Published online Feb 14, 2011. doi: 10.3748/wjg.v17.i6.804
Revised: November 8, 2010
Accepted: November 15, 2010
Published online: February 14, 2011
AIM: To evaluate the effect of hepatitis B virus (HBV) infection on liver metastasis of colorectal cancer.
METHODS: A total of 1298 colorectal cancer patients were recruited from January 2001 to March 2005 in this study. Enzyme-linked immunosorbent assay was used to test serum HBV markers for colorectal cancer. Patients were divided into study (infection) group and control (non-infection) group. Clinical features of patients in two groups were compared.
RESULTS: Liver metastasis was found in 319 out of the 1298 colorectal cancer patients. The incidence of liver metastasis was significantly lower in study group than in control group (14.2% vs 28.2%, P < 0.01). HBV infection significantly decreased the risk of liver metastasis [hazard ratio (HR): 0.50, 95% confidence interval (95% CI): 0.38-0.66], but the incidence of extrahepatic metastasis was significantly higher in study group than in control group (31.9% vs 17.0%, P < 0.01). The HR was the lowest in chronic hepatitis B group (HR: 0.29, 95% CI: 0.12-0.72). The number of liver metastatic lesions was significantly less in study group than in control group with a higher surgical resection rate. However, no significant difference was found in survival rate between the two groups (P = 0.95).
CONCLUSION: HBV infection decreases the risk of liver metastasis in patients with colorectal cancer and elevates the surgical resection rate of liver metastatic lesions.
- Citation: Qiu HB, Zhang LY, Zeng ZL, Wang ZQ, Luo HY, Keshari RP, Zhou ZW, Xu RH. HBV infection decreases risk of liver metastasis in patients with colorectal cancer: A cohort study. World J Gastroenterol 2011; 17(6): 804-808
- URL: https://www.wjgnet.com/1007-9327/full/v17/i6/804.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i6.804
Colorectal cancer (CRC) accounts for 10%-15% of all cancers and is the second leading cause of cancer-related deaths in Western countries[1]. Approximately half of CRC patients develop metastatic disease[2]. Of the CRC patients, 15%-25% present with synchronous liver metastasis and 80%-90% are initially found to have unresectable liver metastatic disease[3]. Metastatic liver disease more frequently develops metachronous metastasis following treatment of CRC. It is estimated that over half of dead CRC patients have liver metastasis at autopsy[4].
Hepatitis B virus (HBV) infection is the most common cause of chronic liver diseases worldwide, an estimated 350 million persons are chronically infected with HBV worldwide, and China is a highly endemic area of HBV infection with approximately 170 million HBV carriers[5]. It has been demonstrated that HBV infection plays an important role in the development of hepatocellular carcinoma (HCC)[6]. It was reported that HBV infection finally reduces the risk of intrahepatic metastasis in HCC patients with a higher survival rate and therefore can be considered an important prognostic factor for HCC patients[7].
Rare reports are available on the relation between HBV infection and hepatic metastasis of CRC. Utsunomiya et al[8] reported that CRC seldom metastasizes to liver of patients infected with HBV or hepatitis C virus (HCV), but most patients in their study were infected with HCV. Song et al[9] showed that chronic HBV infection with viral replication reduces hepatic metastasis of CRC and prolongs the survival time of CRC patients. However, their study was hard to demonstrate the relation between HBV infection and hepatic metastasis of CRC due to its small sample size. Alternatively, investigation of experimentally induced hepatic metastasis of colon cancer demonstrated that activated immune cells residing in livers can effectively kill metastatic tumor cells, indicating that alterations in liver-associated immunity play an important role in hindering hepatic metastasis[10]. Thus, we designed this cohort study to observe the relation between HBV infection and liver metastasis of CRC.
A total of 1298 CRC patients at the age of > 16 years, admitted to Sun Yat-Sen University Cancer Center (Guangzhou, China) from January 2001 to March 2005, were recruited in this study and divided into study (infection) group and control (non-infection) group. All patients gave their written informed consent to receive a test for HBV infection at their first visit. The study was approved by The Ethics Committee of Sun Yat-Sen University Cancer Center.
HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc were detected by enzyme-linked immunosorbent assay and HBV deoxyribonucleic acid (HBV-DNA) was detected by polymerase chain reaction.
Primary colorectal adenocarcinoma was completely removed from all eligible patients with no prior chemotherapy or radiotherapy, and staged according to AJCC Cancer Staging Manual, 6th edition[11]. All patients received 5-fluorouracil-based FOLFOX6 or XELOX regimen. Patients with liver metastasis underwent palliative treatment (including chemotherapy, radiotherapy, surgical resection and radio-frequency ablation) according to the update NCCN Guidelines for CRC[12].
Patients were assessed by abdominal and pelvic computed tomography (CT) scan or magnetic resonance imaging (MRI), thoracic radiography or thoracic CT or MRI before surgery. Patients who underwent surgery were assessed again during operation. All patients, after discharged from hospital, were followed up according to a standard protocol[13]. The patients were followed up every 3 mo in the first 2 years after surgery, during which clinical examination, routine blood test, assessment of tumor markers, and abdominal ultrasonography or CT scan and endoscopy were performed. In the next 3 years, the patients were followed up every 6 mo and underwent endoscopy every 12 mo. The relapse of CRC (defined as local recurrence or metastasis at distant sites) at other sites was detected and staged. The follow-up was terminated in April 2010.
Differences in baseline clinical parameters and treatment outcomes between the two groups were evaluated by chi-square test or Student t test. Hazard ratio (HR) and 95% confidence interval (95% CI) were calculated with the Cox proportional-hazards model. Overall survival (OS) and disease-free survival (DFS) curves were plotted with the Kaplan-Meier method, and compared by log-rank test. OS rate was calculated from the date of discharge to death. DFS time was defined as the time between discharge and first relapse of CRC. A two-tailed P value less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS for Windows V.13.0.
The 1298 patients were divided into study group and control group. Three hundred and thirty-two patients (25.6%) with chronic HBV infection included in study group were further divided into 3 subgroups according to their natural history of HBV infection[14]. Chronic hepatitis B (CHB) was identified in 37 patients (2.9%) according to the presence of HBsAg and HBeAg or HBV-DNA which are markers of active viral replication. Inactive HBsAg carriers (IC), identified in 108 patients (8.3%), were characterized by the presence of HBsAg and anti-HBe and the absence of HBeAg or HBV-DNA. Resolved hepatitis B (RHB) observed in 187 patients (14.4%) was characterized by negative HBsAg and the presence of anti-HBc ± anti-HBs. Nine hundred and sixty-four patients (74.6%) were included in control group. No significant difference was found in sex, age, depth of tumor invasion, lymph-node metastasis, lactate dehydrogenase, γ-glutamyl transpeptidase, alkaline phosphatase, albumin, and total bilirubin between the two groups (Table 1). However, the liver function was significantly worse in study group than in control group.
| Characteristic | Study group | Control group | P |
| No. of patients | 332 (100) | 966 (100) | |
| Gender | NS | ||
| Male | 196 (59.0) | 550 (55.0) | |
| Female | 136 (41.0) | 416 (45.0) | |
| Age (yr) | NS | ||
| Median | 53 | 60 | |
| Range | 16-81 | 16-87 | |
| Depth of tumor invasion1 | 299 (100) | 857 (100) | NS |
| T1 | 21 (6.3) | 40 (4.1) | |
| T2 | 38 (11.4) | 165 (17.1) | |
| T3 | 87 (26.2) | 264 (27.3) | |
| T4 | 153 (46.1) | 388 (40.2) | |
| Lymph-node metastasis1 | 295 (100) | 843 (100) | NS |
| N0 | 160 (48.2) | 490 (58.1) | |
| N1 | 87 (26.2) | 216 (25.6) | |
| N2 | 48 (14.5) | 137 (14.2) | |
| Chronic liver dysfunction2 | 39 (11.7) | 65 (6.7) | < 0.05 |
| Albumin (g/dL) | 39.4 ± 5.5 | 39.4 ± 11.1 | NS |
| Total bilirubin (mg/dL) | 13.0 ± 5.8 | 12.7 ± 6.7 | NS |
| LDH (IU/L) | 191.5 ± 141.7 | 197.5 ± 164.1 | NS |
| ALP (IU/L) | 71.7 ± 35.5 | 75.6 ± 49.7 | NS |
| GGT (IU/L) | 34.7 ± 54.9 | 35.4 ± 55.9 | NS |
The mean follow-up time of patients was 6 mo after operation. The median interval time of patients was 6 mo after operation. The median follow-up time of patients was 57.2 mo (range 0-110.4 mo) after operation.
Liver metastasis occurred in 319 patients including synchronous liver metastasis in 193 cases and metachronous liver metastasis in 127 cases. Of the 193 patients, 39 had synchronous liver metastases. Of the 127 patients, 18 had metachronous liver metastasis. Synchronous or metachronous extrahepatic metastasis occurred in 270 patients was defined as distant metastasis but not as liver metastasis. The incidence of recurrence or metastasis to the distant sites is summarized in Table 2. The incidence of liver and extrahepatic metastasis was comparable between the two groups. The incidence of liver metastasis was significantly lower in study group than in control group (14.2% vs 28.2%, P < 0.01). The Manter-Haenzel χ2 analysis showed that HBV infection significantly decreased the risk of liver metastasis (HR: 0.50, 95% CI: 0.38-0.66). The incidence of extrahepatic metastasis was significantly higher in study group than in control group (31.9% vs 17.0%, P < 0.01). No difference was found in liver metastasis between the two groups.
| Sites of metastasis | Study group (n = 332) | Control group (n = 966) | P value | HR (95% CI) |
| Liver | < 0.01 | 0.50 (0.38-0.66) | ||
| Yes | 47 (14.2) | 272 (28.2) | ||
| No | 285 (85.8) | 694 (71.8) | ||
| Extrahepatic | < 0.01 | 1.88 (1.52-2.33) | ||
| Yes | 106 (31.9) | 164 (17.0) | ||
| No | 226 (68.1) | 802 (83.0) |
The liver metastasis rate in patients with CHB, IC and RHB is listed Tables 3, 4 and 5. CHB, IC and RHB decreased the risk of liver metastasis and increased the risk of extrahepatic metastasis. The HR was the lowest in patients with CHB (HR: 0.29, 95% CI: 0.12-0.72).
| Metastatic sites | CHB group (n = 37) | Control group (n = 966) | P value | HR (95% CI) |
| Liver | < 0.01 | 0.29 (0.12-0.72) | ||
| Yes | 3 (8.1) | 272 (28.2) | ||
| No | 34 (91.9) | 694 (71.8) | ||
| Extrahepatic | < 0.01 | 2.55 (1.77-3.67) | ||
| Yes | 16 (43.2) | 164 (17.0) | ||
| No | 21 (56.8) | 802 (83.0) |
| Metastatic sites | IC group (n = 108) | Control group (n = 966) | P value | HR (95% CI) |
| Liver | < 0.01 | 0.36 (0.22-0.59) | ||
| Yes | 11 (10.2) | 272 (28.2) | ||
| No | 97 (89.8) | 694 (71.8) | ||
| Extrahepatic | < 0.01 | 2.24 (1.66-3.01) | ||
| Yes | 41 (38.0) | 164 (17.0) | ||
| No | 67 (62.0) | 802 (83.0) |
| Metastatic sites | RHB group (n = 187) | Control group (n = 966) | P value | HR (95% CI) |
| Liver | < 0.01 | 0.63 (0.46-0.85) | ||
| Yes | 33 (17.6) | 272 (28.2) | ||
| No | 154 (82.4) | 694 (71.8) | ||
| Extrahepatic | < 0.01 | 1.54 (1.16-2.04) | ||
| Yes | 49 (26.2) | 164 (17.0) | ||
| No | 138 (73.8) | 802 (83.0) |
The number, size and surgical resection rate of metastatic lesions are listed in Table 6. The number of liver metastatic lesions was significantly less in study group than in control group with a higher surgical resection rate (P < 0.05). No significant difference was found in size of liver metastatic lesions between the two groups.
| Metastatic lesion | Study group | Control group | P value |
| Number | 47 | 272 | < 0.05 |
| Single | 17 (36.2) | 73 (26.8) | |
| Multiple | 30 (63.8) | 199 (74.2) | |
| Size (cm) | 3.6 ± 2.0 | 3.9 ± 1.3 | NS |
| Resected | 14 (29.8) | 43 (15.8) | < 0.05 |
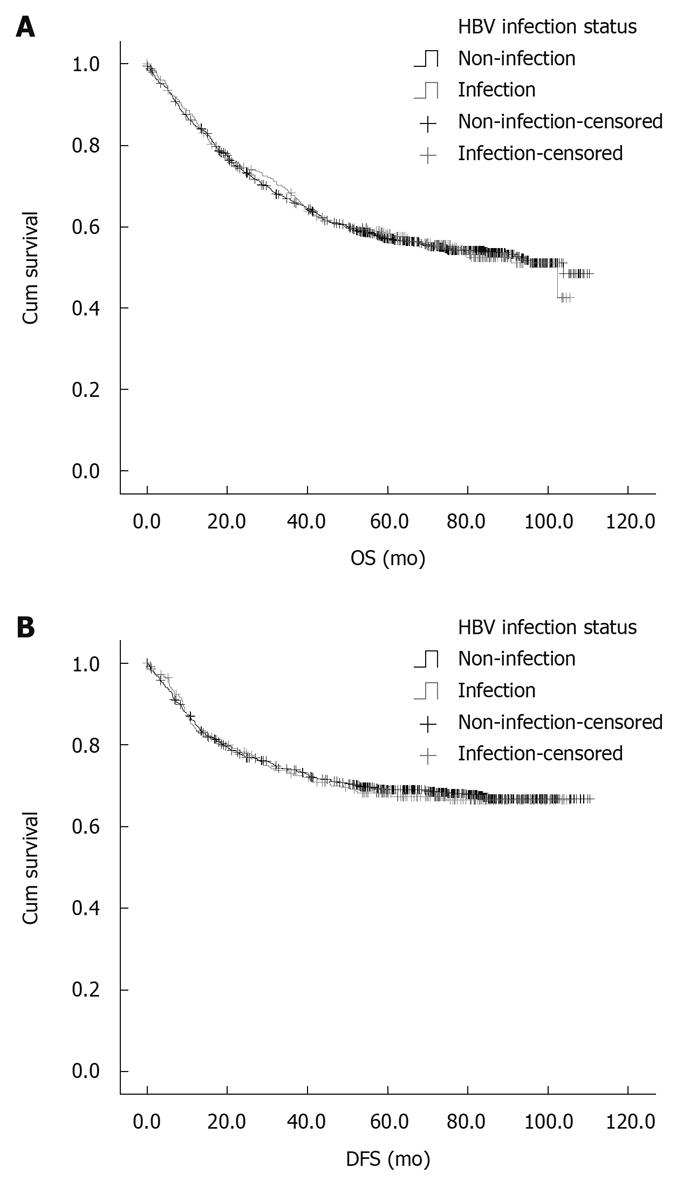
The 5-year survival rate was 57.0% and 58.2%, respectively, for the patients in two groups. No significant difference was found in OS and DFS rate between the two groups (Figure 1A and B).
In the current study, the risk of liver metastasis was significantly lower in study group than in control group (HR: 0.50, 95% CI: 0.38-0.66, P < 0.05). A significant difference was found in extrahepatic metastasis rate and no significant difference was found in survival rate between the two groups, suggesting that HBV infection may have a significant effect on liver metastasis of CRC. It was reported that the liver metastasis rate is low in patients with other malignancies due to HBV infection[7,15].
In this study, the liver metastasis rate of CRC was lower in CHB, IC and RHB subgroups than in control group (P < 0.05). CHB was characterized by positive HBeAg while the serum HBV DNA level and normal aminotransferase level were very low or undetectable in IC. RHB results from previous HBV infection without further virological, biochemical or histological evidence of active virus infection or disease[14,16]. Thus, it is reasonable to postulate that HBV infection with or without virus replication, may affect liver metastasis of CRC. In this study, CHB most significantly decreased the risk of liver metastasis of CRC followed by RHB.
Hepatic resection remains the only curative therapy for liver metastasis of CRC. In this study, the 5-year survival rate of CRC patients was 25%-40% after operation, which is consistent with the reported findings[17,18]. The number of liver metastatic lesions was much less in study group than in control group, leading to a higher surgical resection rate of liver metastatic lesions, indicating that HBV infection plays an important role in the pathogenesis of liver metastasis of CRC. However, no difference was found in overall survival and disease-free survival rate between the two groups, suggesting that liver metastasis of CRC results from the difference in extrahepatic metastasis.
Whether changes in liver-associated immunity contribute to the impediment of CRC colonization in patients infected with HBV remains unclear. The liver has a rich diversity of innate immune cells, particularly lymphocytes including natural killer cells, which respond to altered expression of self-antigens and lyse neoplastic target cells in the absence of additional activating stimuli[19]. A large number of phagocytic and antigen-presenting cells including liver sinusoidal endothelial cells, Kupffer cells and dendritic cells play an important role in local innate immunity of the liver[20]. Furthermore, it was reported that HBV replication enhances the cytotoxicity of immunocytes during chronic HBV infection. Cytotoxic T lymphocytes (CTL) and Kupffer cells are essential for the immune response during HBV infection. HBV replication activates the specific lytic pathways of cell injury by CTL and Kupffer cells[21]. A previous study showed that the hepatic microenvironment in patients with HBV-positive metastatic liver cancer can greatly change their gene expression profiles, and the two significant clusters in the profile revealed notable changes-associated with gene products involved in immune function. In fact, over 30% of the genes in these clusters are related to this process[22]. Another study on tumor and stroma interaction suggested that the propensity of metastatic liver cancer is inherent to the tumor cells and affected by the local environment of metastatic sites[23].
In conclusion, activation of liver-associated immunity due to HBV infection reduces the incidence of liver metastasis in CRC patients.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in Western countries. Metastatic liver disease more frequently develops metachronous metastasis following treatment of CRC. It was reported that hepatitis B virus (HBV) infection finally reduces the risk of intrahepatic metastasis in hepatocellular carcinoma (HCC) patients with a higher survival rate and therefore can be considered an important prognostic factor for HCC patients Rare reports are available on the relation between HBV infection and hepatic metastasis of CRC.
The authors designed a cohort study to observe the relation between HBV infection and liver metastasis of CRC.
The major points summarized in the article can applied in further studies on the correlation between liver metastasis and colorectal cancer.
In this manuscript, the authors evaluated the effect of HBV infection on liver metastases in patients with colorectal cancer. Some discussions should be added and survival curves should be reconsidered.
Peer reviewers: Dr. Lucia Ricci Vitiani, Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena, 299, Rome 00161, Italy; Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Tian L L- Editor Wang XL E- Editor Lin YP
| 1. | Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society 2007; 1-50. [Cited in This Article: ] |
| 2. | Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212-2221. [Cited in This Article: ] |
| 3. | Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243-9249. [Cited in This Article: ] |
| 4. | Foster JH. Treatment of metastatic disease of the liver: a skeptic's view. Semin Liver Dis. 1984;4:170-179. [Cited in This Article: ] |
| 5. | Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol. 2002;67:447-450. [Cited in This Article: ] |
| 6. | De Mitri MS, Cassini R, Bernardi M. Hepatitis B virus-related hepatocarcinogenesis: molecular oncogenic potential of clear or occult infections. Eur J Cancer. 2010;46:2178-2186. [Cited in This Article: ] |
| 7. | Wang J, Li Q, Sun Y, Zheng H, Cui Y, Li H, Zhou H, Hao X. Clinicopathologic features between multicentric occurence and intrahepatic metastasis of multiple hepatocellular carcinomas related to HBV. Surg Oncol. 2009;18:25-30. [Cited in This Article: ] |
| 8. | Utsunomiya T, Saitsu H, Saku M, Yoshida K, Matsumata T, Shimada M, Sugimachi K. Rare occurrence of colorectal cancer metastasis in livers infected with hepatitis B or C virus. Am J Surg. 1999;177:279-281. [Cited in This Article: ] |
| 9. | Song E, Chen J, Ou Q, Su F. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. Am J Surg. 2001;181:529-533. [Cited in This Article: ] |
| 10. | Okuno K, Hirai N, Lee YS, Kawai I, Shigeoka H, Yasutomi M. Involvement of liver-associated immunity in hepatic metastasis formation. J Surg Res. 1998;75:148-152. [Cited in This Article: ] |
| 11. | Greece F, Fleming I, Fritz A. AJCC Cancer Staging Manual. New York: Springer-Verlag 2002; . [Cited in This Article: ] |
| 12. | Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778-831. [Cited in This Article: ] |
| 13. | Desch CE, Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512-8519. [Cited in This Article: ] |
| 14. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [Cited in This Article: ] |
| 15. | Hatanaka K, Kudo M, Fukunaga T, Ueshima K, Chung H, Minami Y, Sakaguchi Y, Hagiwara S, Orino A, Osaki Y. Clinical characteristics of NonBNonC- HCC: Comparison with HBV and HCV related HCC. Intervirology. 2007;50:24-31. [Cited in This Article: ] |
| 16. | Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056-1075. [Cited in This Article: ] |
| 17. | Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, D'Angelica M. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14:1151-1160. [Cited in This Article: ] |
| 18. | Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982. [Cited in This Article: ] |
| 19. | Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314-2321. [Cited in This Article: ] |
| 20. | McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298-1305. [Cited in This Article: ] |
| 21. | Tordjmann T, Soulie A, Guettier C, Schmidt M, Berthou C, Beaugrand M, Sasportes M. Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver. 1998;18:391-397. [Cited in This Article: ] |
| 22. | Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99-111. [Cited in This Article: ] |
| 23. | Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839-849. [Cited in This Article: ] |