Published online Nov 14, 2010. doi: 10.3748/wjg.v16.i42.5297
Revised: July 6, 2010
Accepted: July 13, 2010
Published online: November 14, 2010
AIM: To assess the utility of an autologous CD34+ and CD133+ stem cells infusion as a possible therapeutic modality in patients with end-stage liver diseases.
METHODS: One hundred and forty patients with end-stage liver diseases were randomized into two groups. Group 1, comprising 90 patients, received granulocyte colony stimulating factor for five days followed by autologous CD34+ and CD133+ stem cell infusion in the portal vein. Group 2, comprising 50 patients, received regular liver treatment only and served as a control group.
RESULTS: Near normalization of liver enzymes and improvement in synthetic function were observed in 54.5% of the group 1 patients; 13.6% of the patients showed stable states in the infused group. None of the patients in the control group showed improvement. No adverse effects were noted.
CONCLUSION: Our data showed that a CD34+ and CD133+ stem cells infusion can be used as supportive treatment for end-stage liver disease with satisfactory tolerability.
- Citation: Salama H, Zekri ARN, Bahnassy AA, Medhat E, Halim HA, Ahmed OS, Mohamed G, Alim SAA, Sherif GM. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol 2010; 16(42): 5297-5305
- URL: https://www.wjgnet.com/1007-9327/full/v16/i42/5297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i42.5297
Cirrhosis is the end stage of chronic liver injury caused by viral hepatitis or alcohol intake. In Egypt, most malignant neoplasms of the liver arise on top of chronically damaged livers, typically a cirrhotic liver due to hepatitis C virus (HCV) infection. Two main factors are responsible for the irreversibility of cirrhosis. One factor involves the increased and continuous deposition of the extracellular matrix, due to increased collagen synthesis accompanied by insufficient breakdown of collagen. The other is the impaired capability of the liver to regenerate, which predisposes a patient to postoperative dysfunction or liver failure. Previous studies have shown that a fibrotic liver following hepatic resection regenerates very slowly in comparison with a normal liver. However, little is known about the mechanism(s) of retarded liver growth; though, it has been postulated that growth factors can promote cirrhotic regeneration by an as yet unidentified mechanism.
Until now, effective treatments capable of reversing cirrhosis have not been developed. In the last few years, there has been increasing evidence that adult cells have far greater differentiation plasticity than previously thought, with bone marrow turning into skeletal muscle[1] and skeletal muscle back into bone marrow (BM)[2]; brain into blood[3] and back again[4,5]; BM into liver[6,7] and back again[8]. These phenomena occurred in different mammals (including mice, rats, and sheep); however, it is difficult to know the human relevance of the findings without studying human tissues.
In two recently published studies[9,10], the effects of CD34 hematopoietic stem cell intrahepatic injection and whole BM peripheral infusion have been evaluated in five and nine cirrhotic patients; respectively. The results of these two studies showed that BM stem cells transplantation improved the residual liver function in cirrhotic patients. In the study of Gordon et al[9], a decrease in serum bilirubin and an improvement in serum albumin were observed in three and four of the five patients, respectively, with disappearance of ascites in one patient only.
Similarly, Terai et al[10] reported significant increases in albumin and total protein levels, and an improvement of the Child-Pugh score in all of the nine patients, and a reduction in ascites in six patients. These results, which originated from ongoing research on BM stem cells and the liver, may be considered promising and encouraging, as they demonstrate that clinical results might sometimes precede scientific evidence. However, a more cautious analysis of the results is necessary, considering the clinical course and potential new therapies for complex disorders such as human liver cirrhosis.
These two studies[9,10], however, were conducted on small groups of patients, were not randomized, and also lacked a control group of stem cell untreated patients. The presence of a control group, in particular, is of utmost importance in clinical studies investigating the efficacy of potential new therapies. Using a control group (that should be homogeneous to the experimental group for both epidemiological and clinical characteristics) reduces the likelihood that modifications observed during treatment are accidental and not directly dependent on the therapy administered, and randomization increases the probability that other variables, not considered in the study design, are distributed evenly in both the experimental and control groups.
In the current study, we investigated the possibility of using CD34+ and CD133+ stem cells as a therapeutic modality in patients with end-stage liver diseases.
The ethical committees of Kaser El-Aini School of Medicine and the National Cancer Institute, Cairo University, approved the study protocol and a written informed consent was obtained from each patient.
The present study includes 140 patients with end-stage liver cirrhosis who attended the Hepatology Clinic in Kaser El-Aini School of Medicine, Cairo University, from June 2008 to May 2009.
Patients were randomized into one of two groups: Group 1: 90 patients who received granulocyte colony stimulating factor (G-CSF) (Neupogen, Roche) for five days, followed by autologous CD34+ and CD133+ stem cell infusion in the portal vein; Group 2: 50 patients who served as a control and those received regular liver treatment.
Patients were included in this study if they fulfilled the following criteria: male or female patients aged from 20 to 60 years with evidence of chronic liver insufficiency (decreased serum albumin and/or increased bilirubin and/or increased international normalized ratio-INR), patients unlikely to receive a liver transplant and who had a World Health Organization (WHO) performance score of less than 2 with an ability to give a written consent.
Patients were excluded if they were below the age of 20 or above the age of 60 years, pregnant or lactating women, those with recent recurrent gastrointestinal bleeding, hepatocellular carcinoma or spontaneous bacterial peritonitis, an evidence of human immunodeficiency virus or other life threatening infection, unable to give a written consent, having history of hypersensitivity to G-CSF, or included in any other clinical trial within the previous month. Patients were randomized into either one of these two groups.
Patients in both groups were comparable for baseline characteristics: age and sex, etiology of liver disease and model for end-stage liver disease (MELD) score.
Clinical evaluation was done for all patients in the study, including a detailed medical history and complete clinical examination, with special emphasis on the presence markers of liver cell failure e.g. ascites, jaundice, LL.edema, bleeding tendency, and signs of encephalopathy in addition to the WHO performance score. Abdominal ultrasound scanning was done for both groups using a Hitachi 515 real time scan after overnight fasting.
Laboratory investigations were done for all patients, and included liver biochemical profile [serum bilirubin and albumin, prothrombin time and concentration, INR, alanine transaminase (ALT) and aspartate transaminase (AST)]; blood urea and serum creatinine levels; blood sugar (fasting and two hours post-prandial); complete blood analysis and α-fetoprotein, as well as a hepatitis serological profile (HBsAg, HBcAb, HCV Ab, HCV RNA by quantitative polymerase chain reaction). The MELD score was calculated to assess the degree of hepatic decompensation for every patient.
Group 1: From days one to five, patients received a daily subcutaneous injection of granulocyte colony stimulating factor (G-CSF at 300 μg Neupogen (Roche) - 300 μg/vials) for five days to increase the number of circulating hematopoietic cells. Blood samples were then taken for hematology, biochemical analysis, and coagulation profile on days one and five. Data regarding symptoms and adverse events were collected and recorded. Patients were admitted to the hospital and underwent BM aspiration on day six.
Patient’s skin was cleaned with 70% alcohol at the iliac crest, which is the usual site for puncture in adults. Skin, subcutaneous tissues, and periosteum overlying the selected site for puncture were infiltrated with xylocaine local anesthesia and serial punctures from multiple sites were performed.
With a boring movement, needles (Salah and Klima) were passed perpendicularly into the cavity of the ileum at a point just posterior to anterior superior iliac spine or 2 cm posterior and 2 cm inferior to the anterior superior iliac spine to aspirate 250 mL of the BM.
Following collection, the BM products were transferred to the stem cell laboratory for immuno-magnetic purification of the CD34+ and CD133+ stem cell population. CD34+ and CD133+ cells were isolated using the positive cell selection kit (MACS System Milteny Biotec, Gmbh, Germany (http://www.miltenyibiotec.com). Collected cells were washed twice with phosphate buffered saline pH 7.4, supplemented with 0.5% bovine serum albumin and 5 mmol/L EDTA, and centrifuged at 1500 r/min for 10 min at 4°C. The cell suspension was counted, adjusted to 0.5 × 108, centrifuged, and re-suspended in 100 mL physiological saline.
Patients were admitted to hospital and infused, while fasting, with the purified human stem cells into the portal vein (as previously determined by duplex Doppler scan to assess its potency) under ultrasound guidance using, diazepam 10 mg IV for sedation and xylocaine local infiltration anesthesia.
Group 2: A daily SC injection of distilled water was given for all patients while receiving their usual supportive measures before and after injection.
All patients in both groups were followed up every hour for 24 h, then every week for the first month, and regularly every month for 6 mo. During the follow up, patients were observed for the following: serum bilirubin and albumin, prothrombin time and concentration, INR, ALT and AST; blood urea and serum creatinine levels; blood sugar (fasting and two hours post-prandial); complete blood analysis, together with estimation of the degree of ascites, Child-Pugh score, performance score, hepatic encephalopathy and hematemesis.
All patients’ data were tabulated using Excel XP. Data were processed by SPSS (Statistical Package for Science and Society) version 12.0 for Windows XP. The descriptive statistics were presented as mean ± SD for quantitative variables. All qualitative data were expressed by frequency (number) and percent. Comparisons between groups were done using χ2 test, Fischer’s Exact test or McNemar test when appropriate for qualitative data but independent sample t test and paired sample t test were used for normally distributed quantitative variables, while the non-parametric Mann Whitney test and Wilcoxon singed ranks test were used for abnormally distributed quantitative variables. Percent changes in prognostic variables (e.g. albumin) were calculated and compared using the Mann Whitney test. In all tests, P values lower than 0.05 were considered as statistically significant. Also, linear regression analysis was employed to determine the degree of improvement of clinical features in relation to response rate to the treatment. Multivariate analysis also showed no significant correlation between HCV viral titer changes and serum albumin, prothrombin concentration (PC), Child score, Performance score ascites grade, and hematemesis attack.
One hundred and forty patients (117 males and 23 females) with a mean age of 50.27 ± 6.05 years (range; 45 to 59 years) were included in the study; 90 of them received autologous, BM-derived CD34+ and CD133+ cell transplantation, and 50 were included as a control. In all studied patients, the cells were injected into the portal vein under ultrasound guidance. The etiology, diagnosis, clinical signs, results of investigations, cell concentrations, and post infusion observations for each patient are shown in Tables 1 and 2. After G-CSF treatment, increased total white blood cell counts indicated mobilization of progenitor cells into the circulation in all patients. Patients were then given 0.5 × 108 CD34+ and CD133+ cells as a single bolus.
| Variables | Treated group (n = 90) | Control group (n = 50) |
| Age (yr, mean ± SD) | 50.27 ± 6.05 | 50.9 ± 7.23 |
| Sex | ||
| Male | 78 (86.7) | 39 (78) |
| Female | 12 (13.3) | 11 (22) |
| Residence | ||
| Urban | 30 (33.33) | 20 (40) |
| Rural | 60 (66.66) | 30 (60) |
| Jaundice | 36 (40) | 21 (42) |
| Encephalopathy | 21 (23.33) | 15 (30) |
| Weight loss | 57 (63.33) | 29 (58) |
| Ascites and lower limb edema | 78 (86.66) | 41 (82) |
| Peripheral erythema | 75 (83.33) | 39 (78) |
| Bleeding tendency | 48 (53.33) | 26 (52) |
| Hematemesis | 24 (26.66) | 12 (24) |
| Quantitative HCV RNA PCR (IU/mL) | 1 128 230-1 810 530 | 1 235 650-1 754 150 |
| Variables | Treated group (n = 90) | Control group (n = 50) |
| Liver size | ||
| Average | 27 (30) | 16 (32) |
| Shrunken | 54 (60) | 23 (46) |
| Enlarged | 9 (10) | 11 (22) |
| Liver texture | ||
| Cirrhotic | 90 (100) | 50 (100) |
| Focal lesions | 0 | 0 |
| Spleen | ||
| Average sized | 24 (26.66) | 12 (24) |
| Mild splenomegaly | 48 (53.33) | 29 (58) |
| Moderate splenomegaly | 18 (20) | 9 (18) |
| Huge splenomegaly | 0 | 0 |
| Splenectomy | 0 | 0 |
| Ascites | ||
| Absent | 6 (6.7) | 8 (16) |
| Mild | 12 (13.3) | 5 (10) |
| Moderate | 63 (70) | 32 (64) |
| Massive | 9 (10) | 5 (10) |
No major complications, either local or systemic, occurred as a result of the stem cell transplantation, except for mild pain and discomfort at the site of CD34+ and CD133+ cell infusion. However, a short term fever developed in 15 patients, which subsided in 24 h. Mild bone pain developed in 23 patients after receiving G-CSF, which also subsided spontaneously. However, there was no bleeding, infection, or significant deterioration in liver function. CT scans showed no evidence of focal liver lesions in any of the treated patients, either before or after infusion. Duplex Doppler ultrasound scans showed patent portal veins with normal hepato-pedal flow.
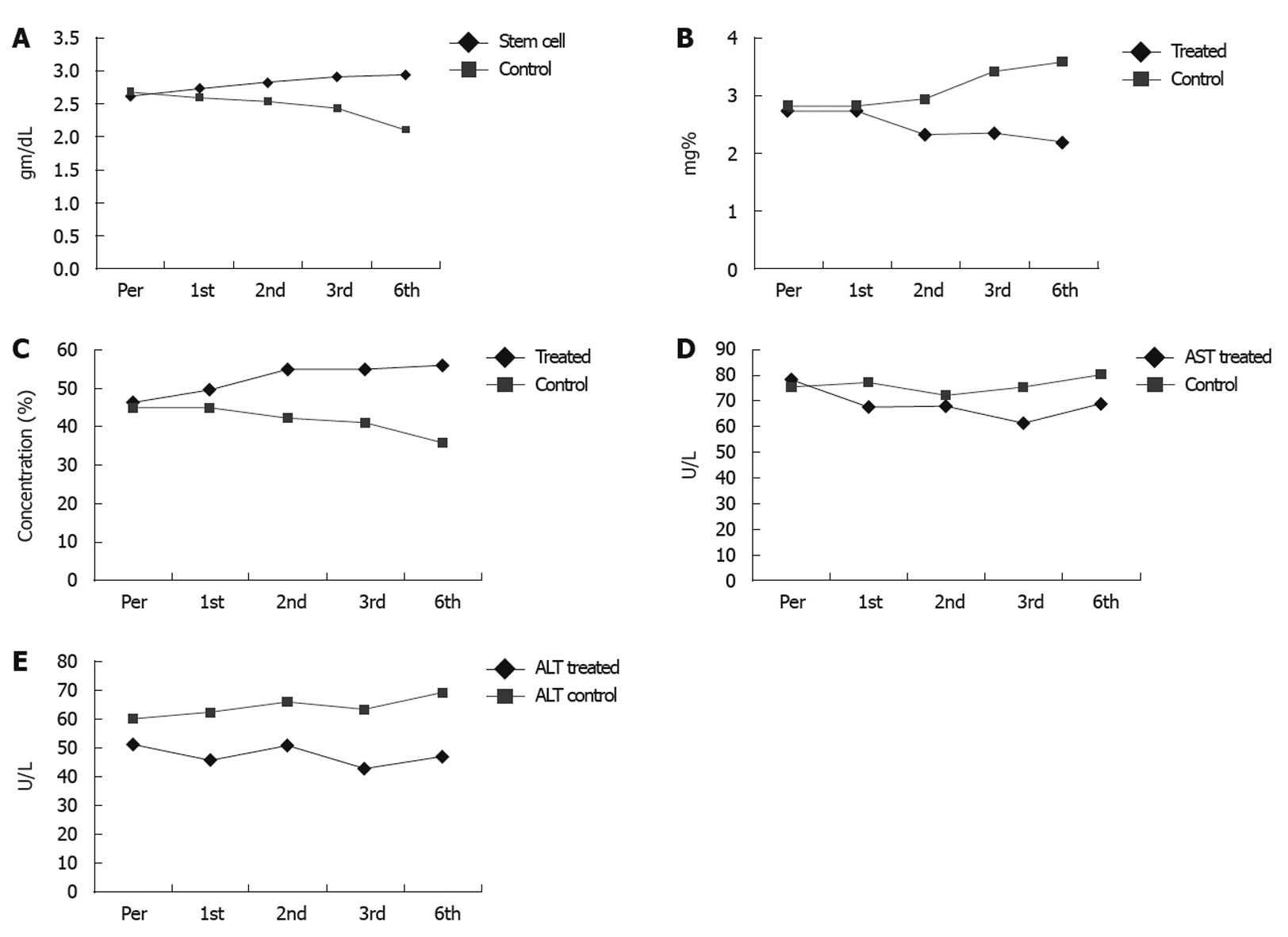
Monthly assessment of changes in the serum level of bilirubin, albumin, liver enzymes, and fluid collection was performed. Serum albumin, bilirubin, transaminases, prothrombin time and concentration, together with estimation of the degree of ascites, Child-Pugh score, performance score, hepatic encephalopathy, and hematemesis were performed every week for the first month, then every month up to 6 mo (Table 3).
| Pre treatment | 1st month | 2nd month | 3rd month | 6th month | |
| Bilirubin (mg%) | |||||
| Patients | 2.51 ± 1.94 | 2.75 ± 1.66 | 2.31 ± 1.33 | 2.34 ± 1.29 | 2.18 ± 1.28 |
| Control | 2.68 ± 1.49 | 2.80 ± 1.51 | 2.92 ± 1.71 | 3.41 ± 1.77 | 3.58 ± 1.56 |
| P < 0.001 | P < 0.002 | P < 0.003 | |||
| Albumin (gm/dL) | |||||
| Patients | 2.61 ± 0.44 | 2.73 ± 0.62 | 2.83 ± 0.39 | 2.92 ± 0.33 | 2.94 ± 0.45 |
| Control | 2.69 ± 0.26 | 2.60 ± 0.30 | 2.54 ± 0.25 | 2.44 ± 0.35 | 2.10 ± 0.34 |
| P < 0.04 | P < 0.001 | ||||
| PC (%) | |||||
| Patients | 46.2 ± 12.5 | 49.6 ± 12.5 | 55.0 ± 14.5 | 54.9 ± 12.9 | 56.1 ± 15.7 |
| Control | 45.1 ± 12.8 | 44.9 ± 13.1 | 42.2 ± 13.5 | 40.9 ± 14.1 | 35.8 ± 15.9 |
| P < 0.005 | P < 0.005 | P < 0.005 | P < 0.005 | ||
| AST (U/L) | |||||
| Patients | 78.2 ± 32.1 | 67.4 ± 25.2 | 68.0 ± 20.2 | 61.0 ± 18.7 | 69.0 ± 29.2 |
| Control | 75.4 ± 35.2 | 77.2 ± 30.1 | 72.1 ± 32.3 | 75.5 ± 33.3 | 80.2 ± 32.4 |
| P < 0.002 | P < 0.002 | P < 0.003 | P < 0.003 | ||
| ALT (U/L) | |||||
| Patients | 51.5 ± 25.5 | 45.7 ± 19.6 | 50.9 ± 27.7 | 42.8 ± 16.1 | 47.0 ± 21.3 |
| Control | 60.1 ± 20.4 | 62.5 ± 21.2 | 66.1 ± 25.1 | 63.5 ± 23.7 | 23.7 ± 22.1 |
| P < 0.001 | P < 0.001 | P < 0.01 | P < 0.01 |
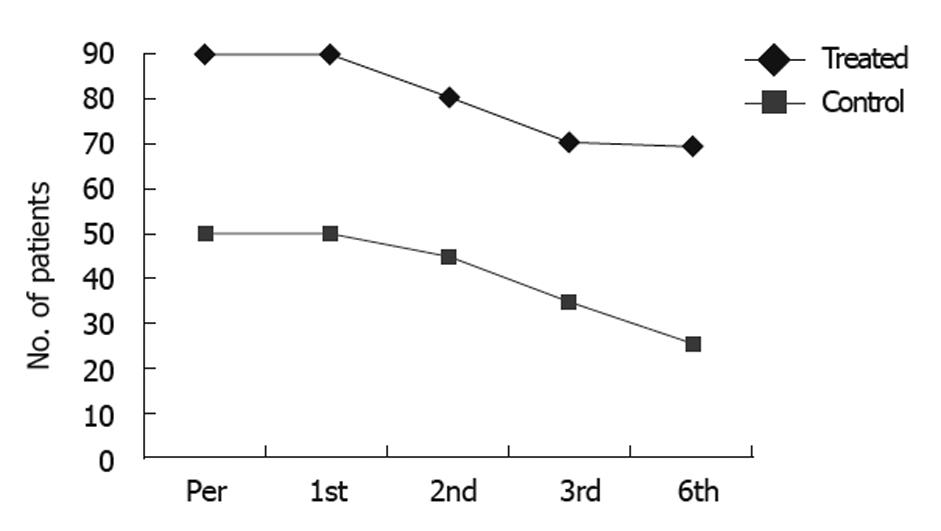
Near normalization of liver enzymes and improvement in the synthetic function of the liver were observed in 54.5% of CD34+ and CD133+ transfused patients and 13.6% of them showed stable states. In comparison, none of the control group showed any improvement (P = 0.069 and 0.023, Table 4 and Figure 1A-E).
| Marker | Responders | Stable | Non-responders | P value | |||||||||
| 1st mo | 2nd mo | 3rd mo | 6th mo | 1st mo | 2nd mo | 3rd mo | 6th mo | 1st mo | 2nd mo | 3rd mo | 6th mo | ||
| Bilirubin | 24 (26.6) | 30 (37.0) | 33 (46.5) | 39 (56.5) | 0 (0) | 9 (11.1) | 9 (12.8 ) | 0 (0) | 66 (73.3) | 42 (51.9) | 29 (40.8) | 30 (43.5) | 0.516 |
| Albumin | 57 (63.3) | 45 (55.6) | 42 (59.2) | 40 (58.0) | 3 (3.33) | 9 (11.1) | 9 (12.7) | 8 (11.6) | 30 (33.3) | 27 (33.3) | 20 (28.1) | 21 (30.4) | 0.069 |
| PC | 66 (73.3) | 57 (70.4) | 47 (66.2) | 48 (65.6) | 0 (0) | 3 (3.6) | 6 (8.5) | 0 (0) | 24 (26.6) | 21 (25.8) | 18 (25.4) | 21 (30.4) | 0.023 |
| AST | 57 (63.3) | 48 (60.3) | 47 (66.2) | 39 (56.5) | 3 (3.33) | 0 (0) | 0 (0) | 0 (0) | 30 (33.3) | 33 (40.7) | 24 (33.8 ) | 30 (43.5) | 0.673 |
| ALT | 54 (60.0) | 48 (59.2) | 47 (66.2) | 30 (43.5) | 0 (0) | 3 (3.7) | 6 (8.5) | 6 (8.7) | 36 (40.0) | 30 (37.1) | 18 (25.3) | 33 (47.8) | 0.550 |
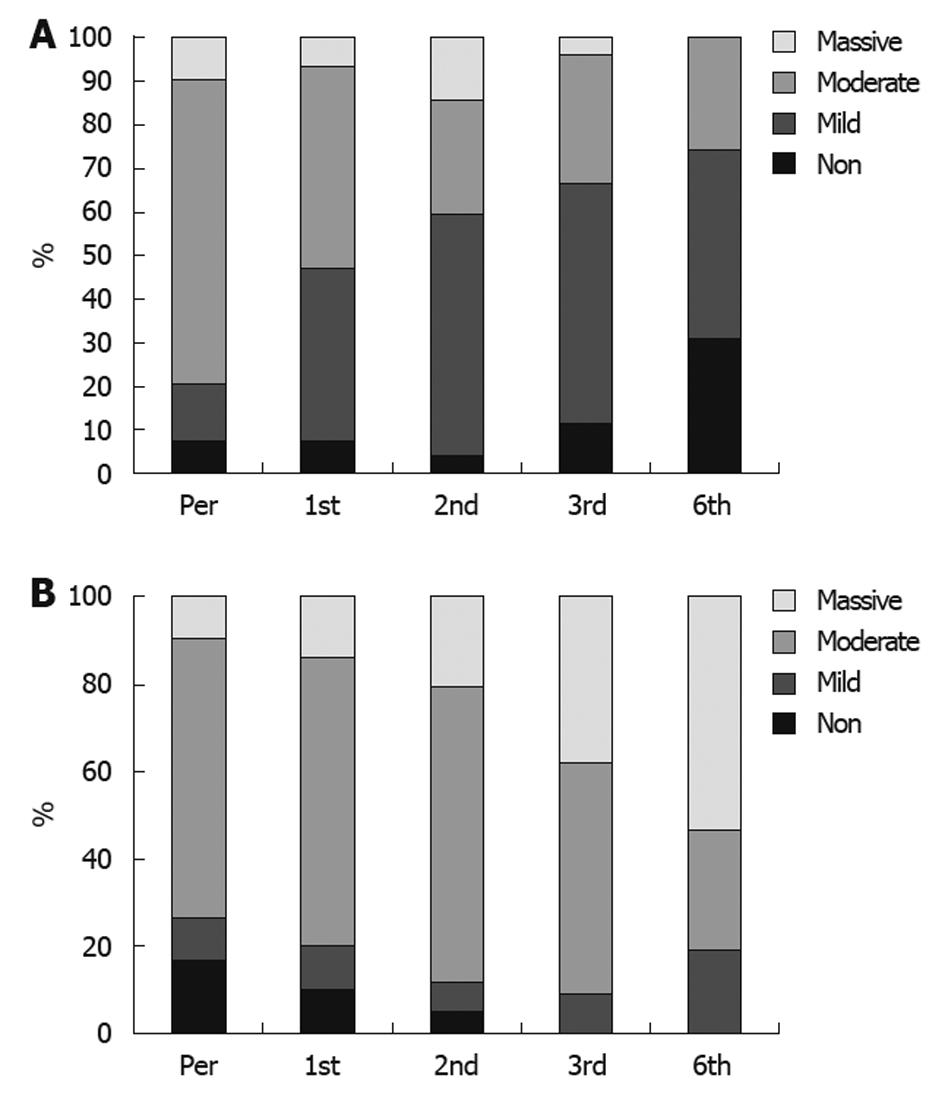
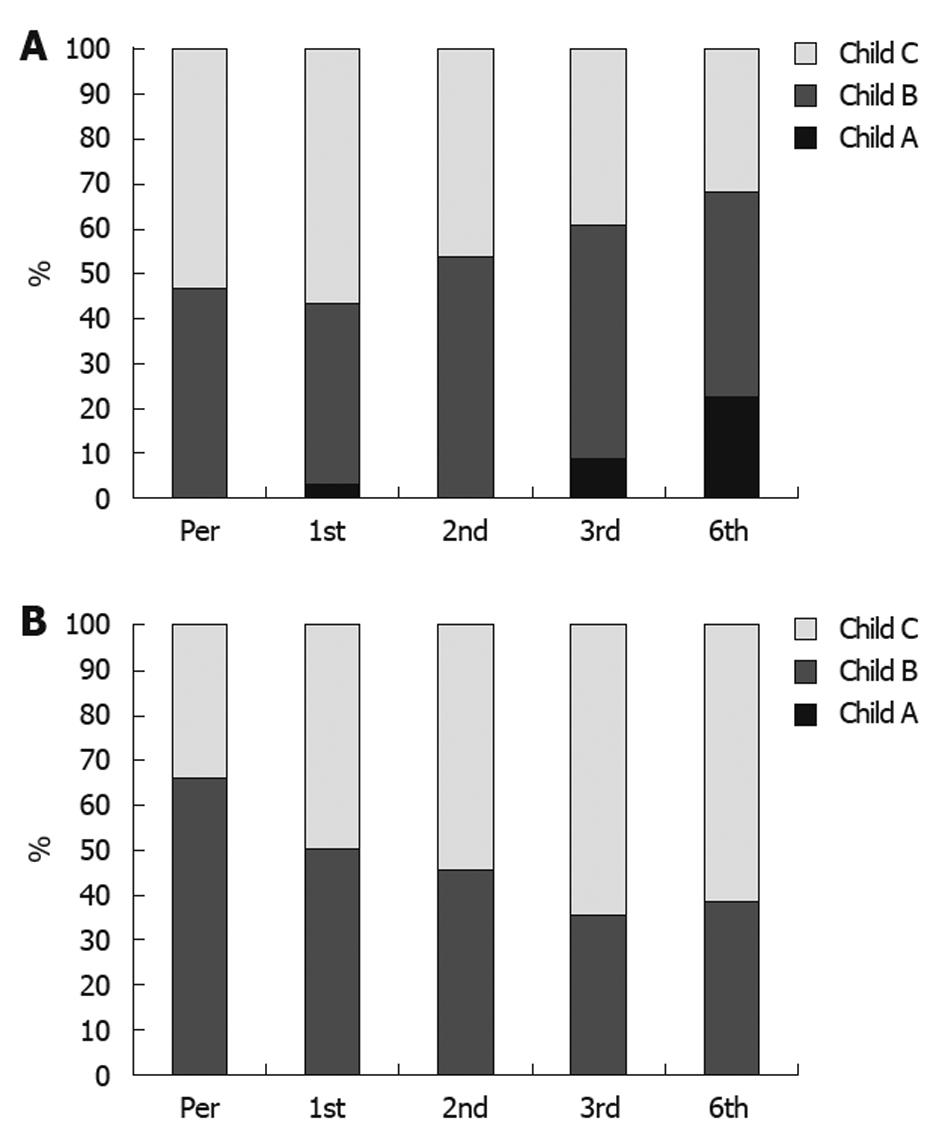
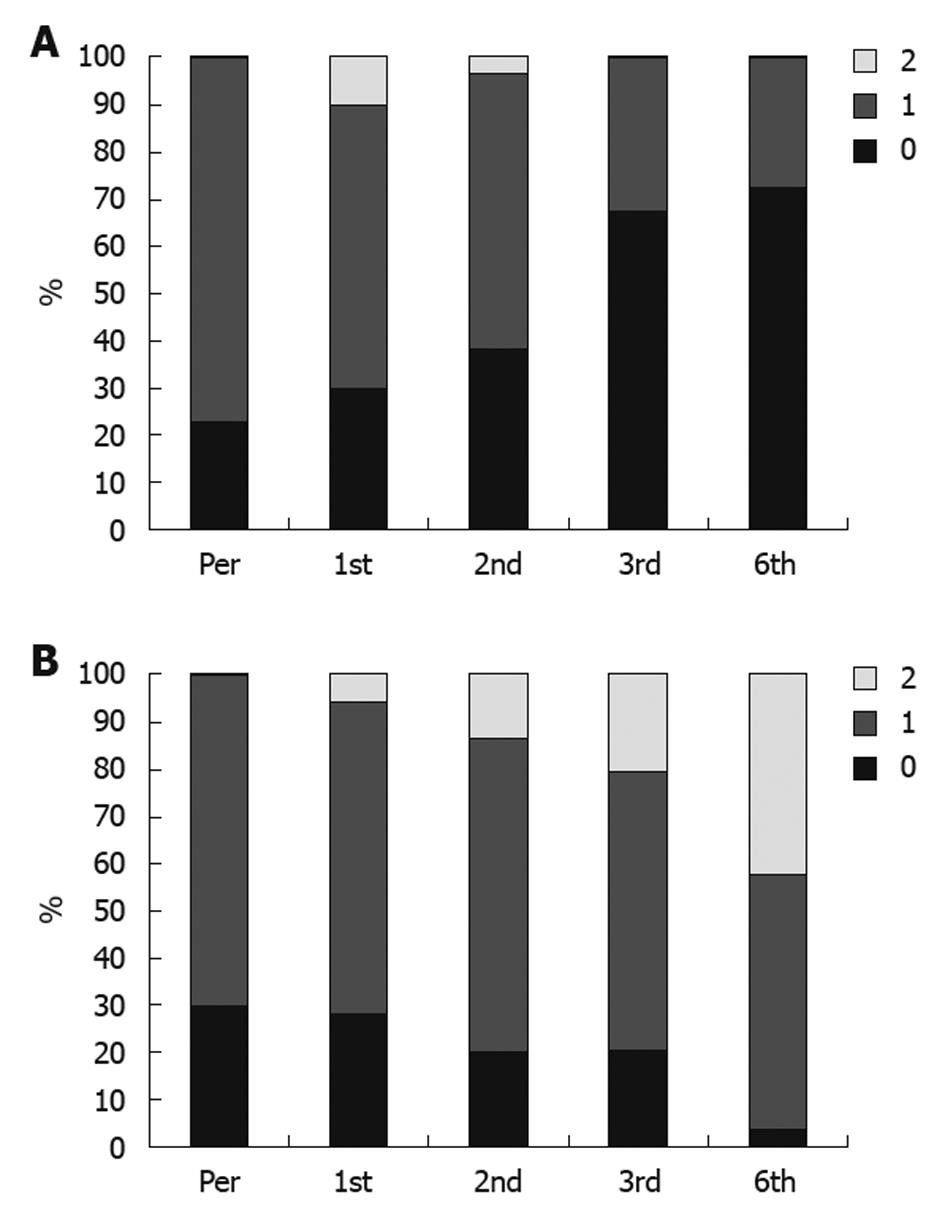
None of the responders or patients with stable disease showed progressive or increased ascites or needed taping, and the treatment schedule for diuretic therapy was dramatically reduced. Disappearance of ascites, as demonstrated by ultrasound scan, was detected in 21 patients, and improvement in the degree of ascites was reported in 51 patients (Figure 2A and B). Also, an improvement in Child-Pugh score was detected in 48 patients (Figure 3A and B); performance score in 52 patients (Figure 4A and B). Improvements in hepatic coma and hematemesis (Table 5) were detected in 91.3% and 87%, respectively. Similarly, a significant improvement was observed in the group of responders compared to the other two groups (Table 6, Figures 2 and 3).
| Pretreatment (n = 90) | 1 mo (n = 90) | 2 mo (n = 81) | 3 mo (n = 71) | 6 mo (n = 69) | ||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
| Coma | ||||||||||
| Studied group | 60 (66.7) | 30 (33.3) | 87 (96.7) | 3 (3.3) | 72 (89) | 9 (11) | 66 (92.9) | 5 (7.1) | 63 (91.3) | 6 (8.7) |
| 50 patients | 50 patients | 44 patients | 34 patients | 26 patients | ||||||
| Control group | 31 (62) | 19 (38) | 29 (48) | 21 (42) | 24 (54.5) | 20 (45.5) | 14 (41.2) | 20 (58.8) | 5 (19.2) | 21 (80.7) |
| Hematemesis | ||||||||||
| Studied group | 72 (80) | 18 (20) | 90 (100) | 0 (0) | 75 (92.6) | 6 (7.4) | 68 (95.8) | 3 (4.2) | 60 (87) | 9 (13) |
| 50 patients | 50 patients | 44 patients | 34 patients | 26 patients | ||||||
| Control group | 35 (70) | 15 (30) | 32 (64) | 18 (36) | 24 (54.5) | 20 (54.5) | 12 (35.3) | 24 (70.6) | 6 (23) | 20 (77) |
| Marker | Responders | Stable | Non-responders | P value | |||||||||
| 1st mo | 2nd mo | 3rd mo | 6th mo | 1st mo | 2nd mo | 3rd mo | 6th mo | 1st mo | 2nd mo | 3rd mo | 6th mo | ||
| Child-Pugh score | 9 (10) | 18 (22.2) | 21 (29.6) | 27 (39.1) | 72 (80) | 48 (59.3) | 45 (63.4) | 39 (56.2) | 3 (10) | 15 (18.5) | 5 (7.0) | 3 (4.4) | 0.03 |
| Performance score | 18 (20) | 27 (33.3) | 42 (59.2) | 42 (60.9) | 51 (56) | 36 (44.4) | 18 (25.4) | 18 (26.1) | 21 (23.2) | 18 (22.2) | 11 (15.4) | 9 (13) | 0.02 |
| Degree of ascites | 33 (36) | 42 (51.8) | 44 (62.0) | 45 (65.2) | 48 (53) | 24 (29.6) | 21 (29.5) | 24 (34.8) | 9 (10) | 15 (18.6) | 6 (8.5) | 0 (0) | 0.02 |
| Hepatitis C coma | 27 (30) | 24 (29.7) | 24 (33.8) | 24 (34.9) | 63 (70) | 54 (66.6) | 47 (66.2) | 42 (60.8) | 0 (0) | 3 (3.7) | 0 (0) | 3 (4.3) | 0.001 |
| Haematemesis | 18 (20) | 21 (25.9) | 21 (29.6) | 18 (26.1) | 72 (80) | 57 (70.4) | 50 (70.4) | 48 (69.6) | 0 (0) | 3 (3.7) | 0 (0) | 3 (4.3) | 0.03 |
In the study population, the mean baseline HCV viral titer value was 1128.23 ± 1810.53 IU/mL. One month after stem cell therapy, it increased to 2749.70 ± 10 533.15 IU/mL, then decreased to 848.88 ± 1375.72 IU/mL two months after the study and then to 592.23 ± 1300.90 IU/mL after three months. After 6 mo of the study, the mean HCV viral titer further decreased to 351.84 ± 1068.73 IU/mL in the treated group. However, no significant change in the viral titer was observed in the control group during the study period.
Linear regression analysis was employed to determine the degree of improvement in the clinical features in relation to the response to treatment. A significant improvement was observed among the responders compared to the control groups (Table 7).
| Beta | T | P value | |
| Child-Pugh score | 27.44 | 1.65 | 0.03 |
| Performance score | 41.2 | 2.6 | 0.02 |
| Degree of ascites | 35.03 | 1.3 | 0.02 |
| Hepatic coma | 29.1 | 1.2 | 0.001 |
| Haematemesis | 26.8 | 1.4 | 0.03 |
Multivariate analysis showed no significant correlation between HCV viral titer and serum albumin, PC, Child score, performance score, ascites grade or hematemesis. However, there was a significant inverse relation between serum bilirubin levels and HCV titer, which reached the highest significance in the 6th month after treatment (r = 0.56, P < 0.002).
Survival after stem cell therapy (6 mo follow-up): Nine patients with post-HCV liver cell failure died (seven from attacks of hematemesis, and two from hepato-renal syndrome) and 13 were considered as lost follow up patients, though still alive, because they did not attend at the regular scheduled time for follow-up. In comparison, 26 patients from the control group died (15 from attacks of haematemesis, five from hepato-renal syndrome, and six from hepatic coma) (Figure 5).
Until now, liver transplantation has been considered the only curative treatment for decompensated cirrhosis[11]. However, this procedure is limited by technical difficulties, high cost, and also by a lack of donors[12].
Stem cells have recently shown promise in cell therapy because they have the capacity for self-renewal and multilineage differentiation, and are applicable to human diseases. The diseased liver may recruit migratory stem cells, particularly from the bone marrow, to generate hepatocyte-like cells either by transdifferentiation or cell fusion. Transplantation of BMSCs can restore liver mass and function, alleviate fibrosis, and correct inherited liver diseases. Therefore, it can significantly improve the liver function of patients with terminal liver disease, with good safety and effectiveness[13]. BMSCs can be delivered via the intraportal vein, systemic infusion, or via intraperitoneal, intrahepatic, and intrasplenic routes.
The current study was designed to evaluate the effects of autologous CD34+ and CD133+ hematopoietic stem cell intrahepatic infusion, and to assess the relationship between HCV viral load and the outcome of stem cell therapy, in patients with post HCV liver cirrhosis. This was the basis for conducting a randomized study on 140 patients with chronic HCV liver disease.
To improve hepatic homing to the liver, CD34+ and CD133+ progenitor cells were applied into the portal vein directly. Our choice of the portal vein infusion was based on a preclinical model, which reported that HSCs, if applied to the portal vein, demonstrate a high percentage of first-pass entrapment in the liver[14].
We found a significant improvement in the mean serum albumin level in the transplanted patients compared to the control group. Similar results were reported by Gordon et al[9]. Similarly, Terai et al[10] reported significant improvements in the average serum albumin levels in nine cirrhotic patients that underwent autologous bone marrow cell infusion from the peripheral vein at 24 wk after infusion. Also, Salama et al[15] conducted a study on 48 Child C cirrhotic patients and demonstrated that there were borderline significant improvements in the serum albumin levels of the transplanted group compared to the control group at the end of the 6 mo study.
The mean serum bilirubin level showed an improvement in our transplanted patients, starting from the second month after stem cell therapy. These results are also consistent with those of Gordon et al[9] and Pai et al[13].
In another study, Levicar et al[16] isolated and injected 1 × 106 to 2 × 108 CD34+ cells into the portal veins or hepatic arteries of five patients, who were followed up for 18 mo. Four patients showed an initial improvement in serum bilirubin level, which was maintained for up to 6 mo. However, a marginal increase in serum bilirubin was reported in three patients at 12 mo and in one patient, the serum bilirubin increased only at 18 mo post-infusion. They concluded that the beneficial effect seemed to last for around 12 mo.
The mean baseline PC value in our studied population started to improve 1 mo after the procedure, and continued to improve until the 6th month. This improvement was statistically significant. These results agree with those of Salama et al[15], who reported that there was an improvement in PC that started in the 2nd month after HSCs transplantation and reached its maximum after 6 mo.
There was a gradual improvement in the hepatic functional reserve of our transplanted patients compared to controls, as assessed by the Child-Pugh score. The maximum improvement in the Child-Pugh score occurred after 6 mo. A statistically significant improvement of Child-Pugh score was demonstrated at four and twenty-four weeks after autologous bone marrow stem cell transplantation therapy by Terai et al[10]. In addition, the Child-Pugh score improved significantly in seven out of nine patients studied by Pai et al[13].
The quality of life (QOL) in the transplanted patients was assessed using the WHO performance score (PS). There was an improvement in the transplanted patients, as regards PS, starting from the 1st month after the HSCs transplantation until the 6th month post-transplantation.
This improvement has been previously reported by Gordon et al[9], who reported a statistically significant improvement in QOL of their patients, as measured by an SF-36 questionnaire after HSCs transplantation.
On the other hand, the present study showed that the number of patients without ascites increased from 6.7% at the start of the study to 27.3% at the end of the study. Maximum improvement occurred after 6 mo, and was of highly significant value as 42 patients (63.6%) showed an improvement in the degree of ascites and 24 patients (36.3%) showed no change in the degree of ascites. However, none of the patients showed deterioration in the degree of ascites. Our results are comparable to those of Terai et al[10] and Pai et al[13].
At the start of the study, the percent of patients with no history of hepatic encephalopathy was 66.7 % and the percent of patients with history of encephalopathy was 33.3 %. The percent of patients with no attacks of encephalopathy became 96.7 %, 80%, 88.5 % and 91.3% after 1, 2, 3, and 6 mo respectively (maximum improvement occurred after 6 mo). Salama et al[15] demonstrated a highly significant improvement in the transplanted group as regards the recurrence of hepatic encephalopathy.
In the study population, the mean baseline HCV viral titer value was 1128.23 ± 1810.53 IU/mL and there was an increase in the transplanted patients during the 1st month only. Thereafter, there was a gradual decrease during the follow-up period. The maximum decrease in HCV viral titer occurred after 6 mo of stem cell transplantation, where 39 patients (59.1%) showed a decrease, 18 patients (27.3%) showed an increase and 9 patients (13.6%) showed no change in their HCV viral titer.
In the study population, the lower pre-transplantation viral load was not due to the intrinsic characteristics of the infective viral strains, but rather to the severity of liver disease. Thus, one month after stem cell transplantation, higher levels of viral replication were restored due to regeneration of liver after stem cell transplantation[17]. Therefore, there was no correlation between HCV viral titer changes and changes in the serum albumin, PC, Child score, performance score, ascites grade or hematemesis attacks. However, there was a moderate negative correlation between HCV titer changes with changes in serum bilirubin in the 3rd month only after stem cell transplantation.
Similarly, there was a negative correlation between HCV titer changes and changes in AST in the 3rd and 6th months only after stem cell transplantation, and a moderate negative correlation between HCV titer changes with changes in ALT in the 2nd month only after stem cell transplantation. These results agree with those of Duvoux et al.[17], who demonstrated that the severity of liver disease is independent of serum levels of HCV.
From the data presented in the current study, which included the largest cohort of HCV-associated end stage liver disease patients to date, we conclude that autologous stem cell transplantation may be safely administered and appears to offer some therapeutic benefit to patients with viral hepatic end-stage liver disease.
Stem cell therapy is an innovation technique with promising application in various human diseases. Stem cell plasticity has enabled different researchers to use them in treating different diseases. Recent success using hematopoietic stem cells to support the failing liver in humans has been reported. Stem cells support of the failing liver might reduce or obviate the need for future liver transplantation.
Stem cells are present in different tissues, with a rich source in the bone marrow. Different manipulation techniques, such stimulation with as granulocyte colony stimulating factor, enabled the scientists to use stem cells effectively to treat different diseases with different etiologies. Due to the high prevalence of hepatitis viruses, such as hepatitis B virus and hepatitis C virus, the proportion of patients with liver cirrhosis and failing liver is increasing linearly. Although liver transplantation is the definitive treatment, its complicated technique, high costs, immune suppression and limited organ supply are major limitations for its wide use. Stem cell therapy is an innovative technique that can offer an effective, safe, and cheap support for patients with failing livers.
Recent reports have highlighted the importance of using stem cells in supporting the failing liver, with promising results for a wide range of etiologies of liver insult. This is the first large, randomized, and controlled study to report the value of using autologous hematopoietic CD34, CD133 stem cells to support the failing liver, with promising 6 mo follow-up results.
This technique exploits the plasticity of stem cells and the ability of hematopoietic stem cells to change into hepatocytes to support the failing liver in patients with end-stage liver disease of various etiologies. In the future this technique might reduce the need for liver transplantation.
Hematopoietic CD34, CD133 Stem cells are bone marrow cells specialized in forming different blood elements and being stem cells, they have the character of plasticity and can change into hepatocytes. Previous human studies and the present one demonstrated, directly and indirectly, the ability of these cells to support the failing liver.
In this manuscript, Salama et al evaluated the therapeutic efficacy of autologous stem cell transplantation in end-stage liver cirrhosis. They evaluated a large number of patients, and found beneficial effects of this therapy. The issue is not novel, but the current study enrolled the largest series of patients ever evaluated. The study design is fair, and the study is well performed. Findings are sound and of potential impact in the field.
Peer reviewer: Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, via A. Manzoni 113, 20089 Rozzano, Milan, Italy
S- Editor Tian L L- Editor Stewart GJ E- Editor Lin YP
| 1. | Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528-1530. [Cited in This Article: ] |
| 2. | Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96:14482-14486. [Cited in This Article: ] |
| 3. | Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534-537. [Cited in This Article: ] |
| 4. | Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711-10716. [Cited in This Article: ] |
| 5. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [Cited in This Article: ] |
| 6. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [Cited in This Article: ] |
| 7. | Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA. 1997;94:4080-4085. [Cited in This Article: ] |
| 8. | Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810-813. [Cited in This Article: ] |
| 9. | Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830. [Cited in This Article: ] |
| 10. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [Cited in This Article: ] |
| 11. | Christensen E. Prognostic models including the Child-Pugh, MELD and Mayo risk scores--where are we and where should we go? J Hepatol. 2004;41:344-350. [Cited in This Article: ] |
| 12. | Fink MA, Berry SR, Gow PJ, Angus PW, Wang BZ, Muralidharan V, Christophi C, Jones RM. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22:119-124. [Cited in This Article: ] |
| 13. | Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952-1958. [Cited in This Article: ] |
| 14. | Fan TX, Hisha H, Jin TN, Yu CZ, Lian ZX, Guo SB, Cui YZ, Feng B, Yang GX, Li Q. Successful allogeneic bone marrow transplantation (BMT) by injection of bone marrow cells via portal vein: stromal cells as BMT-facilitating cells. Stem Cells. 2001;19:144-150. [Cited in This Article: ] |
| 15. | Salama H, Zekri AR, Zern M, Bahnassy A, Loutfy S, Shalaby S, Vigen C, Burke W, Mostafa M, Medhat E. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 2010;Epub ahead of print. [Cited in This Article: ] |
| 16. | Levicar N, Pai M, Habib NA, Tait P, Jiao LR, Marley SB, Davis J, Dazzi F, Smadja C, Jensen SL. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008;41 Suppl 1:115-125. [Cited in This Article: ] |