Published online Dec 28, 2010. doi: 10.3748/wjg.v16.i48.6104
Revised: August 30, 2010
Accepted: September 7, 2010
Published online: December 28, 2010
AIM: To investigate the effect of the G-1666A polymorphism in the multidrug resistance related protein-1 (MRP1) on outcome of hepatocellular carcinoma (HCC).
METHODS: A cohort of 162 patients with surgically resected HCC who received no postsurgical treatment until relapse was studied. Genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism analysis. Electrophoretic mobility shift assay (EMSA) was used to evaluate the influence of the G-1666A polymorphism on the binding affinity of the MRP1 promoter with its putative transcription factors.
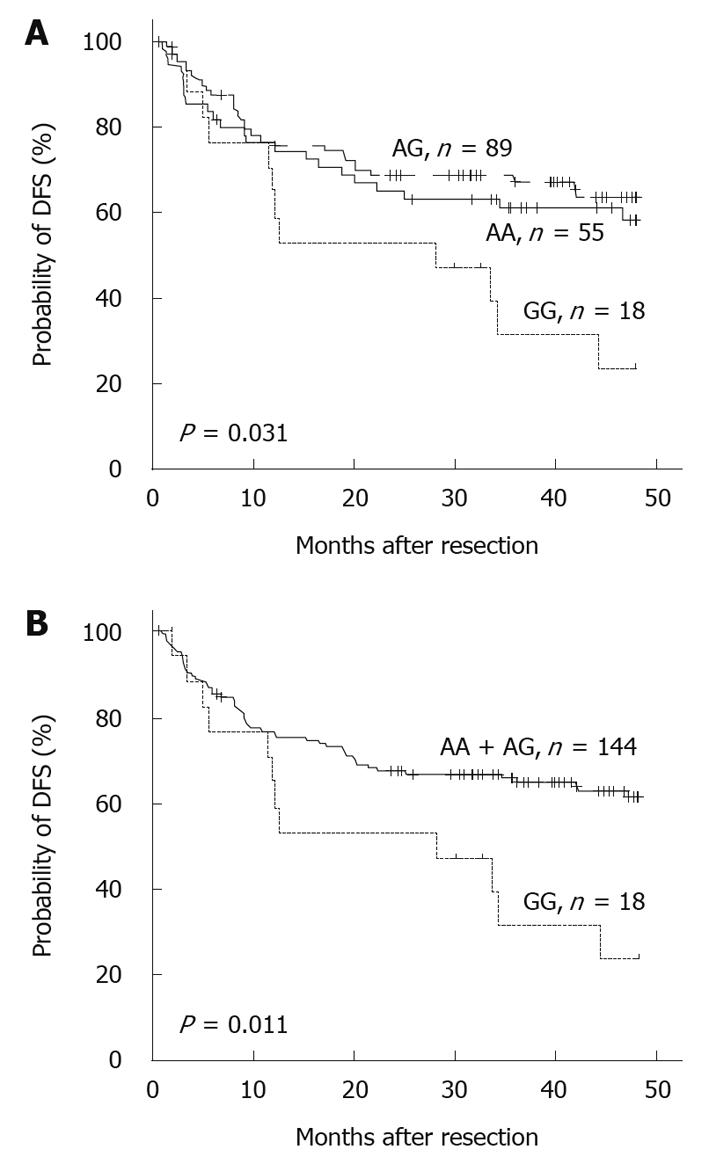
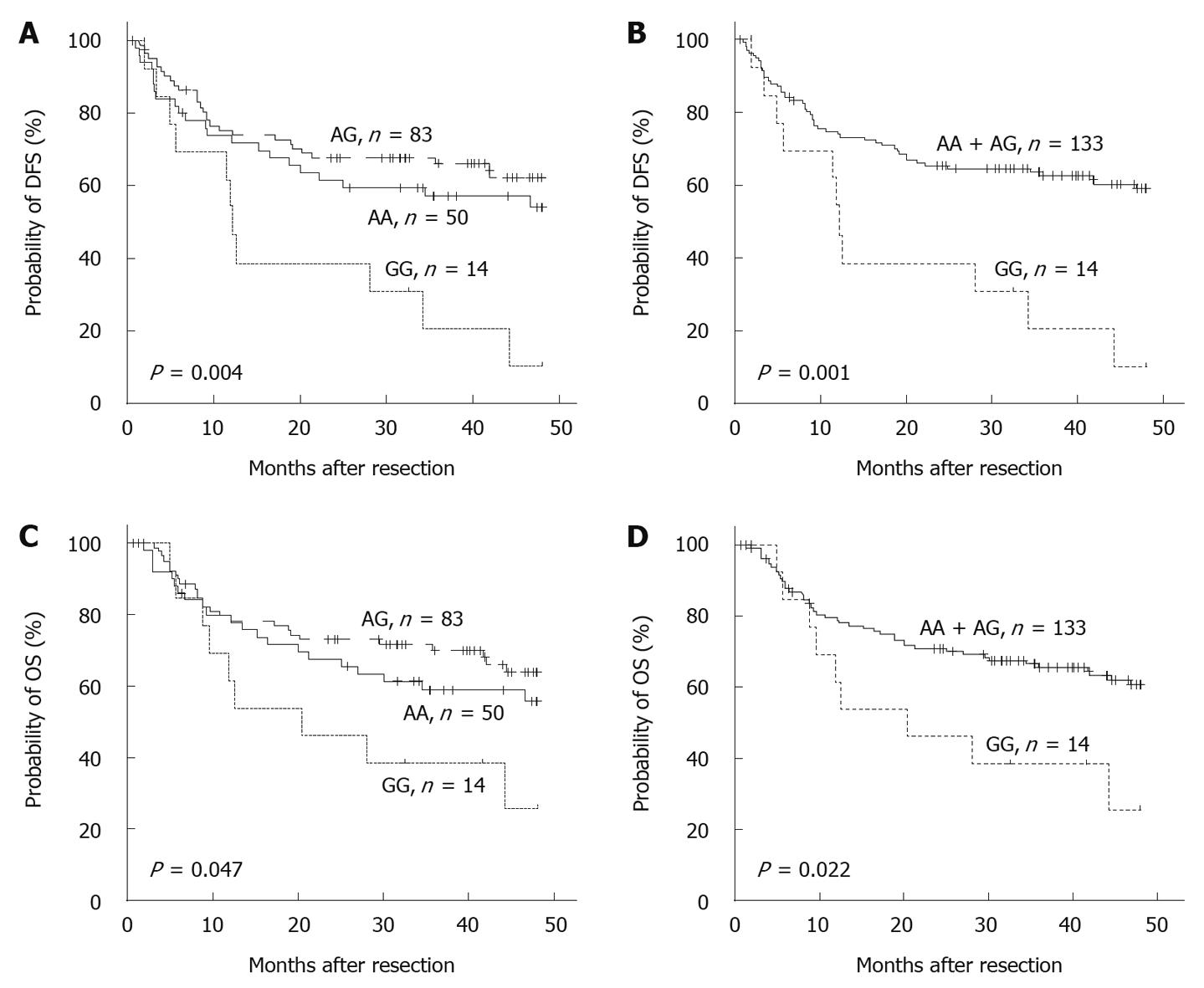
RESULTS: Kaplan-Meier analysis showed that patients with GG homologues had a reduced 4-year disease-free survival compared with those carrying at least one A allele (P = 0.011). Multivariate Cox regression analysis indicated that the -1666GG genotype represented an independent predictor of poorer disease-free survival [hazard ratio (HR) = 3.067, 95% confidence interval (CI): 1.587-5.952, P = 0.001], and this trend became worse in men (HR = 3.154, 95% CI: 1.604-6.201, P = 0.001). A similar association was also observed between 4-year overall survival and the polymorphism in men (HR = 3.342, 95% CI: 1.474-7.576, P = 0.004). Moreover, EMSA suggested that the G allele had a stronger binding affinity to nuclear proteins.
CONCLUSION: The MRP1 -1666GG genotype predicted a worse outcome and was an independent predictor of poor survival in patients with HCC from Southeast China.
- Citation: Zhao J, Yu BY, Wang DY, Yang JE. Promoter polymorphism of MRP1 associated with reduced survival in hepatocellular carcinoma. World J Gastroenterol 2010; 16(48): 6104-6110
- URL: https://www.wjgnet.com/1007-9327/full/v16/i48/6104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i48.6104
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third leading cause of cancer death[1]. Optimal surgical resection is regarded as the best treatment for a curative outcome of HCC. However, long-term survival remains poor because of high rates of tumor recurrence or progression. Substantial effort has been made to identify prognostic factors that can be used for improving therapeutic regimens and survival prediction. However, only a few factors, such as TNM stage or patient performance status, are consistent predictors, and their accuracy remains limited. Therefore, molecular markers that can accurately predict patient outcome are urgently needed.
The human multidrug resistance protein-1 (MRP1), also known as ABCC1, belongs to the ATP-binding cassette superfamily of cell-surface transport proteins. It participates in the transport of a wide variety of endogenously produced and exogenously administered molecules in an adenosine-triphosphate (ATP)-dependent manner[2,3]. Besides its well-known roles in drug resistance, MRP1 is proposed to contribute to the cellular antioxidative defense system by actively extruding glutathione (GSH)-conjugated xenobiotics and GSH-conjugated metabolites from cells[4]. Recent studies have also revealed that MRP1 is involved in inflammatory reactions, such as, dendritic cell differentiation and function[5]. MRP1 is expressed at moderate levels in most normal tissues, including lung, muscle, and kidney, but is barely detectable in normal liver[6-8]. However, in several liver diseases including HCC, its expression in the basolateral membrane is upregulated, which suggests a significant role for this transport protein during carcinogenesis[8,9].
Single nucleotide polymorphisms (SNPs) in the MRP1 gene have been extensively studied in the past few years, and several genetic variants in the coding region have been shown to affect the function of MRP1[10-13]. For example, G2168A (Arg723Gln) can affect patients’ sensitivity to chemotherapy in ovarian cancer[11]. G1299T (Arg433Ser) confers resistance to doxorubicin by reducing intracellular drug accumulation in HeLa cells that stably express mutant MRP1, whereas the G3173A (Arg1058Gln) variation increases the response to etoposide in HEK293 and CHO-K1 cells[12,13]. Recently, it has been observed that SNPs in the gene promoter can affect expression by disturbing the binding affinity of transcription factors, and are associated with disease prognosis[14]. However, whether SNPs in the MRP1 promoter region have any clinical significance remains obscure. The expression level of MRP1 is upregulated in HCC, therefore, we hypothesized that sequence variants in the promoter region potentially affect the expression of the MRP1 gene and the prognosis of cancer, by modulating the efflux of toxins. To test this hypothesis, we investigated the potential of the MRP1 G-1666A polymorphism (rs4148330) as a prognostic marker in a cohort of patients with HCC in Guangdong province of Southeast China.
The study included 162 patients with HCC at the Cancer Center of Sun Yat-sen University (Guangzhou, China) from 2001 to 2005. All patients underwent hepatectomy as initial therapy, and did not receive chemotherapy or radiotherapy as follow-up treatment before recurrence. All samples were histologically confirmed. After surgical resection, the tissue samples were immediately frozen in liquid nitrogen and then stored at -80°C until use.
Clinicopathological details and follow-up information were obtained from hospital records. The patients enrolled in the study were residents of Guangdong Province. Infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) was diagnosed when HBV surface antigen or HCV antibody was detected by enzyme linked immunosorbent assay in the serum isolated from peripheral blood. The TNM criteria and the Edmondson and Steiner grading system were used to classify tumor stages and differentiation grades, respectively. Informed consent was obtained from each patient. This study was approved by the Clinical Research Ethics Committee of Sun Yat-sen University Cancer Center.
Total genomic DNA was isolated with a standard protocol that included proteinase digestion, phenol-chloroform extraction, and ethanol precipitation. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis was used to detect the genotype. A 160-bp fragment that covered the G-1666A polymorphism was generated using sense primer 5'-GCAACAGCATAACTGGCATT-3' and reverse primer 5'-GAGACCTCCCCCCAATCA-3'. PCR was performed as follows: 20 ng genomic DNA was amplified in a 20-μL reaction mixture that contained 2 mmol/L MgCl2, 0.4 mmol/L dNTPs, 0.2 μmol/L each primer, and 0.5 U Taq polymerase (Promega, Madison, WI, USA). After a total of 36 cycles of amplification at an annealing temperature of 58°C, 3 μL PCR products was then incubated overnight at 37°C with 15 U HpaII (MBI Fermentas, Hanover, MD, USA). Digested products were analyzed by 2% agarose gel. PCR fragments that demonstrated altered electrophoretic patterns were purified and characterized by direct DNA sequencing. Results represent two independent experiments.
Liver cancer cell lines Huh7 and Hep3B were obtained from the American Type Culture Collection (Manassas, VA, USA) and grown in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum in a humidified environment of 37°C that contained 50 mL/L CO2. Nuclear protein extracts from Hep3B and Huh7 cells were prepared according to the manufacturer’s protocol (NucBuster Protein Extraction Kit; Novagen, Darmstadt, Germany).
Electrophoretic mobility shift assay (EMSA) was performed with the Gel Shift Assay System (Promega), according to the manufacturer’s instructions. The following oligonucleotides that corresponded to the promoter region of MRP1 and covered the G-1666A polymorphism were synthesized (underline letters indicate polymorphism): -1666A allele, 5'-GGGGGACCCGGGCCAATAAAAAAATCA-3'; -1666G allele, 5'-GGGGGACCCAGGCCAATAAAAAAATCA-3'; nonspecific (scrambled) oligonucleotide, 5'-GAAGCGGTGACACGGAACATCACGAAA-3'. Oligonucleotides were annealed and end-labeled with [γ-32P]-ATP. Five micrograms of Hep3B or Huh7 nuclear extracts were added in each binding reaction. For the competition assay, a 10-, 50- or 100-fold molar excess of unlabeled oligonucleotide was added to the binding reaction mixture as a competitor. The products were separated on pre-electrophoresed 5% polyacrylamide gels at 4°C. The gels were then dried at 80°C for 4 h and exposed to a Storage Phosphor Screen (Amersham Bioscience, Sunnyvale, CA, USA), which was subsequently read with a Typhoon Phosphor Imager (Amersham Bioscience). The putative transcription factors that recognized the sequences that overlapped the G-1666A site were predicted with Alibaba2.1 (http://www.gene-regulation.com/pub/programs/alibaba2/index.html) and the transcription element search software (TESS, http://www.cbil.upenn.edu/cgi-bin/tess/tess).
The χ2 and Fisher’s exact tests were used for the analysis of the relationship between the genotypes and clinicopathological characteristics. Disease-free survival (DFS) was calculated from the day of surgery to either relapse or death without relapse, and it was censored only for patients who were alive and recurrence-free at the last follow-up. Overall survival (OS) was measured from the date of hepatectomy to the time of death or the last follow-up. Survival curves were obtained by the Kaplan-Meier method, and the statistical significance of the differences in survival among subgroups was evaluated with the log-rank test. The Cox proportional hazards model was employed to assess the independent prognostic values of the polymorphisms. Statistical analyses were all performed with SPSS software package (version 13.0; SPSS, Inc., Chicago, IL, USA). All statistical tests were two-sided, and P < 0.05 was considered to be statistically significant.
Demographic and clinicopathological characteristics of the 162 patients with HCC are summarized in Table 1. The mean age at first diagnosis of HCC was 48 years. Consistent with our previous study[15], most patients showed excessive γ-glutamyl transpeptidase and α-fetoprotein, along with liver cirrhosis, and > 80% of the enrolled patients were infected with HBV (140/161, 87.0%), which implicated HBV infection as a leading cause of HCC in Southeast China. In contrast, only a small number of patients were infected with HCV.
| n (%) | |
| Sex | |
| Female | 15 (9.3) |
| Male | 147 (90.7) |
| Age (yr) | |
| < 48 | 77 (47.5) |
| ≥ 48 | 85 (52.5) |
| HBV infection1 | |
| - | 21 (13.0) |
| + | 140 (86.4) |
| HCV infection | |
| - | 158 (97.5) |
| + | 4 (2.5) |
| GGT (U/L)2 | |
| < 50 | 45 (27.8) |
| 50-99 | 48 (29.6) |
| ≥ 100 | 67 (41.4) |
| AFP (ng/mL) | |
| < 20 | 53 (32.7) |
| 20-399 | 45 (27.8) |
| ≥ 400 | 64 (39.5) |
| Tumor size (cm) | |
| < 5 | 53 (32.7) |
| ≥ 5 | 109 (67.3) |
| Ascites3 | |
| - | 148 (91.4) |
| + | 14 (8.6) |
| Cirrhosis | |
| Total | 19 (11.7) |
| Mild | 80 (49.4) |
| Moderate | 49 (30.2) |
| Severe | 14 (8.6) |
| Edmondson grade | |
| I | 11 (6.8) |
| II | 71 (43.8) |
| III | 77 (47.5) |
| IV | 3 (1.9) |
| TNM stage | |
| I | 106 (65.4) |
| II | 7 (4.3) |
| III | 49 (30.3) |
| G-1666A genotype | |
| AA | 55 (34.0) |
| AG | 89 (54.9) |
| GG | 18 (11.1) |
Genotyping was performed by PCR-RFLP. A 160-bp MRP1 promoter region that covered the G-1666A variant was digested with HpaII. After full digestion of the amplified PCR products, those from AA homozygotes still existed as a single 160-bp fragment, whereas those from the GG homozygotes had been divided into two fragments of 71 bp and 89 bp, respectively. The allele frequency of patients with HCC was 0.61 for MRP1 -1666A and 0.39 for -1666G. However, no significant correlations were found between the nucleotide variants and clinical variables (data not shown).
Growing evidence suggests that SNPs are closely related to the risk and outcome of cancer[14,16,17]. To investigate the impact of the G-1666A polymorphism on the prognosis of patients with HCC, we next analyzed the 4-year DFS and OS of patients with different genotypes. A significant correlation between the -1666 polymorphism and postoperative survival was found. The mean survival times of patients with the AA, AG and GG genotypes were 30.4 ± 18.2, 30.7 ± 17.4 and 24.8 ± 17.3 mo, respectively. The survival curves showed that the 4-year rate of DFS among patients who carried GG decreased significantly compared with those who carried the AA or AG allele (P = 0.031, Figure 1A). Moreover, if the patients with AA and AG genotypes were combined, the discrepancy became more obvious (P = 0.011, Figure 1B). Further analysis revealed a similar, albeit non-significant, trend between the -1666 polymorphism and 4-year OS (Table 2). Multivariate Cox proportional hazard analysis was then performed, and the variables that showed significance by univariate analysis were adopted as covariates (Table 2). The results revealed that the MRP1 G-1666A polymorphism was an independent prognostic factor for 4-year DFS [hazard ratio (HR) = 3.067, 95% confidence interval (CI): 1.587-5.952, P = 0.001, Table 3].
| Variable | DFS | OS | ||
| HR (95% CI) | Pa | HR (95% CI) | Pa | |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 4.231 (1.035-17.299) | 0.045 | 7.522 (1.041-54.337) | 0.045 |
| GGT (U/L)1 | ||||
| < 50 | 1 | 1 | ||
| 50-99 | 2.064 (0.920-4.632) | 0.079 | 1.771 (0.734-4.275) | 0.203 |
| ≥ 100 | 3.639 (1.761-7.519) | 0.001 | 3.728 (1.734-8.014) | 0.001 |
| AFP (ng/mL) | ||||
| < 20 | 1 | 1 | ||
| 20-399 | 1.852 (0.954-3.595) | 0.068 | 2.004 (0.989-4.061) | 0.054 |
| ≥ 400 | 1.997 (1.070-3.725) | 0.030 | 2.102 (1.079-4.094) | 0.029 |
| Tumor size (cm) | ||||
| < 5 | 1 | 1 | ||
| ≥ 5 | 2.230 (1.233-4.030) | 0.008 | 2.089 (1.126-3.875) | 0.019 |
| Ascites2 | ||||
| - | 1 | 1 | ||
| + | 2.562 (1.301-5.044) | 0.007 | 2.81 (1.375-5.741) | 0.005 |
| Cirrhosis | ||||
| No | 1 | 1 | ||
| Mild | 2.005 (0.710-5.661) | 0.189 | 2.505 (0.763-8.225) | 0.130 |
| Moderate | 1.832 (0.623-5.387) | 0.271 | 2.287 (0.670-7.806) | 0.187 |
| Severe | 3.230 (0.993-10.505) | 0.051 | 4.796 (1.297-17.738) | 0.019 |
| TNM stage | ||||
| I | 1 | 1 | ||
| II + III | 3.165 (1.940-5.163) | < 0.001 | 3.424 (2.038-5.752) | < 0.001 |
| Genotypes | ||||
| AA | 1 | 1 | ||
| AG | 0.830 (0.479-1.439) | 0.507 | 0.818 (0.463-1.447) | 0.490 |
| GG | 1.988 (0.982-4.024) | 0.056 | 1.491 (0.682-3.258) | 0.317 |
| AA + AG | 1 | 1 | ||
| GG | 2.223 (1.185-4.172) | 0.013 | 1.678 (0.822-3.422) | 0.155 |
One of the key features of HCC is the much higher incidence in men than in women[1]. In our study cohort, the male/female ratio was 9.8:1. Further stratification of the patients by sex revealed an even more pronounced association of the -1666GG genotype with poorer survival (P < 0.05, Figure 2). Multivariate analysis suggested that the -1666GG genotype was an especially powerful independent prognostic factor of 4-year DFS (HR = 3.154, 95% CI: 1.604-6.201, P = 0.001) and OS (HR = 3.342, 95% CI: 1.474-7.576, P = 0.004) in the men with HCC (Table 3).
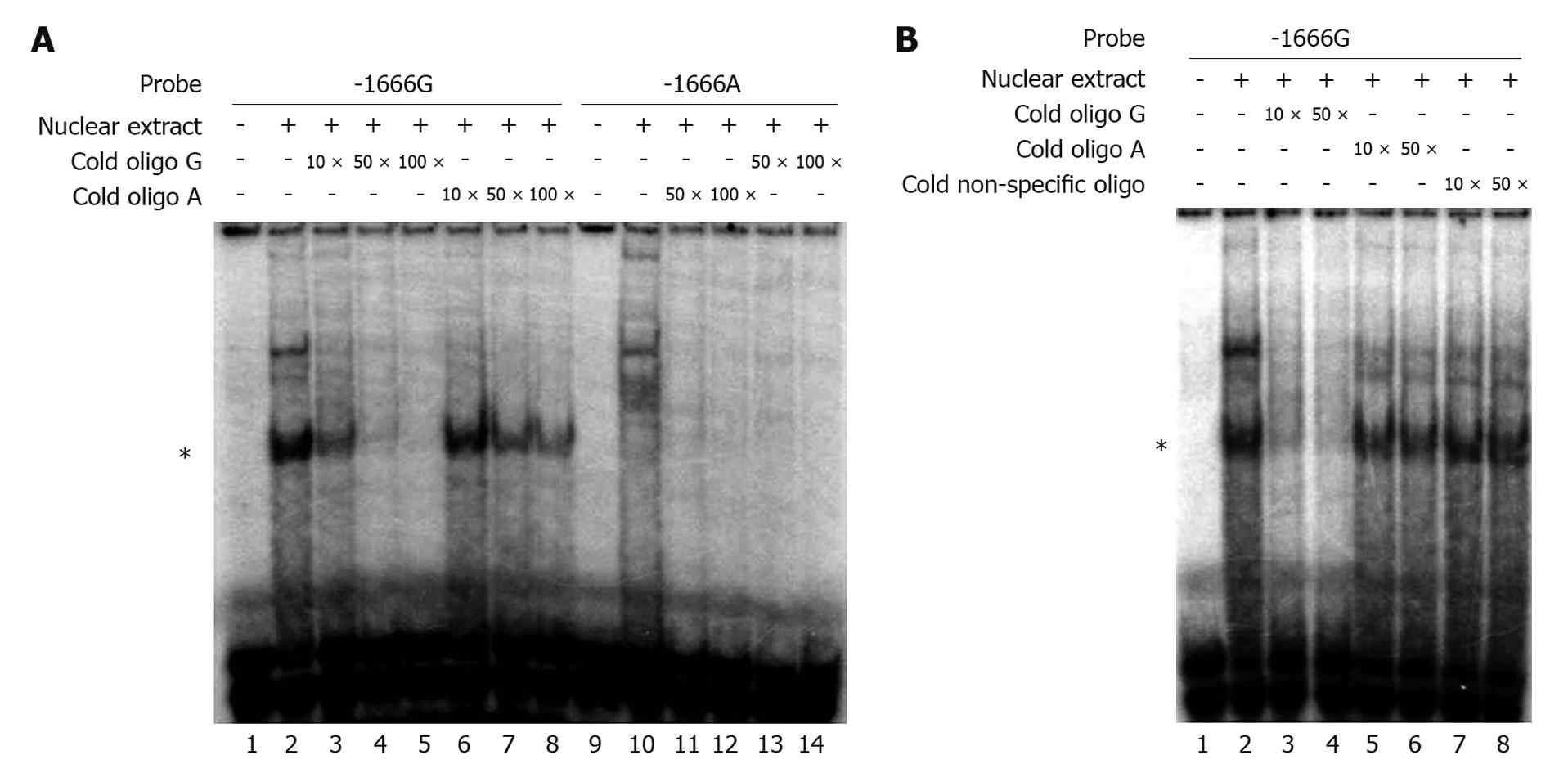
We performed EMSA to evaluate the influence of the G-1666A polymorphism on the binding affinity of the MRP1 promoter with putative transcription factors. The radiolabeled -1666G probe showed strong DNA-protein binding ability in the presence of nuclear proteins extracted from the Hep3B cell line, whereas the -1666A probe barely showed any interaction (Figure 3A, lane 2 and lane 10, respectively). In order to assess the binding specificity and the differences in binding affinity between the G and A alleles, competition assays were performed with unlabeled -1666A and -1666G oligonucleotides. A 50-fold excess of unlabeled -1666A oligonucleotides only partially disrupted the binding of the radiolabeled -1666G probe with nuclear extracts (Figure 3A, lane 7 and Figure 3B, lane 6), whereas this amount of unlabeled -1666G oligonucleotides almost completely abolished the binding (Figure 3A and B, lane 4). In contrast, a non-specific competitor had no effect (Figure 3B, lane 8). Similar results were obtained when using the nuclear extracts from Huh7 cells (data not shown). These data suggested that the G-1666A polymorphism could affect the binding affinity of the MRP1 promoter with transcription factors, and that the G allele had a stronger binding affinity than the A allele.
The multidrug resistance protein family transports a wide range of physiological substrates and diverse therapeutic agents. In the past decade, much effort has been focused on MRP1-mediated drug resistance[18,19]; and emerging evidence indicates that SNPs within the MRP1 gene have prognostic value in predicting the response to chemotherapy in different cancers[11,20]. Notably, MRP1 takes part in the transport of aflatoxin B1, a well-know human liver carcinogen that can induce a characteristic mutation in p53 at codon 249[21,22]; and previous studies have shown that p53 mutations are significantly associated with a poor prognosis for patients with HCC[15,23]. In addition, MRP1 also plays important roles in cellular antioxidant defense and immune cell function[4,5]. These observations and the upregulated expression level of MRP1 gene in several liver diseases, including HCC, suggest the possibility that this protein is involved in tumorigenesis and progression[8,9]. Our present study examined the role of the MRP1 G-1666A polymorphism as a prognostic factor in patients with HCC who were treated only with curative surgery, and proved that the GG genotype was an independent predictor of poor survival, especially in men with HCC. Furthermore, specific binding of nuclear proteins to G allele was found, which suggested a difference in transcription activity between different genotypes.
In the present study, there were 147 men (90.7%) and 15 women with HCC (9.3%), with a male-to-female ratio of 9.8:1. We observed a remarkably significant association of the MRP1 G-1666A polymorphism with 4-year OS in men with HCC, but not in the entire cohort. This phenomenon might result from the interaction between the polymorphism and sexual hormones during carcinogenesis, which has been demonstrated in the example of MDM2 SNP309[24]. Therefore, the correlation between the MRP1 G-1666A polymorphism and the survival of women with HCC requires further investigation to generate a definite conclusion.
SNPs in the promoter region of a gene can potentially alter the affinity of interactions between DNA and nuclear proteins and, in turn, affect the efficiency of transcription. We found that the G allele of the MRP1 G-1666A polymorphism had a stronger binding affinity for nuclear proteins in hepatoma cells than the A allele had. This finding accords with our presumption that the G-1666A polymorphism might dominate the pumping ability of MRP1 by affecting the expression of the protein. Although a G-1666A polymorphism located 1.5 kb upstream of the core promoter of MRP1, and two major regulatory domains had already been found in tandem upstream of the core promoter[25], recent studies have revealed that distal regions (enhancer or suppressor) can influence gene transcription through physical association with the transcription start site[26]. Furthermore, allele G of the G-260C polymorphism could lead to lower activity of the MRP1 promoter in cell lines, which suggests that nucleotide variants in the MRP1 gene account, in part, for inter-individual variations and population differences in cellular efflux[27]. These data suggest that the G-1666A polymorphism functions as a distal element through the folding of the DNA strand.
The three transcription factors Sp1, NF-1 and CTF were predicted to bind to the promoter region, including the G-1666A site, by Alibaba2.1 software, but only the Sp1 consensus motif could partially disrupt the DNA-protein binding in a competition assay, whereas the other two did not show significant influence on the binding (data not shown). Sp1 antibody failed to reveal any super-shift band when added to the EMSA reaction complex (data not shown), which suggested that Sp1 binding to the cis-element, including the G-1666A site, required an interaction between Sp1 and other nuclear proteins. Future work will be required to identify such nuclear proteins.
In summary, our present study shows that the MRP1 G-1666A polymorphism is an independent prognostic factor for patients with HCC, which implies a role for MRP1 in tumor progression. Clearly, much more work remains to be done to confirm our findings and overcome the limitations in our work before this SNP can be used as a marker for poor outcome in HCC.
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third leading cause of cancer death, and long-term survival remains poor because of high rates of tumor recurrence or progression. Therefore, markers that can be used for improving therapeutic regimens and survival prediction are urgently needed.
The finding that human multidrug resistance protein-1 (MRP1) is expressed in unusually large amounts in HCC suggests it has a role in the growth and progression of this cancer. Expression of MRP1 is affected by the genetic sequence in its promoter region, therefore, the authors of this study examined the potential of different sequences (polymorphisms) in the MRP1 promoter to serve as indicators of prognosis and outcome in patients with HCC.
Recent studies have demonstrated that mutations within the MRP1 gene have value in predicting the response to chemotherapy in different cancers, but the clinical significance of such mutations in the MRP1 promoter for patients with HCC is unknown. This is believed to be the first study to identify a polymorphism in the promoter of MRP1 that is an independent prognostic factor for 4-year overall survival in men with HCC. The correlation between the MRP1 polymorphism and the survival of women with HCC requires further investigation. The authors also demonstrated that the polymorphism altered the affinity of nuclear proteins for the DNA in the HCC cells, which might explain the mechanism by which the expression of MRP1 was reducing.
The genetic sequence identified in this study can be used to test tissue samples from patients with HCC to help predict their outcome after therapy. This information can be used to guide treatment decisions and improve therapeutic regimens for individual patients.
MRP1 is one of a family of proteins found on the surface of cells. These proteins transport a wide variety of substances and can contribute to resistance to chemotherapy by transporting anticancer drugs out of cancer cells. The promoter region of a gene is a sequence of nucleotides that regulates whether and how much of a protein is synthesized from that gene.
This is a study of an important area of cancer genomics. The authors found that a single nucleotide polymorphism of MRP1 promoter region is an independent prognostic factor for HCC patients. The study design was well-organized and they reached a conclusion by making full use of the data.
Peer reviewer: Yasuhiro Matsumura, MD, PhD, Research Center for Innovative Oncology, National Cancer Center Hospital East, Investigative Treatment Division, Kashiwa, Chiba 277-8577, Japan
S- Editor Sun H L- Editor Kerr C E- Editor Lin YP
| 1. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [Cited in This Article: ] |
| 2. | Renes J, de Vries EG, Nienhuis EF, Jansen PL, Müller M. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126:681-688. [Cited in This Article: ] |
| 3. | Jedlitschky G, Leier I, Buchholz U, Hummel-Eisenbeiss J, Burchell B, Keppler D. ATP-dependent transport of bilirubin glucuronides by the multidrug resistance protein MRP1 and its hepatocyte canalicular isoform MRP2. Biochem J. 1997;327:305-310. [Cited in This Article: ] |
| 4. | Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci. 2006;27:438-446. [Cited in This Article: ] |
| 5. | van de Ven R, de Jong MC, Reurs AW, Schoonderwoerd AJ, Jansen G, Hooijberg JH, Scheffer GL, de Gruijl TD, Scheper RJ. Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J Immunol. 2006;176:5191-5198. [Cited in This Article: ] |
| 6. | Scheffer GL, Pijnenborg AC, Smit EF, Müller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55:332-339. [Cited in This Article: ] |
| 7. | Sugawara I, Akiyama S, Scheper RJ, Itoyama S. Lung resistance protein (LRP) expression in human normal tissues in comparison with that of MDR1 and MRP. Cancer Lett. 1997;112:23-31. [Cited in This Article: ] |
| 8. | Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J Pathol. 2003;200:553-560. [Cited in This Article: ] |
| 9. | Bonin S, Pascolo L, Crocé LS, Stanta G, Tiribelli C. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. Mol Med. 2002;8:318-325. [Cited in This Article: ] |
| 10. | Mahjoubi F, Akbari S, Montazeri M, Moshyri F. MRP1 polymorphisms (T2684C, C2007T, C2012T, and C2665T) are not associated with multidrug resistance in leukemic patients. Genet Mol Res. 2008;7:1369-1374. [Cited in This Article: ] |
| 11. | Obata H, Yahata T, Quan J, Sekine M, Tanaka K. Association between single nucleotide polymorphisms of drug resistance-associated genes and response to chemotherapy in advanced ovarian cancer. Anticancer Res. 2006;26:2227-2232. [Cited in This Article: ] |
| 12. | Conrad S, Kauffmann HM, Ito K, Leslie EM, Deeley RG, Schrenk D, Cole SP. A naturally occurring mutation in MRP1 results in a selective decrease in organic anion transport and in increased doxorubicin resistance. Pharmacogenetics. 2002;12:321-330. [Cited in This Article: ] |
| 13. | Yin JY, Huang Q, Yang Y, Zhang JT, Zhong MZ, Zhou HH, Liu ZQ. Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population. Pharmacogenet Genomics. 2009;19:206-216. [Cited in This Article: ] |
| 14. | Lehnerdt GF, Franz P, Bankfalvi A, Grehl S, Kelava A, Nückel H, Lang S, Schmid KW, Siffert W, Bachmann HS. The regulatory BCL2 promoter polymorphism (-938C>A) is associated with relapse and survival of patients with oropharyngeal squamous cell carcinoma. Ann Oncol. 2009;20:1094-1099. [Cited in This Article: ] |
| 15. | Su H, Zhao J, Xiong Y, Xu T, Zhou F, Yuan Y, Zhang Y, Zhuang SM. Large-scale analysis of the genetic and epigenetic alterations in hepatocellular carcinoma from Southeast China. Mutat Res. 2008;641:27-35. [Cited in This Article: ] |
| 16. | Schaich M, Kestel L, Pfirrmann M, Robel K, Illmer T, Kramer M, Dill C, Ehninger G, Schackert G, Krex D. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann Oncol. 2009;20:175-181. [Cited in This Article: ] |
| 17. | Xu T, Zhu Y, Wei QK, Yuan Y, Zhou F, Ge YY, Yang JR, Su H, Zhuang SM. A functional polymorphism in the miR-146a gene is associated with the risk for hepatocellular carcinoma. Carcinogenesis. 2008;29:2126-2131. [Cited in This Article: ] |
| 18. | Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105-127. [Cited in This Article: ] |
| 19. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [Cited in This Article: ] |
| 20. | Leslie EM, Létourneau IJ, Deeley RG, Cole SP. Functional and structural consequences of cysteine substitutions in the NH2 proximal region of the human multidrug resistance protein 1 (MRP1/ABCC1). Biochemistry. 2003;42:5214-5224. [Cited in This Article: ] |
| 21. | Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 2006;580:1103-1111. [Cited in This Article: ] |
| 22. | Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. TP53 and liver carcinogenesis. Hum Mutat. 2003;21:201-216. [Cited in This Article: ] |
| 23. | Yano M, Hamatani K, Eguchi H, Hirai Y, MacPhee DG, Sugino K, Dohi K, Itamoto T, Asahara T. Prognosis in patients with hepatocellular carcinoma correlates to mutations of p53 and/or hMSH2 genes. Eur J Cancer. 2007;43:1092-1100. [Cited in This Article: ] |
| 24. | Bond GL, Levine AJ. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene. 2007;26:1317-1323. [Cited in This Article: ] |
| 25. | Zhu Q, Center MS. Cloning and sequence analysis of the promoter region of the MRP gene of HL60 cells isolated for resistance to adriamycin. Cancer Res. 1994;54:4488-4492. [Cited in This Article: ] |
| 26. | Wolf AT, Medcalf RL, Jern C. The t-PA -7351C>T enhancer polymorphism decreases Sp1 and Sp3 protein binding affinity and transcriptional responsiveness to retinoic acid. Blood. 2005;105:1060-1067. [Cited in This Article: ] |
| 27. | Wang Z, Wang B, Tang K, Lee EJ, Chong SS, Lee CG. A functional polymorphism within the MRP1 gene locus identified through its genomic signature of positive selection. Hum Mol Genet. 2005;14:2075-2087. [Cited in This Article: ] |