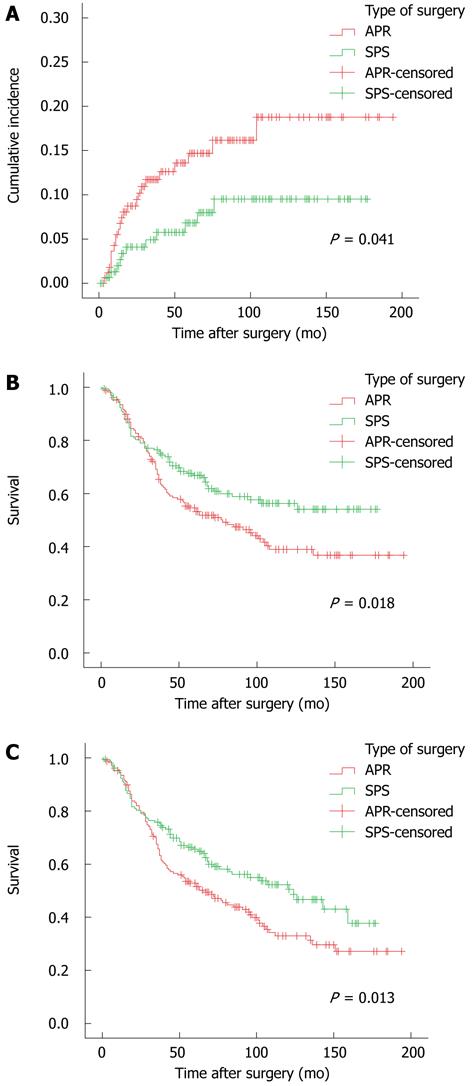
A total of 97 (27.3%) patients experienced recurrence, including 34 (10.3%) local, 58 (17.5%) distant, and 5 (1.5%) combined recurrence. Seventy-five patients died of cancer recurrence. The median disease-free interval for the 35 patients with local recurrence was 15.5 mo (range, 4 mo-104 mo). For the 51 patients with distant metastasis, the median time to metastasis was 25 mo (range, 4 mo-123 mo). The overall 5-year cumulative local recurrence rate was 10.9%. The estimated local recurrence rate at 5 years was 14.7% for APR and 6.8% for SPS (P = 0.041, Figure 1A).
The five-year overall (OS) and disease-free survival (DFS) were 60.6% and 59.1%, respectively. The five-year OS was 54.6% after APR and 66.8% after SPS (P = 0.018, Figure 1B). The 5-year DFS was 52.9% after APR and 65.5% after SPS (P = 0.013, Figure 1C).
In univariate analysis, OS and DFS were both significantly influenced by tumor size, type of surgery, resection margins, tumor histopathologic grade, pT stage, and pTNM stage (Table 2).
| Characteristic | No. of patients | 5-year OS | 5-year DFS | ||
| % | P value | % | P value | ||
| Gender | 0.987 | 0.768 | |||
| Male | 211 | 61.2 | 59.2 | ||
| Female | 120 | 59.6 | 58.9 | ||
| Age (yr) | 0.707 | 0.987 | |||
| < 60 | 182 | 58.8 | 56.4 | ||
| ≥ 60 | 149 | 62.7 | 62.2 | ||
| Tumor size (cm) | 0.011 | 0.003 | |||
| < 4 | 103 | 74.8 | 74.0 | ||
| ≥ 4 | 228 | 54.1 | 52.3 | ||
| Distance of tumor from the anal verge (cm) | 0.189 | 0.197 | |||
| < 3.5 | 117 | 60.1 | 58.1 | ||
| ≥ 3.5 | 214 | 60.9 | 59.6 | ||
| Type of surgery | 0.018 | 0.013 | |||
| APR | 172 | 54.6 | 52.9 | ||
| SPS | 159 | 66.8 | 65.6 | ||
| Resection margins | < 0.001 | < 0.001 | |||
| Negative | 298 | 63.8 | 62.1 | ||
| Positive | 33 | 32.5 | 32.5 | ||
| Histopathologic grade | 0.006 | 0.002 | |||
| Well | 61 | 66.5 | 66.5 | ||
| Moderate | 210 | 63.6 | 61.9 | ||
| Poor | 60 | 43.6 | 41.6 | ||
| pT stage | < 0.001 | < 0.001 | |||
| 1-2 | 125 | 74.0 | 71.9 | ||
| 3-4 | 206 | 52.4 | 51.2 | ||
| pTNM stage | < 0.001 | < 0.001 | |||
| I-II | 174 | 75.4 | 73.3 | ||
| III-IV | 157 | 44.0 | 43.1 | ||
| Mucin production | 0.212 | 0.121 | |||
| No | 307 | 61.7 | 60.3 | ||
| Yes | 24 | 44.8 | 42.6 | ||
| Anastomotic leakage1 | 0.310 | 0.386 | |||
| No | 153 | 67.6 | 66.3 | ||
| Yes | 6 | 50.0 | 50.0 | ||
| Adjuvant chemotherapy | 0.950 | 0.573 | |||
| No | 268 | 60.2 | 58.4 | ||
| Yes | 63 | 62.2 | 62.2 | ||
Multivariate analysis was performed for those factors which were statistically significantly associated with OS and DFS in the univariate analysis. In backward multivariate Cox regression analysis, poor OS was significantly related to positive resection margins [hazard ratio (HR) = 1.644, P = 0.031], pT3-4 (HR = 1.781, P = 0.003), and pTNM III-IV (HR = 2.153, P < 0.001). Positive resection margins (HR = 1.728, P = 0.012), pT3-4 (HR = 1.669, P = 0.006), pTNM III-IV (HR = 1.839, P < 0.001), and poor tumor differentiation (HR = 1.665, P = 0.034) were significantly associated with poor DFS (Table 3).
| Characteristic | OS | DFS | ||||
| HR | 95% CIfor HR | Pvalue | HR | 95% CIfor HR | P value | |
| Tumor size (cm) | 0.131 | 0.067 | ||||
| < 4 | 1.000 | 1.000 | ||||
| ≥ 4 | 1.327 | 0.920-1.915 | 1.388 | 0.977-1.971 | ||
| Type of surgery | 0.091 | 0.112 | ||||
| APR | 1.000 | 1.000 | ||||
| SPS | 0.754 | 0.544-1.047 | 0.778 | 0.572-1.060 | ||
| Resection margins | 0.031 | 0.012 | ||||
| Negative | 1.000 | 1.000 | ||||
| Positive | 1.644 | 1.047-2.582 | 1.728 | 1.129-2.644 | ||
| Histopathologic grade | 0.150 | 0.047 | ||||
| Well | 1.000 | 1.000 | ||||
| Moderate | 1.061 | 0.688-1.637 | 0.788 | 1.108 | 0.735-1.670 | 0.624 |
| Poor | 1.515 | 0.917-2.504 | 0.105 | 1.665 | 1.039-2.666 | 0.034 |
| pT stage | 0.003 | 0.006 | ||||
| 1-2 | 1.000 | 1.000 | ||||
| 3-4 | 1.781 | 1.212-2.616 | 1.669 | 1.162-2.396 | ||
| pTNM stage | < 0.001 | < 0.001 | ||||
| I-II | 1.000 | 1.000 | ||||
| III-IV | 2.153 | 1.515 | 3.058 | 1.839 | 1.332-2.540 | |
Curative resection of local rectal cancer consists of complete removal of the primary tumor and its lymphatic drainage by sharp mesorectal excision with or without sphincter preservation. The choice of APR or SPS may be affected by different opinions regarding safe distal margin, views on the functional aspects associated with intersphincteric dissection, a variety of institution-specific cultural features and the availability of staple anastomosis[12-15]. Our study demonstrated that the rate of sphincter-preserving surgery increased from 40.9% before 2001 to 59.1% after 2001. This may be attributed to the acceptance of a shorter distal margin (1-2 cm) and the availability of the double stapling technique.
This study shows that more patients had positive resection margins after APR than after SPS, which is in line with other studies[6,10,16-18]. Nevertheless, a positive margin should lead to a worse prognosis, and this was confirmed by the multivariate analysis in this study. Inadequate resection at the level of the pelvic floor with TME in APR may result in an increased risk for positive resection margins, contributing to the observed higher risk for positive circumferential resection margins and local recurrence after APR than after SPS. The five-year OS and DFS after APR were inferior to those after SPS, and the OS and DFS were both significantly influenced by the type of surgery in the univariate analysis. However, after adjustment for other potential risk factors, we failed to find a significant association between the type of surgery and survival.
Low rectal cancers lie at the pelvic floor and close to the anal sphincter, which makes completion of a radical operation challenging. To reduce the incidence of circumferential resection margin involvement and intra-operative tumor perforation, some surgeons have made a change in their approach to APR. West et al[14] applied cylindrical abdominoperineal excision of the rectum and anus, entailing resection of the levator muscles en bloc with the lower rectum and anal canal, for patients with low rectal cancer, leading to a decrease in circumferential resection margin involvement from 40.6% after traditional APR to 14.8%. They also reduced the rate of intra-operative perforations from 22.8% to 3.7%. The cylindrical technique may possess the potential to improve patient outcomes, and close attention should be paid to the perineal part of the APR surgery.
It has been reported that preoperative CRT has a strong influence on the prognosis in rectal cancer[18,19]. However, only 17 patients in this study received preoperative CRT due to economic considerations and lack of acceptance of it as a major part of the management of rectal cancer. It was not until recently that we began to include preoperative CRT widely for advanced rectal cancers. We also investigated other variables for poor survival outcome and determined that the pathologic TNM stage remained the strongest risk factors for OS and DFS, which is consisted with previous studies[20-21].
In conclusion, this consecutive cohort study from a single institution in China demonstrates that a higher risk of positive resection margins and local failure was observed in patients after APR. The survival after APR is inferior to that after SPS. However, after adjustment for other covariates, we failed to confirm that the type of surgery is an independent risk factor for survival outcome. To reduce positive resection margins and local recurrence, there has been a call to change the approach to APR.
Risk factors for survival in low rectal cancer patients still need to be clarified. There is evidence that the type of surgery, sphincter-preserving surgery (SPS) or abdominoperineal resection (APR), may influence the prognosis in low rectal cancer.
A retrospective, consecutive cohort study was conducted, attempting to demonstrate the oncologic outcomes of low rectal cancer and to clarify the risk factors for survival. Univariate and multivariate Cox regression analyses were performed to determine whether gender, age, tumor size, distance of the tumor from the anal verge, operative procedure, resection margins, histopathologic grade, pathologic stage, mucin production, and adjuvant therapy were associated with the prognosis of low rectal cancer.
A higher risk for positive resection margins and local failures were observed in APR. The survival outcome after APR is inferior to that after SPS. However, after adjustment for other covariates, the type of surgery was not identified as an independent risk factor for survival.
To reduce positive resection margins and local recurrence, there has been a call for a change in the approach to APR.
Total mesorectum excision (TME) is the standard technique for the treatment of rectal cancer. It was devised some 20 years ago by Professor Bill Heald at the Basingstoke District Hospital in the United Kingdom. TME is accomplished by precise sharp dissection under direct visualization with the true pelvis around the integral mesentery enveloping the entire mid-rectum and with preservation of the hypogastric plexus.
This is an important paper, comparing the outcome of low mesorectal anterior resections for carcinoma of the rectum with abdominoperineal resections.
Peer reviewer: Frank I Tovey, OBE, ChM, FRCS, Honorary Research Fellow, Department of Surgery, University College London, London WC1E 6BT, United Kingdom
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10002] [Cited by in F6Publishing: 10353] [Article Influence: 739.5] [Reference Citation Analysis (0)] |
| 2. | Yu BM. Treatment of low rectal cancer. Zhonghua Weichang Waike Zazhi. 2004;29:87-90. [Cited in This Article: ] |
| 3. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1818] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 4. | Cohen Z, Myers E, Langer B, Taylor B, Railton RH, Jamieson C. Double stapling technique for low anterior resection. Dis Colon Rectum. 1983;26:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kwok SP, Lau WY, Leung KL, Liew CT, Li AK. Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. Br J Surg. 1996;83:969-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257-9264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, Dixon MF, Mapstone NP, Sebag-Montefiore D, Scott N. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Haward RA, Morris E, Monson JR, Johnston C, Forman D. The long term survival of rectal cancer patients following abdominoperineal and anterior resection: results of a population-based observational study. Eur J Surg Oncol. 2005;31:22-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Anderin C, Martling A, Hellborg H, Holm T. A population-based study on outcome in relation to the type of resection in low rectal cancer. Dis Colon Rectum. 2010;53:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Silberfein EJ, Kattepogu KM, Hu CY, Skibber JM, Rodriguez-Bigas MA, Feig B, Das P, Krishnan S, Crane C, Kopetz S. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010;17:2863-2869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259-264. [PubMed] [Cited in This Article: ] |
| 12. | Rullier E, Laurent C, Bretagnol F, Rullier A, Vendrely V, Zerbib F. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg. 2005;241:465-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Mulsow J, Winter DC. Sphincter preservation for distal rectal cancer--a goal worth achieving at all costs? World J Gastroenterol. 2011;17:855-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | West NP, Finan PJ, Anderin C, Lindholm J, Holm T, Quirke P. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26:3517-3522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Temple LK, Romanus D, Niland J, Veer AT, Weiser MR, Skibber J, Wilson J, Rajput A, Benson A, Wong YN. Factors associated with sphincter-preserving surgery for rectal cancer at national comprehensive cancer network centers. Ann Surg. 2009;250:260-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Chuwa EW, Seow-Choen F. Outcomes for abdominoperineal resections are not worse than those of anterior resections. Dis Colon Rectum. 2006;49:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Luna-Pérez P, Bustos-Cholico E, Alvarado I, Maffuz A, Rodríguez-Ramírez S, Gutiérrez de la Barrera M, Labastida S. Prognostic significance of circumferential margin involvement in rectal adenocarcinoma treated with preoperative chemoradiotherapy and low anterior resection. J Surg Oncol. 2005;90:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1313] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 20. | Jörgren F, Johansson R, Damber L, Lindmark G. Risk factors of rectal cancer local recurrence: population-based survey and validation of the Swedish rectal cancer registry. Colorectal Dis. 2010;12:977-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Syk E, Glimelius B, Nilsson PJ. Factors influencing local failure in rectal cancer: analysis of 2315 patients from a population-based series. Dis Colon Rectum. 2010;53:744-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |