Published online Jan 28, 2010. doi: 10.3748/wjg.v16.i4.467
Revised: November 13, 2009
Accepted: November 20, 2009
Published online: January 28, 2010
AIM: To study the impact of an endoscopy-based long-term study on the quality of life in healthy volunteers (HV).
METHODS: Ten HV were included into a long-term prospective endoscopy-based placebo-controlled trial with 15 endoscopic examinations per person in 5 different drug phases. Participants completed short form-36 (SF-36) and visual analog scale-based questionnaires (VAS) for different abdominal symptoms at days 0, 7 and 14 of each drug phase. Analyses were performed according to short- and long-term changes and compared to the control group.
RESULTS: All HV completed the study with duration of more than 6 mo. Initial quality of life score was comparable to a general population. Analyses of the SF-36 questionnaires showed no significant changes in physical, mental and total scores, either in a short-term perspective due to different medications, or to potentially endoscopic procedure-associated long-term cumulative changes. Analogous to SF-36, VAS revealed no significant changes in total scores for pathological abdominal symptoms and remained unchanged over the time course and when compared to the control population.
CONCLUSION: This study demonstrates that quality of life in HV is not significantly affected by a long-term endoscopy-based study with multiple endoscopic procedures.
- Citation: Link A, Treiber G, Peters B, Wex T, Malfertheiner P. Impact of endoscopy-based research on quality of life in healthy volunteers. World J Gastroenterol 2010; 16(4): 467-473
- URL: https://www.wjgnet.com/1007-9327/full/v16/i4/467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i4.467
Endoscopy is the main diagnostic tool for examination of the upper gastrointestinal tract. The main advantage of endoscopy in comparison to other non-invasive procedures lies in its ability to obtain tissue biopsies, which permit histological evaluation of tissues for clinical as well as basic research[1].
The majority of endoscopy-based pharmacological studies are performed in patients with or without a specific disease. Investigations surrounding this focused patient population yields answers for only a limited number of questions. Studies designed around healthy volunteers (HV) may resolve some of those issues (e.g. by reducing inter-individual differences); although at the same time may open a new series of questions. One of the main differences between patients and HV is hidden in the dilemma “treat the disease” in patients and “do no harm” in HV[2,3]. “Harm” can arise from a number of sources such as the study-related treatment, the endoscopy procedure itself, and/or other related discomfort (e.g. multiple blood samples, restriction of daily activities).
Upper GI-endoscopy is a relatively well-accepted procedure. The magnitude of discomfort to the examined person depends upon several factors such as previous contact with the examiner, appropriate elucidation of the procedure, sedation, investigator’s experience, duration of examination and the expected benefit from the procedure[4]. Because of the potential therapeutic benefit, it is easier to justify the examination of the patients. Since in most cases, HV can not expect any health-related advantages from the procedure, the involvement of HV in endoscopy-based research is a matter of ethical concern[5].
Updated recommendations on ethics in gastrointestinal endoscopy-based research were discussed and summarized in the recent Workshop of the European Society of Gastrointestinal Endoscopy (ESGE)[6]. The main ethical considerations were as follows: first, there must be a research issue that can not be adequately addressed by the involvement of patients; and second, the inherent risk for HV is regarded to be “acceptable”. However the term “acceptable” is not well defined and it is not uncommon that different scientific and ethical committees make different conclusions concerning endoscopy-based studies. Objective methods for ethical evaluation could potentially be helpful in narrowing such differences in opinions. Targeting the quality of life (QOL) in volunteers could potentially contribute to objectivity in ethical questions, however, there are no data regarding this issue.
For this reason we aimed to evaluate the QOL in HV during endoscopy-based research. To answer these questions, we used the previously well-validated short-form 36 (SF-36) and visual analog scale-based questionnaires (VAS). In this pilot study, we demonstrate that an endoscopy-based drug trial has no significant influence on the QOL in healthy participants even under rigorous conditions and a long-term protocol.
The study design was approved by the local ethics committee of Magdeburg University Hospital and by government authorities. HV, as defined below, were recruited for the study in accordance with the Declaration of Helsinki principles and recommendations of the ESGE-workshop on the ethics of gastrointestinal endoscopy-based research[6]. The recruitment of HV was performed through the distribution of brochures or information posters avoiding affiliation or dependency of HV to the investigators. The purpose, risks of the procedure and the drug-related side effects were fully explained to participants and included in the written informed consent (e.g. risk of bleeding due to platelet inhibitory drugs). HV were informed about the opportunity to revoke study consent and cancel participation at any time. At least 24 h consideration time was provided to the HV before all participants provided written informed consent. Due to the extensive study protocol, all HV were financially reimbursed for their time, effort and contribution to the research. Financial compensation was equal in all HV and was modestly calculated based on generally used factors including compensation for time (or possible income shortfall due to participation in the study), risk, discomfort and inconvenience.
Ten HV [8 men and 2 women, age 27.8 years (22-37) and body mass index 24.7 kg/m2 (22.3-27.2)] were included in the study based on previously described inclusion/exclusion criteria[7]. Briefly, HV had no abnormality at physical examination or during routine laboratory examination, no history of relevant illnesses especially history of peptic ulceration, no pregnancy in women and were negative for H. pylori infection.
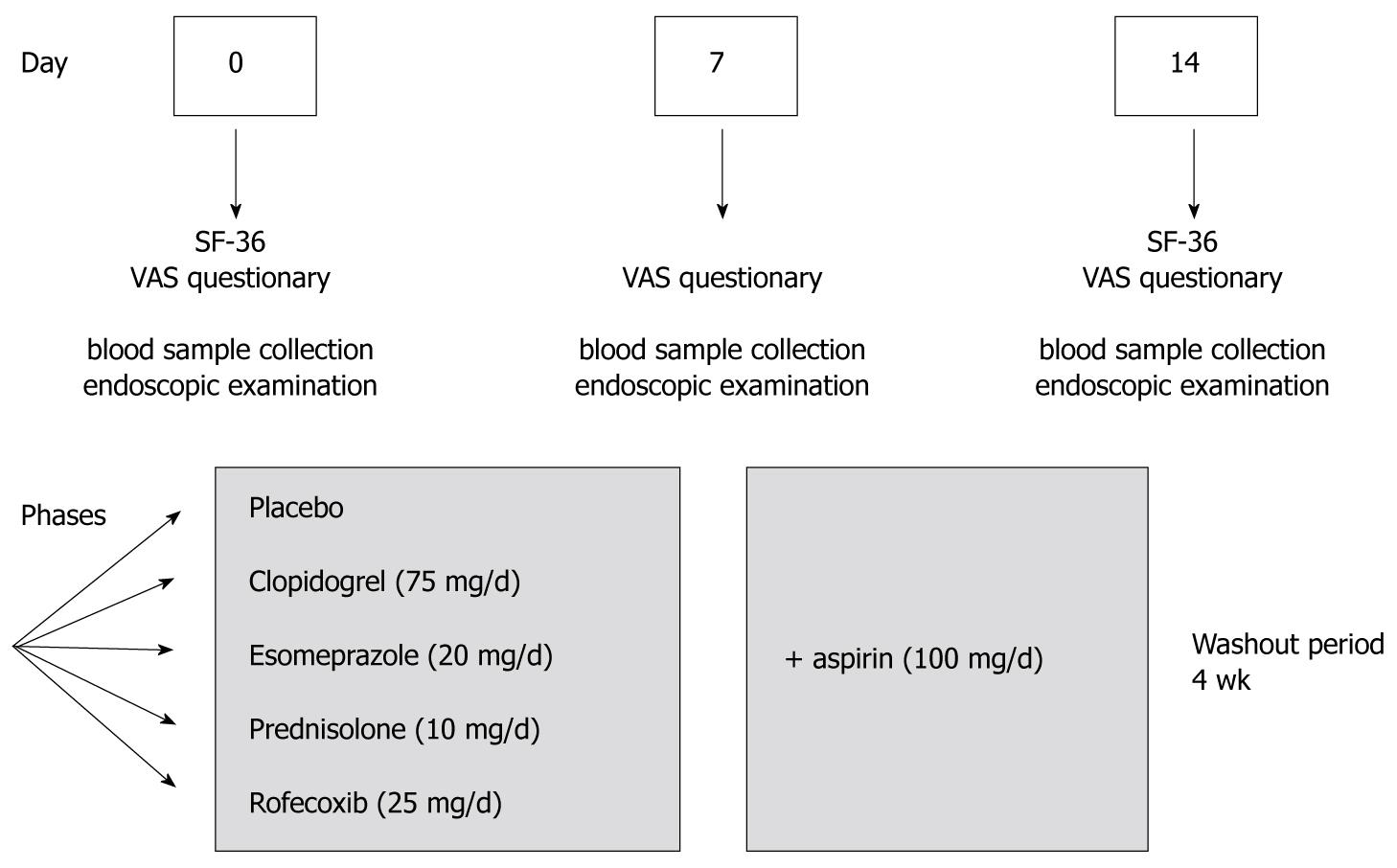
The study was conducted as a prospective double-blind, placebo-controlled study with cross-over design. The main aim of the trial was to study the influence of several frequently used drugs on the healing of gastroduodenal lesions (preliminary clinical results were reported elsewhere)[8]. In addition, we aimed to evaluate the impact of the endoscopy-based trial on QOL in HV. All volunteers were asked to fill out the questionnaires on the day of each endoscopy, describing the subjective symptoms and QOL. Rofecoxib, clopidogrel, prednisolone, esomeprazole or placebo were given alone and/or together with acetylsalicylic acid (ASA). The endoscopic procedures were performed on days 0, 7 and 14 (Figure 1). The volunteers received one of the test drugs for one week and the same drug together with ASA for another week. Total duration of the study was over 6 mo including a 4-wk washout period between 5 different drug phases.
After overnight fasting, the endoscopic examination was performed by an experienced endoscopist (G.T.) using a standard endoscope (GIF 145, Olympus, Germany). All HV received topical lidocaine 2% for local anesthesia and 20 mg of butylscopolaminiumbromid intravenously (iv) to inhibit gastric and small bowel propagation. Additionally, midazolam 5 mg was given iv if requested by the participants. During the procedure, patients were monitored for heart rate and oxygen saturation. After a systematic examination of the stomach and duodenum, multiple biopsies (10 per region, a total of 30 per endoscopy) were taken using standard forceps (5 mm maximum open diameter) from the distal duodenal bulb, antrum and corpus.
To determine the QOL in HV, we used a validated German version of the SF-36 (short form) questionnaire which includes an 8-scale profile: physical functioning, role physical, bodily pain, general health, vitality, social functioning, emotional role and mental health. These scales further represent two distinct higher-ordered clusters due to physical and mental health variance. A lower score in SF-36 indicates a greater impairment and can range from 0 (worst health) to 100 (best health). To determine the abdominal symptoms of HV, we used a previously validated visual analog scale (VAS) based questionnaire[9] with the following dimensions of symptoms: abdominal pain, bloating, reflux, nausea, vomiting, diarrhea and loss of appetite. The summary symptom score (0-70) was calculated by adding up the values of the single scores. A single score value from 1-10 was defined as presence, and 0-1 as the absence of any symptoms. As shown in Figure 1, VAS questionnaires were filled out on days 0, 7 and 14 and the SF-36 questionnaires were filled out before and after each drug phase. The score parameters of HV were compared to a previously evaluated control population of 28 patients, who underwent a single endoscopic examination and in the absence of acid suppressive drugs no visible macroscopic or histological abnormalities were found[9].
All data were analyzed using the SPSS 10 (Chicago, IL, USA) and Graph Pad Prism 4.0 (San Diego, CA, USA). The differences between the groups were analyzed according to corresponding formula and rules, using where appropriate paired or unpaired t-test and Friedman’s test (FT) for non-normal distributed data. For all comparisons, a P-value (two-sided) of < 0.05 was regarded as significant.
All 10 HV completed all 5 phases of the study with 15 endoscopies per HV. With the exception of 2 individuals who either experienced a prolonged bleeding after biopsies had been taken or melena (due to the combination of ASA and clopidogrel) without development of anemia, there were no further drug- or endoscopy-related complications. The VAS and SF-36 questionnaires were completed and returned in 99.5% and in 88% of all cases/time points, respectively. In 91% of endoscopies, HV declined to receive midazolam for sedation and received only topical anesthesia with lidocaine 2%.
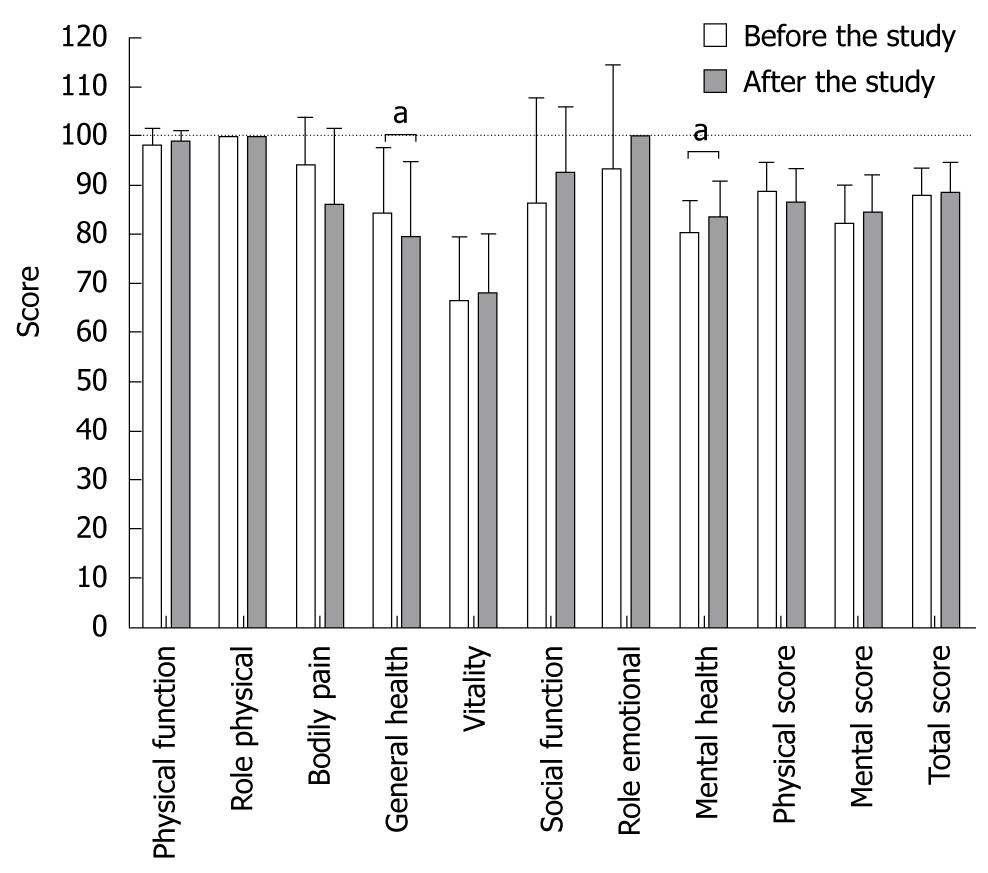
SF-36 questionnaires were analyzed in regard to different aspects, such as influence of study drugs, different time points, as well as long term changes in QOL. The physical, mental and total scores, determined by the short form SF-36 questionnaire ranged between 65.6 to 97.0, 65.3 to 96.0 and 75.6 to 97.1, respectively. We found no significant changes in physical, mental or total scores either between different medications or due to participation in each drug phase (data not shown). Overall changes in physical, mental and total scores are shown in Table 1. For the long-term impact of the intensive endoscopy-based trial on the QOL in HV, the SF-36 assessed data were compared between baseline (day 0 during the first phase) and the last time point of the last phase more than 6 mo later. Remarkably, physical (88.4 ± 6.0 vs 86.4 ± 6.7), mental (82.1 ± 8.1 vs 84.6 ± 7.4) and total scores (88.0 ± 5.7 vs 88.6 ± 6.1) showed no significant changes over the 6-mo period (Figure 2). Single score analysis revealed that only general health decreased from 84.4 ± 13.1 to 79.4 ± 15.4 (mean ± SD; paired t-test P = 0.023) and mental health increased from 80.0 ± 6.8 to 83.2 ± 7.5 (P = 0.022).
| Test drug day | Physical score | P | Mental score | P | Total score | P | |||
| 0 | 14 | 0 | 14 | 0 | 14 | ||||
| Placebo | 88.7 ± 5.9 | 88.2 ± 4.1 | NS | 85.7 ± 8.5 | 86.0 ± 7.8 | NS | 89.9 ± 6.2 | 89.8 ± 4.8 | NS |
| Esomeprazole | 85.7 ± 7.7 | 84.1 ± 6.0 | NS | 83.3 ± 6.2 | 83.0 ± 6.0 | NS | 86.2 ± 7.2 | 87.4 ± 4.8 | NS |
| Clopidogrel | 86.6 ± 6.5 | 85.5 ± 6.7 | NS | 84.0 ± 7.3 | 83.8 ± 8.2 | NS | 88.1 ± 6.1 | 87.0 ± 6.3 | NS |
| Prednisolone | 85.1 ± 5.2 | 82.2 ± 9.6 | NS | 81.9 ± 7.0 | 82.6 ± 6.4 | NS | 87.0 ± 5.4 | 85.7 ± 6.7 | NS |
| Rofecoxib | 86.2 ± 8.2 | 86.4 ± 3.1 | NS | 85.0 ± 6.2 | 84.5 ± 4.7 | NS | 88.3 ± 6.5 | 88.4 ± 3.1 | NS |
| Total Scores | 86.4 ± 1.3 | 85.4 ± 2.0 | NS | 83.5 ± 1.3 | 84.2 ± 1.2 | NS | 87.8 ± 1.2 | 87.8 ± 1.5 | NS |
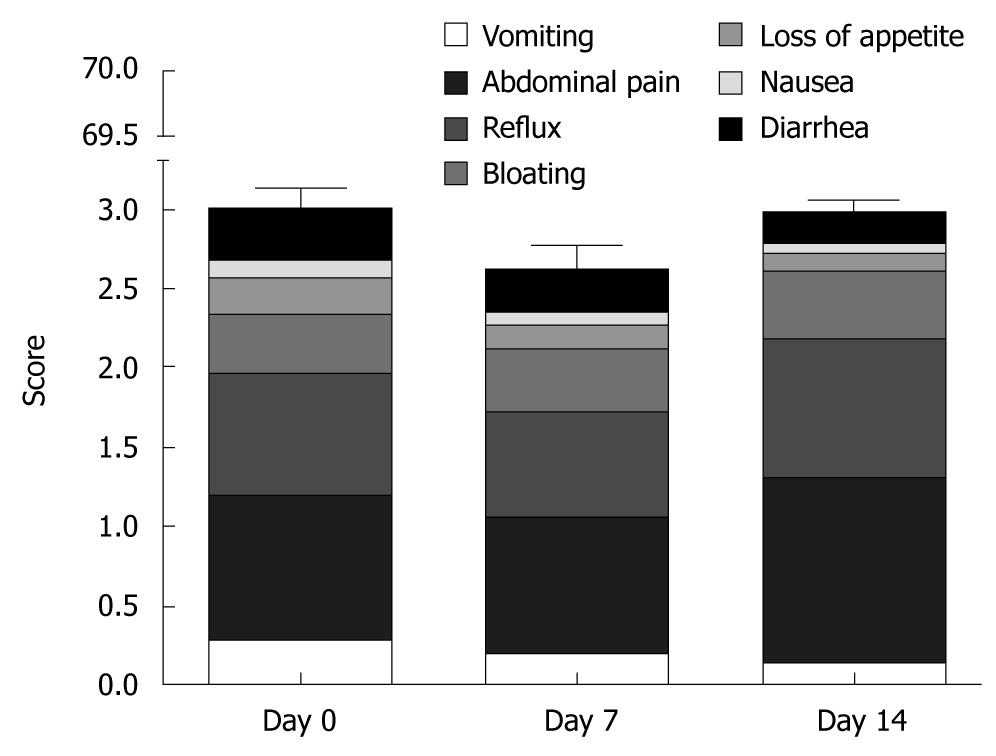
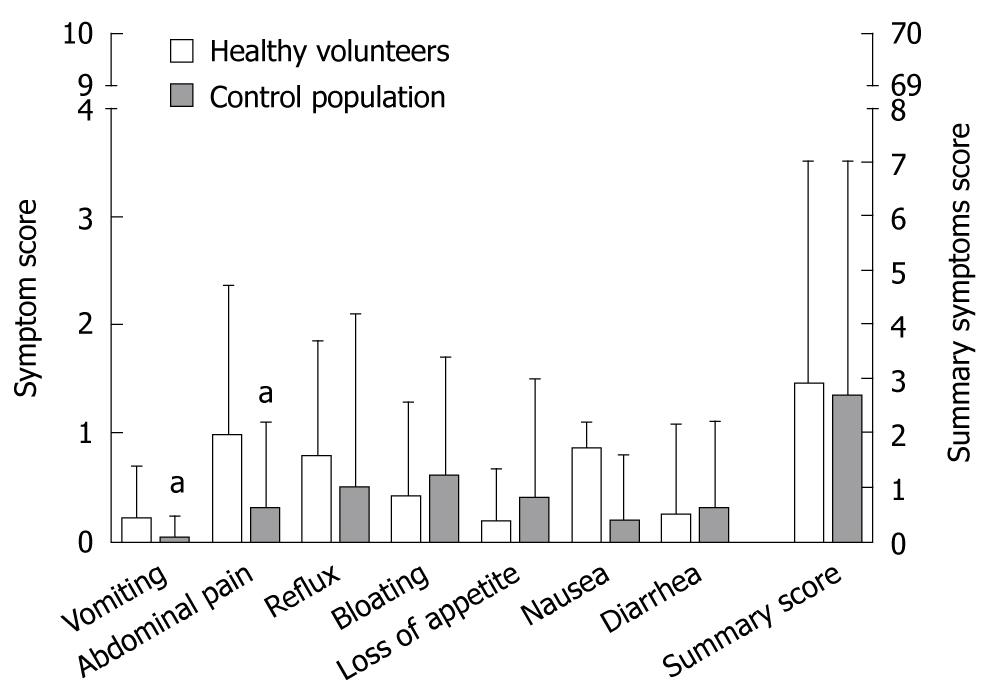
Physical complaints were assessed for different symptoms with VAS and analyzed in a similar manner to the SF-36 questionnaires. As anticipated, subjective physical and emotional perception even at baseline differed from one person to another (high inter-individual variability, data not shown). Almost 60% of all complaints were due to abdominal pain and reflux, but these scores showed no significant changes when compared with baseline (Figure 3). The rarest complaints with < 10% of the maximal score were nausea and loss of appetite. To evaluate the global changes we constructed a summary score; values at baseline (3.2 ± 5.1) were comparable to scores at day 7 (2.6 ± 3.7) and day 14 (3.0 ± 3.4) and were less than 5% of the theoretical maximal score.
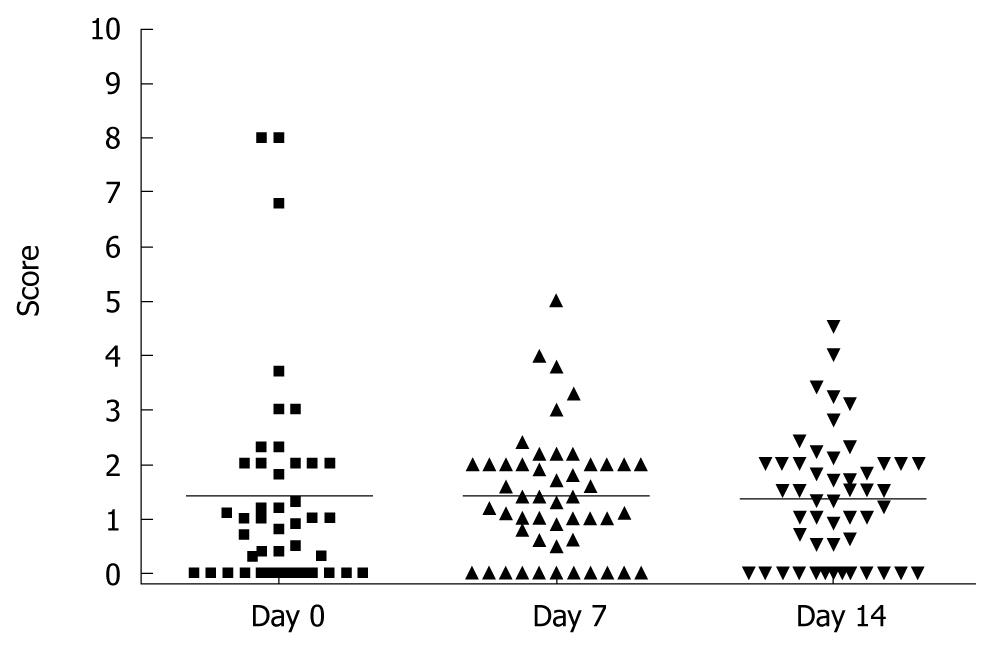
To analyze the subjective endoscopy-related impairment in HV, we used VAS which allows the volunteers to score the procedure-oriented discomfort. In analogy to physical complains VAS, we observed high inter-individual variability with the overall range varying from absence to 80% of maximum possible impairment during the endoscopy. As demonstrated in Figure 4, there was no significant difference between self-assessed scores for complaints about endoscopic procedures (1.4 ± 2.0, 1.4 ± 1.1 and 1.35 ± 1.1 for day 0, 7 and 14, RM ANOVA, P > 0.05).
The VAS-scores from HV were compared with previously published VAS-scores from a control population[9]. The overall mean for summary symptom scores of HV and controls were comparable (HV 2.9 ± 4 and controls 2.7 ± 4.4, t-test: P > 0.05) and was lower than 5% of the theoretically possible value. We found significantly more complaints for abdominal pain (0.98 ± 1.37 vs 0.3 ± 0.8, P = 0.012) and vomiting (0.22 ± 0.5 vs 0.03 ± 0.2, P = 0.046) in the HV group (Figure 5), but due to the limited number of HV we could not perform age adjustment for the symptoms. The difference between both scores were below the defined cut-off value for the presence or absence of symptoms and were significantly lower than a symptomatic population as previously published[9].
QOL of HV is one of the major ethical concerns in endoscopy-based research. Due to the lack of objective data, the decision to include or not to include HV into a clinical trial is made rather arbitrarily and is mostly based on the individual judgment of clinicians and the opinion of expert committees. Therefore, there is a strong need for prospective data that would help to gain knowledge directly related to HV. To test if the endoscopy-based study impacted the QOL of HV, we prospectively evaluated QOL in HV under rigorous conditions. During the study of multiple potentially GI-harmful medications, HV underwent multiple (150) endoscopic examinations with over 4500 biopsies in 5 independent phases. We demonstrated that by accurate implementation of recommendations and guidelines, even a long-term endoscopy-based study has only a little or no impact on the QOL in HV.
Several different methods to measure QOL, for example Sickness Impact Profile, Medical Outcome Study Short-Form 36 (SF-36), Nottingham Health Profile, Quality of Well Being Scale and other disease-specific scales have been previously validated[10]. We decided to use the SF-36 questionnaire for its brevity and its comprehensiveness. SF-36 is one of the best validated and widely used questionnaires both in clinical practice and research work in gastroenterology[11]. To increase the reliability of the study, we also used VAS which was previously validated in dyspeptic patients by our group[9]. Both questionnaires are based on self-evaluation which minimizes the investigator-related impact, and further contributes to reliability of the outcome results.
In this pilot study, we found that the endoscopy-based trial was not associated with significant changes in QOL scores. These scores were comparable between different phases and time points as well as when compared to the general population[12] and to the control population[9]. Based on our results and previous knowledge, several conclusions can be made[6]. First, implementation of the current guidelines and recommendation are a reliable fundament for the successful realization of an endoscopy-based study. Second, careful selection of volunteers might be important, especially taking into account the motivation of the HV. Motivation is - at least in part - based on an idealistic character, even if financial compensation is provided. However, especially in developing countries, financial compensation may become the dominant stimulus for participation which may overcome the study-related discomfort[5,13]. In our study, inclusion of HV was based on a “first come, first served” principle, however, there is still a chance of creating a bias due to personal motivation, willingness to participate in the study or even financial interest[5,13]. In this regard it is important to mention that analogous to other studies, all the HV were reimbursed for their time, effort and contribution to the research, but financial compensation was modest at best. Since personal motivation may vary considerably, testing HV with higher and lower tolerance to endoscopy-based studies, especially in relation to financial reward, could add interesting and valuable information and should be considered in future studies. Besides motivation, physician/clinician and nursing care (GCP) also plays an important role in subjective cognition of impaired QOL. All endoscopic examinations were performed by an experienced endoscopy team which could have influenced the satisfaction, and thereby tolerance to the procedure[14].
To the best of our knowledge, the only comparable study that has addressed a similar issue was from Adachi et al[15]. This group analyzed cardiac stress in HV without sedation, and showed that endoscopic examination of HV without sedation increased cardiac stress (without affecting cardiac output) by 66%[15]. Although evaluation of objective physiological parameters may add valuable information, especially if correlated to VAS or even SF-36, the correlation to personal impairment is still unknown. The VAS impairment score in our study was constant throughout the study, with mean impairment of 15% as a maximal value. Although premedication would improve tolerance to endoscopic procedures, premedication with midazolam was only used in 9% of procedures. From another perspective, the “social life” of HV may also have had an impact on the decision to use or not use premedication. Necessary daily activities like study, work or transportation could be significantly influenced by premedication, especially because the duration of this study was 6 mo. Furthermore, it is worth mentioning that none of the HV interrupted the study, and all of them declared that they would re-participate. Some of them did participate in a later endoscopy-based study with 4 endoscopic procedures within 2 h[7].
It was not the aim of our study to clarify whether endoscopy-based research is ethical or not, but primarily to evaluate QOL in HV following the implementation of current recommendations and guidelines. Nevertheless, this information could indirectly contribute to the ethical considerations of endoscopy-based research, if the guidelines and recommendations are acceptable. Therefore, safety standards including experienced endoscopists are a pre-requisite in such studies. Endoscopy-based studies have some risks for complications, which in the worse case can be lethal, even if volunteers are young and healthy[16]. However, the risk is negligible if the procedure is carried out with maximum accuracy and according to the recommendations for standards of sedation and monitoring of patients during GI-endoscopy[3,17,18].
In summary, in this pilot study we show for the first time that participation in long-term endoscopy-based trials is not necessarily associated with significant changes in QOL in HV. Evaluation of QOL might be a valuable tool regarding ethical questions in further studies. Larger studies are warranted to confirm the data and to further analyze the interaction between QOL and motivation of HV.
Involvement of healthy volunteers (HV) is absolutely essential in gaining valuable knowledge in clinical and preclinical research. The involvement of HV (HV) has a long history, but many unresolved issues of ethical, methodological or even legal concerns in this regard still remain unanswered. The importance of this becomes even more pronounced when invasive procedures like GI endoscopy are undertaken. Existing ethical recommendations and guidelines on this topic are mostly based on the opinion of experts, but many of these questions have not been studied in a systematic manner.
Multiple studies have shown that the motivation of HV plays a key role in recruiting volunteers. Financial reward is one of the crucial motivating factors in HV. Participation in research studies might be associated with study-related risks, discomfort and inconvenience that might become even more relevant in endoscopy-based studies. Impairment in the quality of life in HV may play a crucial role in understanding these ethical concerns, however, so far no study has addressed the impact of endoscopy-based research on the quality of life of HV.
In this pilot study the authors evaluate the changes in quality of life in HV in a long-term endoscopy-based drug study. The study was conducted according to existing ethical recommendation and guidelines. Using the well-validated SF-36 and visual analog scale-based questionnaires, they show that endoscopy-based research has no significant impact on quality of life in HV even under rigorous conditions.
This study provides valuable and important information regarding the quality of life of HV who participate in endoscopy-based studies, and supports the existing recommendation and guidelines on this topic in Gastroenterology.
Healthy volunteer: is a person who voluntarily participates in a research study. Volunteer come from volunteering which means working, participating or being involved in something without being motivated by financial or material gain. The historical intention, such as to promote good, serve society and improve human quality of life show static movement in paid volunteerism. Endoscopy-based research: are studies or trials performed on individuals which involve endoscopic techniques. Short Form 36: is a well-validated and widely used short survey which includes 36 specific questions on health-related quality of life.
This study examines the impact of a demanding endoscopy protocol on gastrointestinal symptoms and quality of life in 10 human volunteers. The study involved 15 endoscopic procedures over a period of 6 mo.
Peer reviewers: Ian C Roberts-Thomson, Professor, Department of Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South 5011, Australia; William Dickey, Professor, Altnagelvin Hospital, Londonderry, BT47 6SB, Northern Ireland, United Kingdom
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Swain CP. New Thechnology in diagnostic and therapeutic endoscopy. Gastroenterological endoscopy. Stuttgart: George Thieme Verlag 2002; 62-69. [Cited in This Article: ] |
| 2. | Hope T, McMillan J. Challenge studies of human volunteers: ethical issues. J Med Ethics. 2004;30:110-116. [Cited in This Article: ] |
| 3. | Treiber G, Malfertheiner P. Endoscopy-based research in healthy volunteers. Dig Dis. 2002;20:266-270. [Cited in This Article: ] |
| 4. | Ko HH, Zhang H, Telford JJ, Enns R. Factors influencing patient satisfaction when undergoing endoscopic procedures. Gastrointest Endosc. 2009;69:883-891, quiz 891.e1. [Cited in This Article: ] |
| 5. | van Gelderen CE, Savelkoul TJ, van Dokkum W, Meulenbelt J. Motives and perception of healthy volunteers who participate in experiments. Eur J Clin Pharmacol. 1993;45:15-21. [Cited in This Article: ] |
| 6. | Malfertheiner P, Mantzaris GJ, Farhing M, Niv Y, Escourrou J, Treiber G, Di Mario F, Reymond MA. Recommendations of the ESGE workshop on Ethics in Gastrointestinal Endoscopy-Based Research. First European Symposium on Ethics in Gastroenterology and Digestive Endoscopy, Kos, Greece, June 2003. Endoscopy. 2003;35:775-777. [Cited in This Article: ] |
| 7. | Treiber G, Wex T, Link A, Vieth M, Laufer S, Malfertheiner P. The effect of single-dose naproxen on eicosanoid formation in human gastroduodenal mucosa. Aliment Pharmacol Ther. 2006;23:155-167. [Cited in This Article: ] |
| 8. | Treiber G, Vieth M, Wex T, Malfertheiner P. Impact of Aspirin and Comedications On Healing of Biopsy-Induced Gastroduodenal Erosions. Digestive Disease Week 2006. Los Angeles, CA, USA. Gastroenterology. 2006;130:267. [Cited in This Article: ] |
| 9. | Treiber G, Schwabe M, Ammon S, Walker S, Klotz U, Malfertheiner P. Dyspeptic symptoms associated with Helicobacter pylori infection are influenced by strain and host specific factors. Aliment Pharmacol Ther. 2004;19:219-231. [Cited in This Article: ] |
| 10. | Yacavone RF, Locke GR 3rd, Provenzale DT, Eisen GM. Quality of life measurement in gastroenterology: what is available? Am J Gastroenterol. 2001;96:285-297. [Cited in This Article: ] |
| 11. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [Cited in This Article: ] |
| 12. | Brazier JE, Harper R, Jones NM, O‘Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160-164. [Cited in This Article: ] |
| 13. | Tishler CL, Bartholomae S. The recruitment of normal healthy volunteers: a review of the literature on the use of financial incentives. J Clin Pharmacol. 2002;42:365-375. [Cited in This Article: ] |
| 14. | Yacavone RF, Locke GR 3rd, Gostout CJ, Rockwood TH, Thieling S, Zinsmeister AR. Factors influencing patient satisfaction with GI endoscopy. Gastrointest Endosc. 2001;53:703-710. [Cited in This Article: ] |
| 15. | Adachi W, Yazawa K, Owa M, Koide N, Hanazaki K, Kajikawa S, Kobayashi S, Amano J. Quantification of cardiac stress during EGD without sedation. Gastrointest Endosc. 2002;55:58-64. [Cited in This Article: ] |
| 16. | Pentz RD. Spreading it around: money for researcher and research participants. Mt Sinai J Med. 2004;71:266-270. [Cited in This Article: ] |
| 17. | Aisenberg J, Cohen LB. Sedation in endoscopic practice. Gastrointest Endosc Clin N Am. 2006;16:695-708. [Cited in This Article: ] |
| 18. | Orme M, Harry J, Routledge P, Hobson S. Healthy volunteer studies in Great Britain: the results of a survey into 12 months activity in this field. Br J Clin Pharmacol. 1989;27:125-133. [Cited in This Article: ] |