Published online Feb 14, 2010. doi: 10.3748/wjg.v16.i6.770
Revised: December 31, 2009
Accepted: January 7, 2010
Published online: February 14, 2010
AIM: To evaluate the efficacy of antiviral or corticosteroid treatment on hepatitis B virus-associated glomerulonephritis (HBV-GN).
METHODS: Six and five trials were used respectively to evaluate the efficacy of either antiviral or corticosteroid treatment on HBV-GN. Pediatric patients were pooled separately to assess their response to the above treatment modalities. The primary and secondary outcomes were remission of proteinuria and clearance of Hepatitis B e-antigen (HBeAg), respectively. A fixed or random effect model was established to collect the data.
RESULTS: The remission rate of proteinuria (RR = 1.69, 95% CI: 1.08-2.65) and the clearance rate of HBeAg (RR = 6.44, 95% CI: 3.11-13.35) were significantly higher in antiviral treatment group than in control group. The proteinuria remission was significantly associated with HBeAg clearance (P = 0.002). However, the difference in proteinuria remission rate was not statistically significant between corticosteroid treatment group and control group (RR = 1.45, 95% CI: 0.68-3.11). Antiviral therapy could significantly promote the HBeAg clearance in pediatric patients, but neither antiviral nor corticosteroid therapy could significantly decrease proteinuria in pediatric patients compared to controls.
CONCLUSION: Antiviral but not corticosteroid treatment can decrease proteinuria and promote HBeAg clearance in HBV-GN patients.
- Citation: Zhang Y, Zhou JH, Yin XL, Wang FY. Treatment of hepatitis B virus-associated glomerulonephritis: A meta-analysis. World J Gastroenterol 2010; 16(6): 770-777
- URL: https://www.wjgnet.com/1007-9327/full/v16/i6/770.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i6.770
Hepatitis B virus-associated glomerulonephritis (HBV-GN) remains one of the most common secondary glomerular diseases in Chinese children, although its incidence seems to decrease nowadays after the popularization of HBV vaccination[1,2]. Most HBV-GN patients present with nephrotic syndrome and some show mild to moderate proteinuria with hematuria[3]. Although spontaneous remission has been reported in many pediatric patients[3], some still develop progressive renal failure[4-6]. Therefore, it is very important to attenuate proteinuria and slow down renal disease progression in HBV-GN patients.
HBV-GN is treated with either antiviral drugs including interferon, lamivudine, and entecavir or with corticosteroids and even immunosuppressive agents like mycophenolate mofetil, leflunomide[7,8]. It has been shown that antiviral therapy can promote the clearance of HBV and improve the coexisting renal disease[3], but the efficacy of interferon on HBV-GN has not been confirmed[9,10]. Moreover, interferon therapy is not as successful for HBV-GN in children as for HBV-GN in adults[3]. Thus, the efficacy of antiviral therapy on HBV-GN remains to have been established, especially in pediatric patients. Corticosteroids are also used in treatment of some patients with nephrotic syndrome. However, it is argued that corticosteroid and immunosuppressive agents are unfavorable for HBV-GN since they inhibit the immune system and activate latent HBV, leading to active replication of HBV and deterioration of renal lesions[3,11]. So the efficacy of these treatment modalities on HBV-GN is still uncertain. Up to date, we are not sure if patients with HBV-GN can be treated with antiviral drugs alone and if nephrotic patients can be treated with corticosteroids.
Unfortunately, the data available in studies on HBV-GN treatment are limited and often provide inconsistent results, which can be explained by many factors like variable sample size, racial differences, disease variation as well as interference of other treatment. These inconsistencies can be solved by meta-analysis. In a meta-analysis[12] of antiviral therapy for HBV-GN published in 2006, 2 of the 6 trials included were non-controlled studies, other treatments like corticosteroids and pediatric patients were not analyzed. Thus, we performed a meta-analysis including just controlled trials to evaluate the effects of antiviral drugs and corticosteroids on HBV-GN both in adults and in children.
All eligible articles in English and Chinese published prior to November 2008 were searched from PubMed, EMBASE, Cochrane Library and CNKI. The terms, including hepatitis B virus (or hepatitis B), nephropathy, nephrotic syndrome and therapy, interferon, lamivudine, corticosteroid, prednisolone, etc, were crossed. Furthermore, bibliographies of retrieved articles, proceedings of major recent meetings on nephrology and hepatology and related dissertations in English or Chinese were manually searched.
Controlled clinical trials, cohort studies, and case-control studies were searched for this systematic review. The diagnosis of HBV-GN was established based on renal pathology. The primary and secondary outcomes were remission of proteinuria and clearance of Hepatitis B e-antigen (HBeAg), respectively. Only dissertations, conference papers and full-text papers published in peer-reviewed journals concerning the treatment of HBV-GN were included in the study. The decision was made based on the quality of studies rather than on their results.
Publications were excluded if they were non-controlled studies or on treatment of HBV-GN with Chinese herbal drugs. For serial reports of the same patients, only those who provided the most comprehensive information were included.
The assessed outcomes included clinical and virologic responses. Clinical responses were divided into complete remission and partial remission, which were respectively defined as disappearance of proteinuria (< 0.3 g/d) and reduction in urine protein excretion. Virologic response was defined as clearance of HBeAg from serum.
Two reviewers independently selected the studies, and extracted data and outcomes according to the inclusion criteria. In case of disagreement between the two reviewers, a third reviewer was introduced to discuss with the two reviewers and extracted the data when all the three reviewers reached a consensus.
Meta-analysis was performed using fixed-effect or random-effect methods, depending on the absence or presence of significant heterogeneity. Statistical heterogeneity between trials was evaluated by the Cochran χ2 test and significance was considered when P < 0.10. In the absence of statistically significant heterogeneity, the Mantel-Haenszel method in the fixed-effect model was used for meta-analysis. Otherwise, the DerSimonian and Laird method[13] in the random-effect model was selected. The relative risk (RR) with 95% confidence interval (CI) was used to assess the treatment efficacy. The combined result was an average RR and 95% CI weighted according to the standard error of the RR of the trial. P < 0.05 was considered statistically significant. We used funnel plots to assess the publication bias, and tested for funnel plot asymmetry using Egger’s test[14] and Begg’s test[15]. All analyses were performed with STATA version 9.0 (Stata Corp, College Station, Tx) and Review Manager version 4.2 (RevMan, Cochrane Collaboration, Oxford, England).
Of the 998 studies we identified in the search, 55 and 943 articles were published in English and Chinese, respectively. After a review of the titles and abstracts or full texts, 989 articles were excluded and 9 articles[16-24] (8 in English and 1 in Chinese) were included based on the pre-specified criteria. One of them was randomized controlled trial (RCT)[16], others were cohort studies. Among the 9 articles, 5 (55.6%) were from China, corresponding to the high incidence of HBV-GN in China and the low incidence in Europe and North American. The characteristics of 9 clinical trials included are shown in Table 1, and the details of intervention methods like dose and duration of drugs, main outcomes, and follow-up time in each study are provided in Tables 2 and 3.
| Study | Country or region | Patients | Study design | |
| Gender | Age (yr) | |||
| Lin[16], 1995 | Taiwan, China | 29M, 11F | 6.2 ± 2.4 | RCT (3 score) |
| Bhimma et al[17], 2002 | South Africa | 34M, 5F | 8.7, 9.2 | Cohort study |
| Lai et al[18], 1991 | Hong Kong, China | 14M, 2F | 27.2 ± 6.2 | Cohort study |
| Tang et al[19], 2005 | Hong Kong, China | 14M, 8F | 48.3 ± 12.8, 43.1 ± 22.8 | Cohort study |
| Panomsak et al[20], 2006 | Thailand | 14M, 10F | 39.8 | Cohort study |
| Yang et al[21], 2003 | Wenzhou, China | 28M, 5F | 8.01 ± 1.23 | Cohort study |
| Lai et al[22], 1990 | Hong Kong, China | 10M, 5F | 22.8 ± 14.4, 17.2 ± 8.2 | Cohort study |
| Ozdamar et al[23], 2003 | Turkey | 11M, 3F | 10 | Cohort study |
| Peña et al[24], 2001 | Spain | 11M, 1F | 4.52 ± 2.34 | Cohort study |
| Author | Group | Case (n) | Intervention | Dropped-out (n) | Outcome | Follow-up | ||
| CR | VR | Renal insufficiency (n) | ||||||
| Lin[16], 1995 | Control | 20 | The same supportive treatment as treatment group | 0 | 7 complete remission, 10 partial remission | 0 HBeAg clearance | UA | 24 mo |
| Treatment | 20 | rIFNα, 5 mU (weight < 20 kg), 8 mU (weight ≥ 20 kg), 3 t/w for 12 mo | 0 | 20 complete remission | 16 HBeAg clearance | UA | ||
| Bhimma et al[17], 2002 | Control | 20 | Anti-hypertension and diuretics if needed | 0 | 0 complete remission, 5 partial remission | 1 HBeAg clearance | 0 | 40 wk |
| Treatment | 24 | rIFNα-2b, 10 mU/m2, 3 t/w for 16 wk | 5 | 10 complete remission, 4 partial remission | 10 HBeAg clearance, 4 reverters, 5 failures | 2 | ||
| Lai et al[18], 1991 | Control | 11 | Diuretic agents or dipyridamole or none | 0 | 0 complete remission, 8 partial remission | 0 HBeAg clearance | 4 | 60 mo |
| Treatment | 5 | 2 wk of prednisolone 40 mg/d followed by 12 wk of rIFNα-2b 3 mU, 3 t/w | 0 | 1 complete remission, 4 partial remission | 1 HBeAg seroconversion | 1 | ||
| Tang et al[19], 2005 | Control | 12 | ACEI or ARB | 0 | 2 complete remission, 2 partial remission | 1 HBeAg clearance, 2 HBeAg seroconversion | 5 ESRD | 49.2 ± 16.5 mo |
| Treatment | 10 | 3TC, 100 mg/d, 49.2 ± 16.5 mo, plus ACEI or ARB | 0 | 7 complete remission, 3 partial remission | 8 HBV-DNA clearance (5 HBeAg clearance) | 0 | ||
| Panomsak et al[20], 2006 | Control | 10 | ACEI, fish oil, or neither | 3 | 2 complete remission | 0 HBeAg clearance | 2 ESRD | 5-120 mo |
| Treatment | 7 | 1 month of prednisolone followed by 3TC in 6 case and IFNα in one case | 0 | 2 complete remission, 5 partial remission | 1 HBeAg seroconversion | 0 | ||
| Yang et al[21], 2003 | Control | 14 | The supportive or symptomatic treatment | 0 | 9 complete remission, 2 partial remission | 3 HBeAg seroconversion | 0 | 3.8 ± 2.4 yr |
| Treatment | 6 | rIFNα, 1-3 mU, 3 t/w for 3-6 mo | 0 | 3 complete remission, 2 partial remission | 3 HBeAg seroconversion | 0 | ||
| Author | Group | Case (n) | Intervention | Dropped-out (n) | Outcome | Follow-up | |
| CR | Renal insufficiency (n) | ||||||
| Lai et al[22], 1990 | Control | 7 | Diuretic agents | 0 | 2 complete remission | UA | 14-37 mo |
| Treatment | 8 | Prednisolone 60 mg/d (adult), 40 mg/m2 per day (< 15 yr), for 6 mo | 0 | 3 complete remission, 4 partial remission, 1 relapse | UA | ||
| Ozdamar et al[23], 2003 | Control | 4 | None | 0 | 4 complete remission | UA | 5-120 mo |
| Treatment | 8 | Prednisolone, 2 mg/kg per day | 2 | 1 complete remission, 4 partial remission, 1 death due to sepsis | UA | ||
| Panomsak et al[20], 2006 | Control | 10 | ACEI, fish oil, or neither | 3 | 2 complete remission | 2 ESRD | 5-120 mo |
| Treatment | 6 | Prednisolone, 2 mg/kg per day | 1 | 3 complete remission, 2 partial remission | 0 | ||
| Yang et al[21], 2003 | Control | 14 | The same supportive and symptomatic treatment as treatment group | 0 | 9 complete remission, 2 partial remission | 0 | 3.8 ± 2.4 yr |
| Treatment | 8 | Prednisolone, 1.5-2 mg/kg per day, for 3 mo | 0 | 4 complete remission, 2 partial remission | 0 | ||
| Peña et al[24], 2001 | Control | 4 | Symptomatic treatment | 0 | 4 complete remission | UA | 9.95 ± 5.88 yr |
| Treatment | 7 | Prednisone, 1.5-2 mg/kg per day, a minimun of 4 wk (1 case prednisone + CTX) | 0 | 7 steroid-resistant during therapy, but all complete remission at the end of follow-up | UA | ||
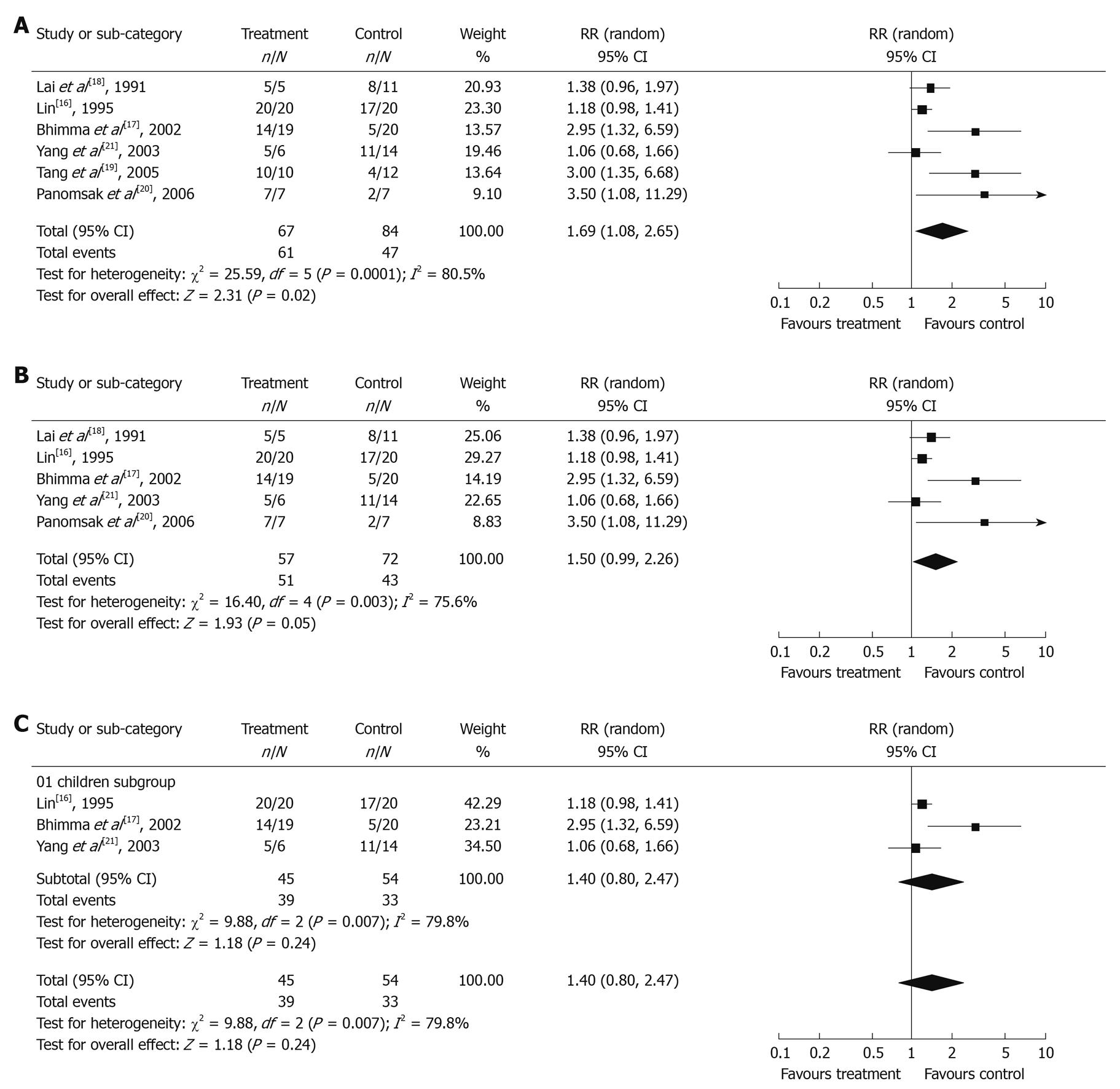
The efficacy of antiviral therapy on HBV-GN was assessed using 6 trials[16-21], including 1 RCT[16] and 5 cohort studies[17-21]. The total number of patients was 159 (72 in treatment group with 5 dropped out, 87 in control group with 3 dropped out). Among the 159 patients, 133 presented with nephrotic syndrome and 134 with membranous nephropathy. The mean follow-up time was five months to ten years, significantly different between trials.
Clinical response in antiviral treatment group and control group: The χ2 test of heterogeneity was highly significant (P = 0.0001). Accordingly, a random-effect model was used. The remission rate of proteinuria was significantly higher in antiviral treatment group (91.0%) than in control group (56.0%) with a combined RR of 1.69 (95% CI: 1.08-2.65, Figure 1A). The result of sensitivity analysis remained unchanged even if lamivudine treatment studies were excluded (RR = 1.50, 95% CI: 0.99-2.26, Figure 1B), indicating that the result is stable.
Furthermore, three trials[16,17,21] on pediatric patients were analyzed. The χ2 test of heterogeneity was also highly significant (P = 0.007), so a random-effect model was selected. As shown in Figure 1C, the remission rate of proteinuria in pediatric patients was slightly higher in treatment group (86.7%) than in control group (61.1%) with a combined RR of 1.40 (95% CI: 0.80-2.47), but the difference was not statistically significant (P = 0.24).
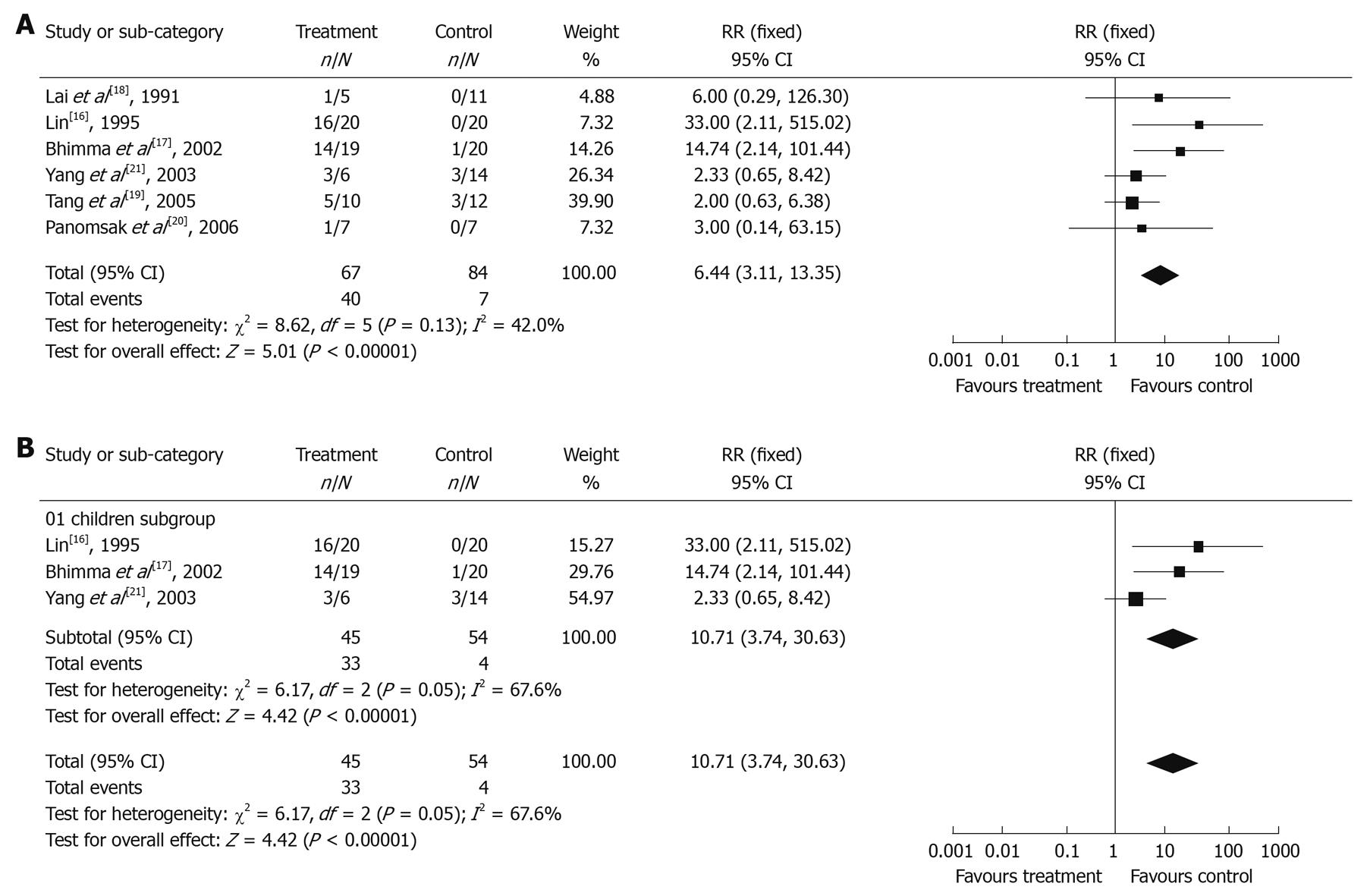
Virologic response in antiviral treatment group and control group: The χ2 test of heterogeneity was not significant (P = 0.13), therefore a fixed-effect model was selected. The clearance rate of HBeAg was significantly higher in antiviral treatment group (59.7%) than in control group (8.33%) with a RR of 6.44 (95% CI: 3.11-13.35, Figure 2A).
In addition, 3 trials[16,17,21] on pediatric patients were separately analyzed for virologic response. The χ2 test of heterogeneity was significant (P = 0.05), therefore a random-effect model was used. The clearance rate of HBeAg was significantly higher in antiviral treatment group (73.3%) than in control group (7.4%) with a RR of 10.71 (95% CI: 3.74-30.63, Figure 2B).
Consistency analysis of clinical and virologic responses: Kappa analysis showed that proteinuria remission was significantly related with HBeAg clearance after antiviral therapy (kappa = 0.285, P = 0.002)
The renal function of patients was observed in 5 of the 6 trials during the follow-up (Table 2). Renal insufficiency was found in only 3 of 47 (6.38%) patients in the antiviral treatment group and in 11 of 64 (17.2%) patients in the control group, respectively.
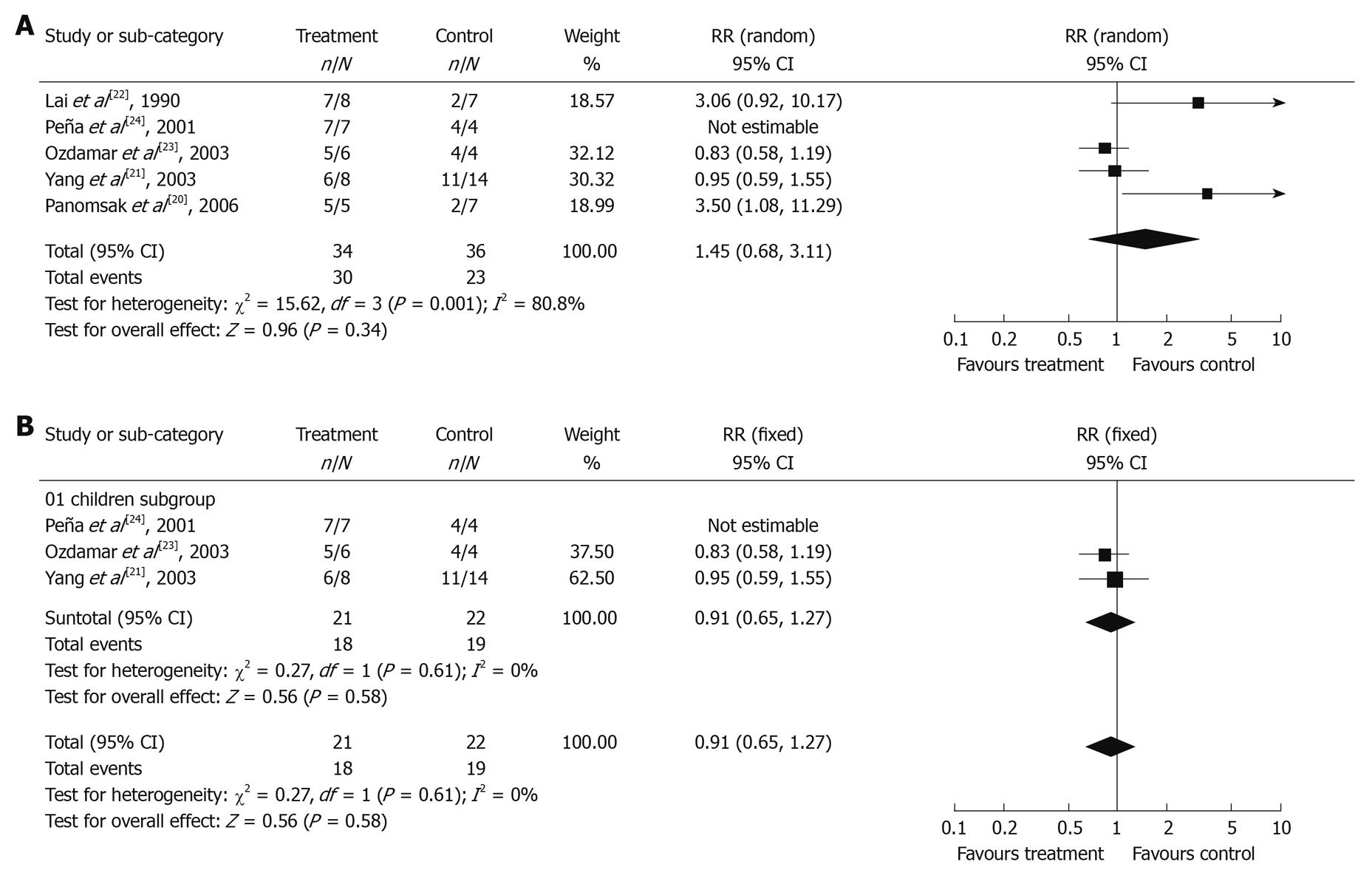
The efficacy of corticosteroid treatment on HBV-GN was assessed in 5 out of 9 articles[20-24]. Two of them were included in meta-analysis of antiviral therapy efficacy. Of the 23 patients in Panomsak’s study[20], 7, 6 and 10 were treated with antiviral drugs, prednisolone and symptomatic treatment, respectively. Of the 28 patients in Yang’s study[21], 6, 8 and 14 were treated with antiviral drugs, prednisolone and symptomatic treatment, respectively. All the 5 trials were cohort studies. The clinical response at the end of follow-up is shown in Figure 3A. Of the 76 patients analyzed, 37 were in corticosteroid treatment group with 3 dropped out, 39 were in control group with 3 dropped out. Among them, 52 presented with nephrotic syndrome and 56 with membranous nephropathy. The χ2 test of heterogeneity was highly significant (P = 0.001), so a random-effect model was used. The combined RR was 1.45 with a 95% CI of 0.68-3.11 (P = 0.34), indicating that there is no significant difference in proteinuria remission rate between corticosteroid treatment group and control group. However, this result should be carefully interpreted since a limited number of clinical trials can affect the conclusion of meta-analysis. Besides, it was difficult to assess the protective effect of corticosteroid treatment on renal function since only 2 of 5 clinical trials described the renal function during the followed-up.
Moreover, 3 trials[21,23,24] on pediatric patients were separately pooled to analyze the efficacy of corticosteroid treatment. The χ2 test of heterogeneity was not significant (P = 0.61), so a fixed-effect model was used. The combined RR was 0.91 with a 95% CI of 0.65-1.27 (P = 0.58), indicating that the difference in the remission rate of proteinuria was also not significant between corticosteroid treatment group and control group (Figure 3B).
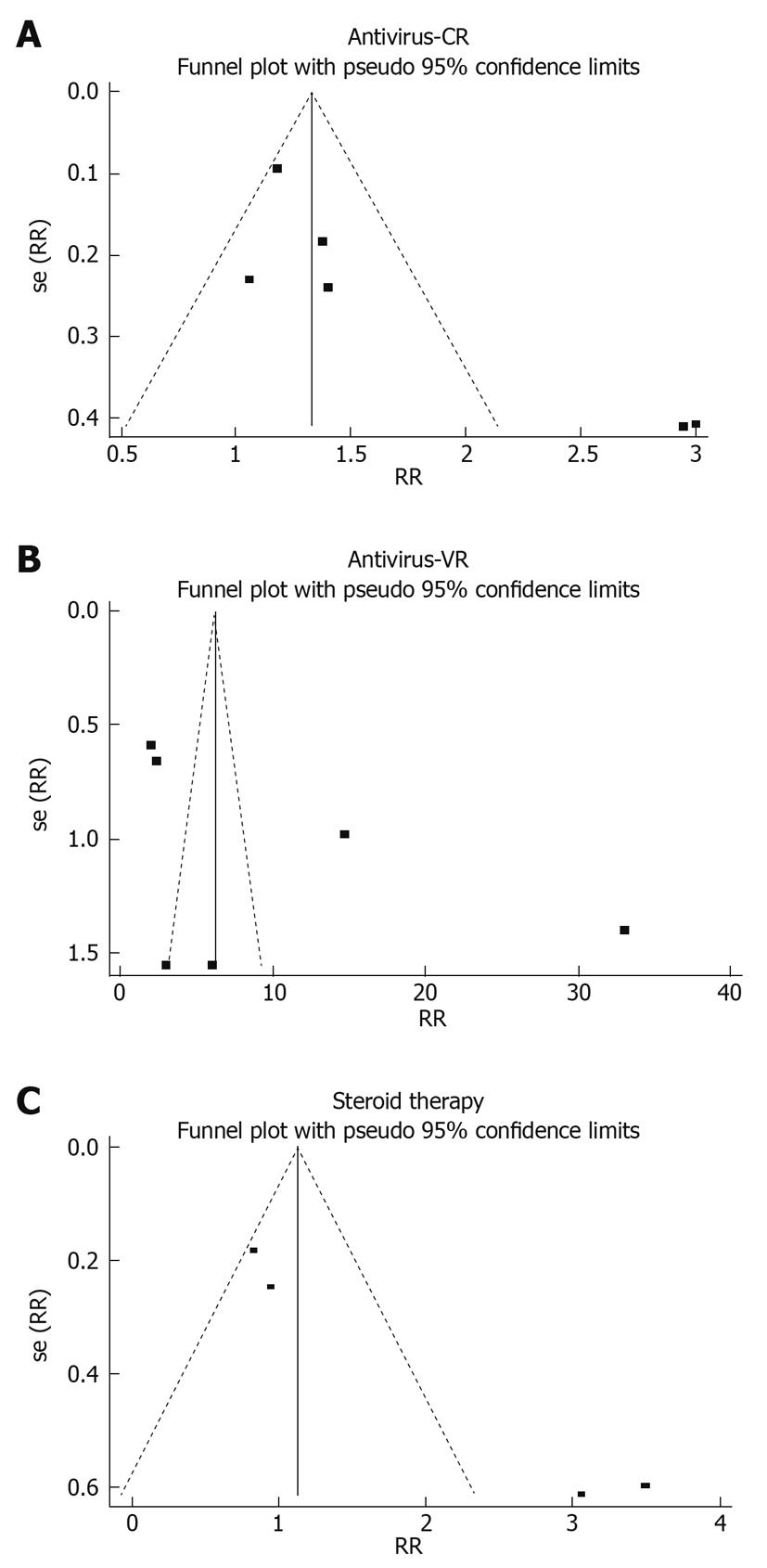
Publication bias may exist when no significant findings remain unpublished, thus artificially inflating the apparent magnitude of an effect. Egger and Begg tests showed that the risk of having missed trials was acceptably low, since the P values for the clinical and virologic responses to antiviral therapy and the clinical response to corticosteroid treatment were greater than 0.05. The funnel plots of study results against precision are shown in Figure 4.
Since adverse events were reported inconsistently in across studies and the relevant information in these studies was incomplete, we did not evaluate their incidence and severity of adverse events of these drugs. Some adverse events such as influenza-like illness, anemia, leucopenia, etc, were reported in patients treated with IFN. Almost all patients showed good tolerance to long-term administration of lamivudine, although some patients complained of headache, dizziness, local myalgia, paresthesia, etc.
Most HBV-GN patients presented with nephrotic syndrome, many of them, especially pediatric patients showed a spontaneous remission trend, so whether the patients should be treated with antiviral drugs or with immunosuppressive agents remains to be elucidated. Antiviral therapy has been recommended in many studies for HBV-GN since it can effectively inhibit HBV replication and attenuate proteinuria[9,25-33]. Our results demonstrated that antiviral therapy could significantly improve the remission rate of proteinuria, the clearance rate of HBeAg, and renal progression. Moreover, Kappa analysis showed that proteinuria remission is significantly related with HBeAg clearance after antiviral therapy. Only 5 patients were dropped out in antiviral treatment group due to economical reasons. Almost all patients were tolerable to antiviral drugs. Our results are consistent with Fabrizi’s study[12]. Since each trial used different kinds, dosages and treatment courses of antiviral drugs, the meta-analysis proved the efficacy of antiviral treatment but did not necessarily mean that an exact treatment protocol should be recommended. The meta-analysis of pediatric patients showed that antiviral therapy could significantly increase the clearance rate of HBeAg, but not remarkably improve proteinuria, which is not consistent with our above findings possibly due the limited sample size. Large-scale randomized controlled trials on pediatric patients are needed to clarify if antiviral therapy can induce remission of proteinuria.
Corticosteroid is the first-line drug for idiopathic nephrotic syndrome, but it may activate potent HBV infection leading to deterioration of liver and renal lesion[22,34-36]. Our meta-analysis showed that corticosteroid treatment could not significantly improve proteinuria. The effect of corticosteroids on proteinuria remission was not better than nonspecific symptomatic treatment, but its potent risk could not be neglected. Therefore, based on the results of this meta-analysis, corticosteroid should not be recommended for HBV-GN patients solely, especially for those with a high viral load and abnormal liver functions. Theoretically, corticosteroid in combination with antiviral drugs is certainly superior over corticosteroid alone, but no trials are available. So corticosteroid may only be used cautiously on the basis of antiviral therapy with viral load closely monitored.
As with all meta-analyses, our study had certain limitations of publication bias[37]. The number of high-quality clinical trials and enrolled patients was limited in this study. Moreover, the time of treatment was not long enough to evaluate its effects on chronic HBV-GN.
In conclusion, the efficacy and safety of antiviral therapy (including IFN and lamivudine) on HBV-GN are good. Antiviral therapy is effective on remission of proteinuria, and HBeAg clearance, delaying renal function deterioration. However, corticosteroids cannot ameliorate HBV-GN.
Hepatitis B virus-associated glomerulonephritis (HBV-GN) is one of the common secondary glomerular diseases in China. Although spontaneous remission can occur in many pediatric patients, some still develop progressive renal failure. Therefore, it is very important to attenuate proteinuria and delay renal disease progression.
So far HBV-GN has been treated like hepatitis B with antiviral drugs including interferon, lamivudine, entecavir or like primary nephrotic syndrome with corticosteroids and even immunosuppressive agents such as mycophenolate mofetil, leflunomide, etc. However, it is still uncertain up to now about the efficacy of these treatment modalities.
The data available in previous studies on HBV-GN treatment are limited and often provide inconsistent results. So far only one meta-analysis of antiviral therapy for HBV-GN was published in 2006, but 2 of the 6 trials included were non-controlled studies. The meta-analysis including controlled studies is the first to evaluate the effects of antiviral drugs and corticosteroids on HBV-GN. Moreover, pediatric patients were separately assessed.
The results of this study suggest that antiviral but not corticosteroid treatment can decrease proteinuria and promote Hepatitis B e-antigen clearance in HBV-associated glomerulonephritis patients. It may help doctors to optimally treat HBV-GN patients.
Hepatitis B virus-associated glomerulonephritis is an immune-mediated secondary glomerular disease characterized by deposits of hepatitis B viral antigens, immunoglobulins and C3 in the glomerular capillary wall and mesangium. Nephrotic syndrome, proteinuria and/or hematuria are the most common renal manifestations.
The present manuscript describes a meta-analysis for the evaluation of clinical and virologic responses to antiviral and corticosteroid treatment of hepatitis B-associated nephritis. Overall, the methods are appropriate and the results are believable.
Peer reviewer: Eva Herrmann, Professor, Department of Internal Medicine, Biomathematics Saarland University, Faculty of Medicine, Kirrberger Str., 66421 Homburg/Saar, Germany
S- Editor Tian L L- Editor Wang XL E- Editor Lin YP
| 1. | Bhimma R, Coovadia HM, Adhikari M, Connolly CA. The impact of the hepatitis B virus vaccine on the incidence of hepatitis B virus-associated membranous nephropathy. Arch Pediatr Adolesc Med. 2003;157:1025-1030. [Cited in This Article: ] |
| 2. | Xu H, Sun L, Zhou LJ, Fang LJ, Sheng FY, Guo YQ. The effect of hepatitis B vaccination on the incidence of childhood HBV-associated nephritis. Pediatr Nephrol. 2003;18:1216-1219. [Cited in This Article: ] |
| 3. | Walters S, Levin M. Infectious diseases and the kidney. Pediatric Nephrology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins 1999; 1088-1090. [Cited in This Article: ] |
| 4. | Lai KN, Lai FM, Chan KW, Chow CB, Tong KL, Vallance-Owen J. The clinico-pathologic features of hepatitis B virus-associated glomerulonephritis. Q J Med. 1987;63:323-333. [Cited in This Article: ] |
| 5. | Gilbert RD, Wiggelinkhuizen J. The clinical course of hepatitis B virus-associated nephropathy. Pediatr Nephrol. 1994;8:11-14. [Cited in This Article: ] |
| 6. | Lin CY. Hepatitis B virus-associated membraneous nephropathy: clinical features, immunological profiles and outcome. Nephron. 1990;55:37-44. [Cited in This Article: ] |
| 7. | Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol. 2004;24:198-211. [Cited in This Article: ] |
| 8. | Zhou JH, Zhang Y. Treatment of Hepatitis B Virus Associated Nephritis in Children. Shiyong Erke Linchuang Zazhi. 2008;23:329-332. [Cited in This Article: ] |
| 9. | Chung DR, Yang WS, Kim SB, Yu E, Chung YH, Lee Y, Park JS. Treatment of hepatitis B virus associated glomerulonephritis with recombinant human alpha interferon. Am J Nephrol. 1997;17:112-117. [Cited in This Article: ] |
| 10. | Wong SN, Yu EC, Lok AS, Chan KW, Lau YL. Interferon treatment for hepatitis B-associated membranous glomerulonephritis in two Chinese children. Pediatr Nephrol. 1992;6:417-420. [Cited in This Article: ] |
| 11. | Appel GB, Radhakrishnan J, D’Agati V. Secondary glomerular disease. The Kidney. 6th ed. Philadelphia, PA: WB Saunders 2000; 1411. [Cited in This Article: ] |
| 12. | Fabrizi F, Dixit V, Martin P. Meta-analysis: anti-viral therapy of hepatitis B virus-associated glomerulonephritis. Aliment Pharmacol Ther. 2006;24:781-788. [Cited in This Article: ] |
| 13. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [Cited in This Article: ] |
| 14. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [Cited in This Article: ] |
| 15. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [Cited in This Article: ] |
| 16. | Lin CY. Treatment of hepatitis B virus-associated membranous nephropathy with recombinant alpha-interferon. Kidney Int. 1995;47:225-230. [Cited in This Article: ] |
| 17. | Bhimma R, Coovadia HM, Kramvis A, Adhikari M, Kew MC. Treatment of hepatitis B virus-associated nephropathy in black children. Pediatr Nephrol. 2002;17:393-399. [Cited in This Article: ] |
| 18. | Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324:1457-1463. [Cited in This Article: ] |
| 19. | Tang S, Lai FM, Lui YH, Tang CS, Kung NN, Ho YW, Chan KW, Leung JC, Lai KN. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750-1758. [Cited in This Article: ] |
| 20. | Panomsak S, Lewsuwan S, Eiam-Ong S, Kanjanabuch T. Hepatitis-B virus-associated nephropathies in adults: a clinical study in Thailand. J Med Assoc Thai. 2006;89 Suppl 2:S151-S156. [Cited in This Article: ] |
| 21. | Yang Q, Zhuang JQ, Lin RX, Yang YZ, Wang ZX. Clinicopathological Features and Treatment of Hepatitis B Virus Associated Membranous Nephropathy in Children. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi. 2003;4:706-708. [Cited in This Article: ] |
| 22. | Lai KN, Tam JS, Lin HJ, Lai FM. The therapeutic dilemma of the usage of corticosteroid in patients with membranous nephropathy and persistent hepatitis B virus surface antigenaemia. Nephron. 1990;54:12-17. [Cited in This Article: ] |
| 23. | Ozdamar SO, Gucer S, Tinaztepe K. Hepatitis-B virus associated nephropathies: a clinicopathological study in 14 children. Pediatr Nephrol. 2003;18:23-28. [Cited in This Article: ] |
| 24. | Peña A, Débora MJ, Melgosa M, Luz Picazo M, Navarro M. Membranous nephropathy associated with hepatitis B in Spanish children. Clin Nephrol. 2001;55:25-30. [Cited in This Article: ] |
| 25. | Shapiro RJ, Steinbrecher UP, Magil A. Remission of nephrotic syndrome of HBV-associated membranous glomerulopathy following treatment with interferon. Am J Nephrol. 1995;15:343-347. [Cited in This Article: ] |
| 26. | Kavukçu S, Başdemir G, Eroğlu Y, Türkmen M, Eser F, Büyükgebiz B. Interferon treatment in hepatitis B virus-associated membranous glomerulopathy. Pediatr Nephrol. 1995;9:539-540. [Cited in This Article: ] |
| 27. | Conjeevaram HS, Hoofnagle JH, Austin HA, Park Y, Fried MW, Di Bisceglie AM. Long-term outcome of hepatitis B virus-related glomerulonephritis after therapy with interferon alfa. Gastroenterology. 1995;109:540-546. [Cited in This Article: ] |
| 28. | Connor FL, Rosenberg AR, Kennedy SE, Bohane TD. HBV associated nephrotic syndrome: resolution with oral lamivudine. Arch Dis Child. 2003;88:446-449. [Cited in This Article: ] |
| 29. | Filler G, Feber J, Weiler G, Le Saux N. Another case of HBV associated membranous glomerulonephritis resolving on lamivudine. Arch Dis Child. 2003;88:460. [Cited in This Article: ] |
| 30. | Gan SI, Devlin SM, Scott-Douglas NW, Burak KW. Lamivudine for the treatment of membranous glomerulopathy secondary to chronic Hepatitis B infection. Can J Gastroenterol. 2005;19:625-629. [Cited in This Article: ] |
| 31. | Kanaan N, Horsmans Y, Goffin E. Lamivudine for nephrotic syndrome related to hepatitis B virus (HBV) infection. Clin Nephrol. 2006;65:208-210. [Cited in This Article: ] |
| 32. | Okuse C, Yotsuyanagi H, Yamada N, Ikeda H, Takahashi H, Suzuki M, Kondo S, Kimura K, Koike J, Itoh F. Successful treatment of hepatitis B virus-associated membranous nephropathy with lamivudine. Clin Nephrol. 2006;65:53-56. [Cited in This Article: ] |
| 33. | Wen YK, Chen ML. Remission of hepatitis B virus-associated membranoproliferative glomerulonephritis in a cirrhotic patient after lamivudine therapy. Clin Nephrol. 2006;65:211-215. [Cited in This Article: ] |
| 34. | Taskapan H, Oymak O, Dogukan A, Ozbakir O, Utas C. Transformation of hepatitis B virus-related membranous glomerulonephritis to crescentic form. Clin Nephrol. 2000;54:161-163. [Cited in This Article: ] |
| 35. | Lai FM, Tam JS, Li PK, Lai KN. Replication of hepatitis B virus with corticosteroid therapy in hepatitis B virus related membranous nephropathy. Virchows Arch A Pathol Anat Histopathol. 1989;414:279-284. [Cited in This Article: ] |
| 36. | Lin CY. Clinical features and natural course of HBV-related glomerulopathy in children. Kidney Int Suppl. 1991;35:S46-S53. [Cited in This Article: ] |
| 37. | Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207-216. [Cited in This Article: ] |