Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3642
Revised: May 29, 2010
Accepted: June 5, 2010
Published online: August 7, 2010
AIM: To investigate the effects of the free radical scavenger bis(1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl)decandioate (IAC) in the dextran sodium sulphate (DSS) experimental model of ulcerative colitis.
METHODS: Colitis was induced in Sprague Dawley male rats by administration of 5% DSS in drinking water. IAC (30 mg/kg, lipophilic or hydrophilic form) was administered daily (orally or ip) for 6 d until sacrifice. Colonic damage was assessed by means of indirect (Disease Activity Index score) and direct measures (macroscopic and microscopic scores) and myeloperoxidase (MPO) activity. Neutrophil infiltration within the tissue and glutathione S-transferase activity were also investigated.
RESULTS: DSS-induced colitis impaired body weight gain and markedly increased all inflammatory parameters. Six-day treatment with lipophilic IAC significantly reduced intestinal damage caused by inflammation, induced a down-regulation in MPO activity (0.72 ± 0.12 and 0.45 ± 0.12 with lipophilic IAC po and ip, respectively, vs 1.10 ± 0.27 in untreated DSS colitis animals) and minimized DSS-induced neutrophil infiltration, while hydrophilic IAC administered orally did not ameliorate DSS-induced damage.
CONCLUSION: These results support the hypothesis that reactive oxygen metabolites contribute to inflammation and that the radical scavenger IAC has therapeutic potential in inflammatory bowel disease.
- Citation: Vasina V, Broccoli M, Ursino MG, Canistro D, Valgimigli L, Soleti A, Paolini M, Ponti FD. Non-peptidyl low molecular weight radical scavenger IAC attenuates DSS-induced colitis in rats. World J Gastroenterol 2010; 16(29): 3642-3650
- URL: https://www.wjgnet.com/1007-9327/full/v16/i29/3642.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i29.3642
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory diseases of the gut of unclear etiology: environmental and genetic factors regulating mucosal immune response, mucosal barrier function and response to intestinal microflora are all thought to contribute to the pathogenesis of these diseases, characterized by mucosal inflammatory infiltrates, intestinal barrier function alteration and erosive loss of mucosa and submucosa[1,2].
Because of extensive mucosal damage and massive infiltration of polymorphonuclear and mononuclear leukocytes[1,2], reactive oxygen and nitrogen radical species are produced and released, resulting in potential oxidation and peroxidation of a large number of molecules (e.g. proteins, lipids and DNA). Indeed, the intestinal mucosa of patients with inflammatory bowel disease (IBD) is characterized by radical species overproduction and imbalance of the most important antioxidants[3,4] leading to oxidative damage; self-sustaining cycles of oxidant production may amplify inflammation and mucosal injury in UC, where activated neutrophils and macrophages mainly contribute to active lesions[5,6]. The phagocytes present in the mucosa of IBD patients, indeed, can produce reactive oxygen metabolites such as superoxide and hydrogen peroxide, through both respiration burst and prostaglandin and leukotriene metabolism. Radical species released during inflammation increase mucosal permeability and contribute to the recruitment and activation of further neutrophils, thus initiating and/or propagating inflammation and tissue damage[6].
Antioxidant compounds and free radical scavengers improved colitis in several experimental models[7,8]. Recently, in a proof of concept study, we have shown that the radical scavenger bis(1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl)decandioate (IAC) protects the rat colon from DNBS-induced colitis, which mimics human CD[9]. DNBS is a contact sensitizing allergen which causes immunological activation; acute pathological features include focal necrosis and acute inflammation, followed by a chronic infiltration of mononuclear cells[10].
In this present study, we investigated the effects of IAC in a different model of colitis, i.e. one that is induced by dextran sodium sulphate (DSS), which differs from the DNBS model in terms of tissue inflammatory and immunological activation, as well as severity of the inflammatory process, and is thought to closely mimic human UC[10]. In the DSS model, as in UC, only the mucosa is affected by inflammation; early damage includes shortening and dropout of crypts, particularly over lymphoid aggregates, progressing to focal ulceration, mononuclear cell and neutrophil infiltration. Similar to UC, DSS provokes acute inflammation and macrophage activation, with consequent epithelial cell injury and activation of innate immune responses by luminal bacterial components and eventual activation of T cells[2,11,12].
We tested two different forms of IAC in DSS colitis, with either hydrophilic or lipophilic character. Due to its peculiar physico-chemical properties, IAC readily diffuses through the cellular membrane and can reach virtually any compartment where the production of free radicals occurs, possessing equal radical scavenging activity[13]. This represents an advantage, because it can directly react with free radicals.
Male Sprague Dawley rats (180-200 g body weight; Harlan Italy, S. Pietro al Natisone, Udine, Italy) were used in this study. Animals were housed in a controlled environment and had free access to food and water throughout the study. Before starting any experimental procedure, in order to minimize stress, animals of all experimental groups were weighed and gently manipulated in the laboratory environment for 30 min every day for at least 1 wk. All experiments were carried out according to the guidelines set forth by EEC Directive 86/609 on the care and use of experimental animals. The protocol for induction of colitis was reviewed by the Institutional Committee on the care and use of experimental animals of the University of Bologna and was authorized by the Italian Ministry of Health. A persistently hunched posture and labored respiration, a markedly erect coat and a weight loss of more than 20% were considered as humane end-points at which to euthanize the animals.
Colitis was induced using a previously described method[14]. DSS (molecular weight, 40 kilodaltons; ICN Biomedicals Inc, Aurora, OH) was added to drinking water at a final concentration of 5% (wt/vol) for 5 d. Controls were all time-matched and consisted of rats receiving normal drinking water only. The DSS solution was replenished daily and mean DSS consumption was noted per cage at the end of 5-d treatment.
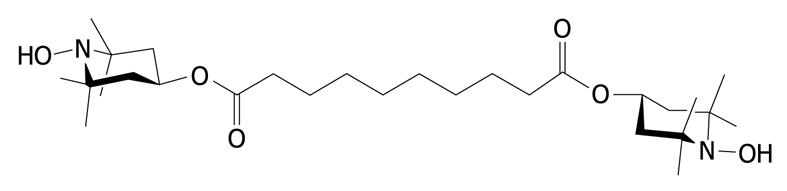
We studied groups of rats with and without colitis (n = 6-12 per group), which were treated with the radical scavenger IAC (Figure 1) synthesized in our laboratory[13], starting the day before the induction of colitis for 5 d. IAC (30 mg/kg), hydrophilic or lipophilic form, was administered once daily at the same time (orally or ip) as water solution or suspension. In our previous work, we observed that treatment with hydrophilic IAC po in DNBS-induced colitis induced only a minor protective effect, while when administered ip, it was unable to reduce inflammation, therefore we decided to only test the hydrophilic form po in this study[9].
The dose of 30 mg/kg was selected on the basis of previous studies, in which IAC showed the best antioxidant activity[15] and protective effect in DNBS-induced colitis[9]. At the end of this 7-d period, the animals were killed and their colons collected for further analysis.
Before tissue samples were collected, the entire colon was removed, the length starting from 1 cm above the anus to the top of the cecum and the weight of the colon still containing fecal contents were measured. Then the colon was opened longitudinally and washed with phosphate buffered saline (PBS) to remove luminal contents; at this time, the consistency of any stools found within the colon and the gross macroscopic appearance of the colon were noted. Whole-wall colonic samples were pinned flat on wax, fixed in cold neutral 4% formalin and then placed in 25% sucrose in PBS at 4°C for cryoprotection and embedded in optimal cutting temperature tissue freezing medium. Seven-micron-thick sections of colon were cut, serially mounted on glass and processed for routine hematoxylin-eosin (HE) staining and naphthol AS-D chloroacetate esterase assay. Specimens of colonic tissue were also removed, snap frozen in liquid nitrogen and stored at -80°C until subsequent assays.
Disease activity index: Disease activity index (DAI) scores have historically well correlated with pathological findings in a DSS-induced model of IBD[16]. DAI is the combined score of weight loss, stool consistency and bleeding, as detailed in Table 1. All parameters were scored from day 0 to day 5 during DSS treatment.
| Stool consistency | Bleeding | Weight loss | Maximum score |
| 0 = formed | 0 = normal color stool | 0 = no weight loss | 10 |
| 1 = mild-soft | 1 = brown color stool | 1 = 5%-10% weight loss | |
| 2 = very soft | 2 = reddish color stool | 2 = 11%-15% weight loss | |
| 3 = watery stool | 3 = bloody stool | 3 = 16%-20% weight loss | |
| 4 = > 20% weight loss |
Total macroscopic score: When rats were sacrificed, the colon was removed, opened longitudinally and washed with PBS to remove luminal contents and macroscopic damage was immediately assessed. The macroscopic parameters analyzed before tissue samples were collected were: colon length starting 1 cm above the anus to the top of the cecum, weight of the colon still containing fecal contents, stool consistency and gross macroscopic appearance of the colon. Decreases in filled colon weight are indicative of colonic hypermotility[17]. Indeed, colons from animals with severe colitis can be seen to be nearly devoid of fecal contents. Colon shrinkage is also commonly observed in DSS colitis and is indicative of colonic smooth muscle contraction[17,18]. Total macroscopic score was assigned according to a previously described scoring system[16]; details for each parameter are reported in Table 2.
| Stool consistency | Bleeding | Colon damage score | Colon weight score | Colon length score | Maximum score |
| 0 = formed | 0 = absent | 0 = no inflammation | 0 = < 5% weight loss | 0 = < 5% shortening | 14 |
| 1 = loose | 1 = present | 1 = hyperemia | 1 = 5%-14% weight loss | 1 = 5%-14% shortening | |
| 2 = liquid | 2 = slight erosion | 2 = 15%-24% weight loss | 2 = 15%-24% shortening | ||
| 3 = extensive erosion/ulceration | 3 = 25%-35% weight loss | 3 = 25%-35% shortening | |||
| 4 = > 35% weight loss | 4 = > 35% shortening |
Histology: Seven-micron-thick sections of colon were cut, serially mounted on glass and processed for routine HE staining. Colonic damage was scored based on a published scoring system that considers amount of inflammation (from 0, none, to 3, severe), extent of inflammation (from 0, none, to 3, transmural), crypt damage (from 0, none, to 4, entire crypt and epithelium lost) and tissue regeneration (from 4, no tissue repair, to 0, complete regeneration or normal tissue)[11].
Specimens of colonic tissue (50 mg) were assayed using a previously described method[19]. Myeloperoxidase (MPO) is a granule-associated enzyme present in neutrophils and other cells of myeloid origin, and widely used as a marker of intestinal inflammation. Colonic tissues were homogenized in ice-cold potassium phosphate buffer (pH 6.0) and centrifuged for 10 min at 6000 g at 4°C. The supernatants were then collected, added to a solution of O-dianisidine (Sigma-Aldrich, Milan, Italy) and hydrogen peroxide and assayed to assess MPO activity.
MPO was expressed in units per milligram of tissue, where 1 U corresponds to the activity required to degrade 1 μmol of hydrogen peroxide in 1 min at room temperature.
Colonic samples (25 mg) were obtained using a previously described method[9]. The supernatant was collected and then assayed for glutathione S-transferase (GST) activity[20]. Assessment of protein content in colonic samples was performed using the Quick Start™ Bradford Protein Assay (BIO-RAD, Hercules, CA, USA); the protein-dye complex absorbance was read using a spectrophotometer at 595 nm. GST activity was expressed as μmol/mg of protein per min.
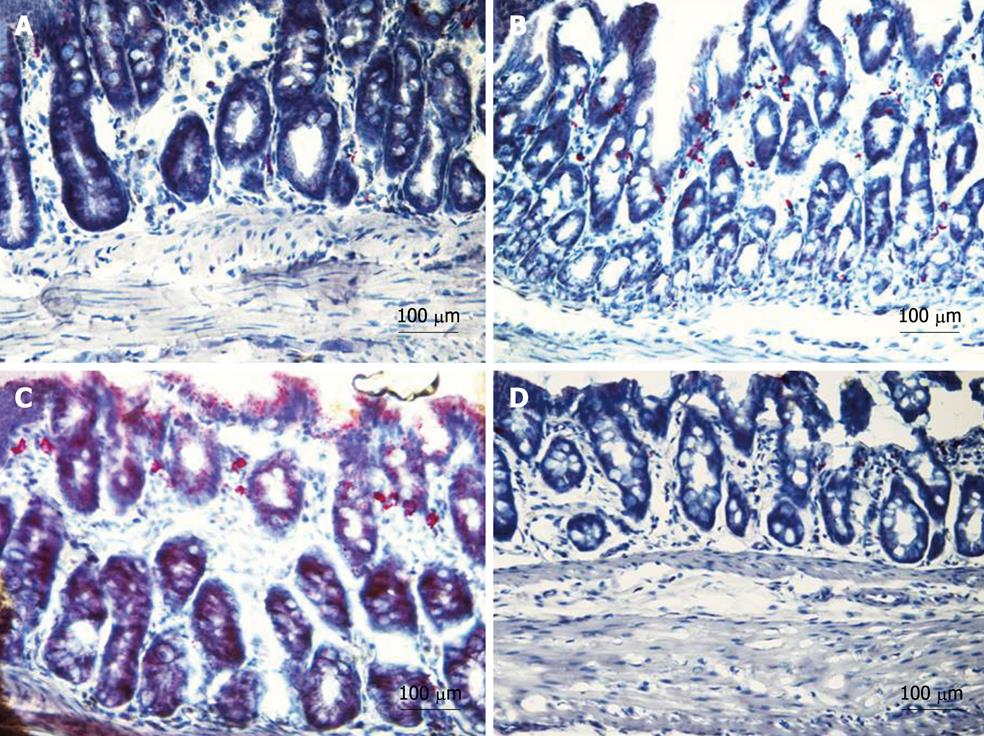
In order to identify neutrophil infiltration within the tissue, we used a commercially available kit (naphthol AS-D chloroacetate esterase; 91C, Sigma-Aldrich, Milan, Italy). This enzyme is considered specific for cells of granulocytic lineage and its sites of activity show bright red granulation. Seven-micron-thick sections of colon were used for this assay. Briefly, tissue sections were fixed in citrate-acetone-formaldehyde solution and assayed according to the manufacturer’s protocols. Specimens were mounted with mounting media (glycerol-PBS, 9:1), examined by light microscope (ECLIPSE 90i, Nikon Instruments, Calenzano, Italy) and representative photomicrographs were taken by DS-5M digital camera (Nikon Instruments, Calenzano, Italy).
Results are expressed as mean ± SE. Statistical analysis was performed using analysis of variance (one-way or two-way, as appropriate, with the Bonferroni’s correction for multiple comparisons). A P value < 0.05 was considered significant. N refers to the number of animals used for each experiment (n = 8-16). Calculations were performed using GraphPad Prism™ (version 5.01, GraphPad Software Inc., San Diego, CA, USA).
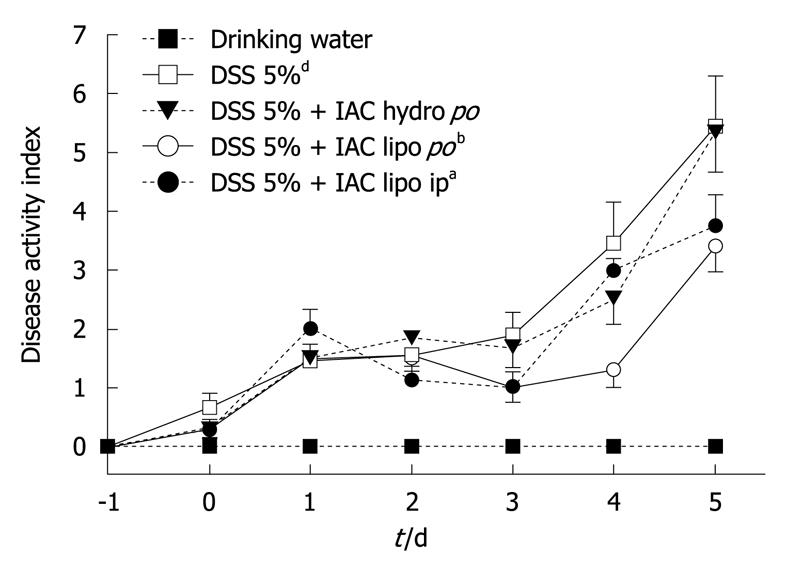
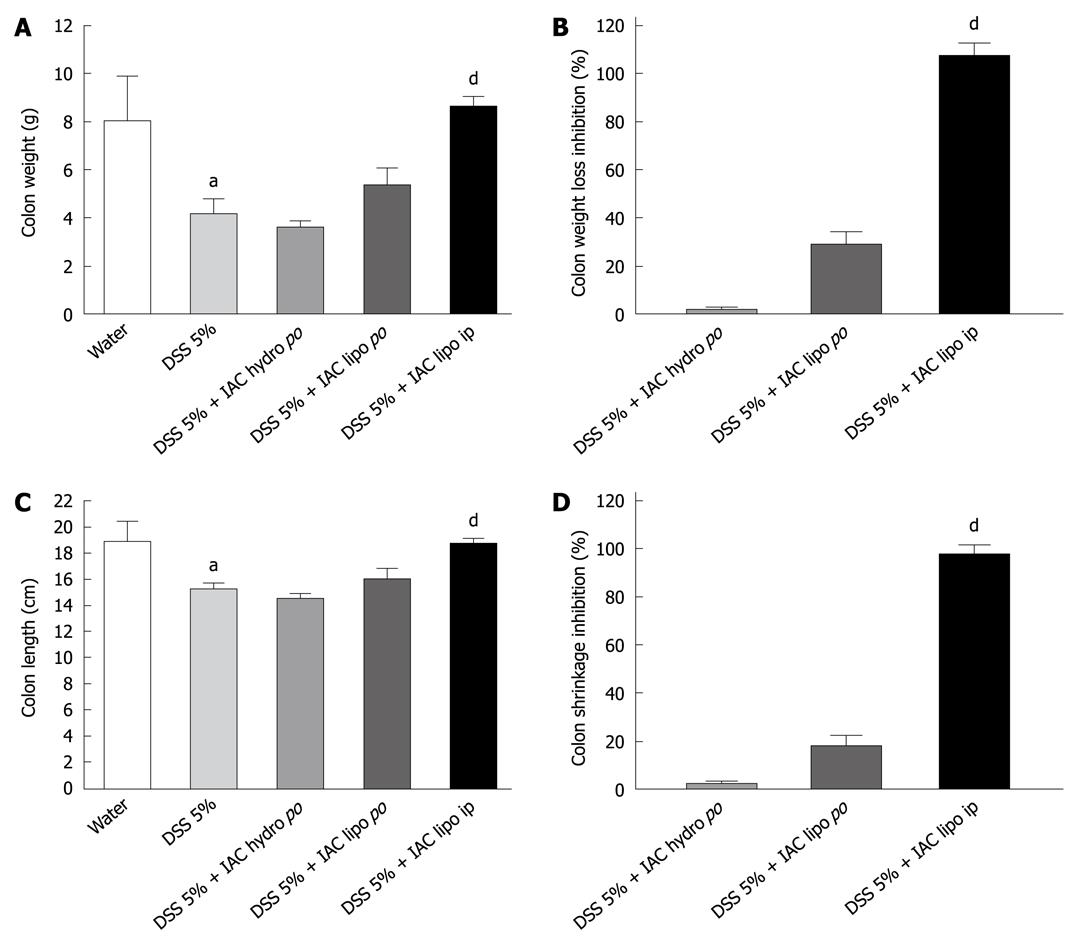
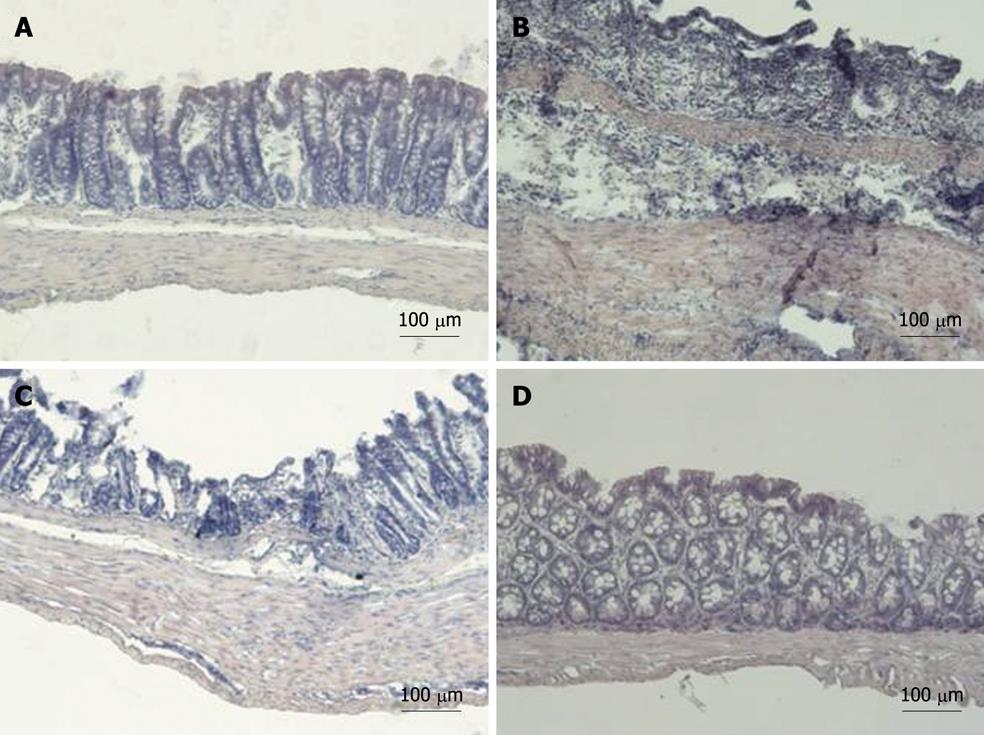
All rats with DSS colitis progressively lost weight and manifested bloody diarrhea. DAI was significantly increased together with all inflammatory parameters (Figures 2, 3, 4, 5 and 6). On day 5 after DSS administration, the colonic mucosa of inflamed rats was edematous and erythematous with occasional areas of mucosal erosion. Compared to non-inflamed rats, the colon weight was decreased, the colon length was significantly shorter and a marked increase in total macroscopic damage score was noted (Figure 3, panel A). Colitis was characterized by mucosal ulceration, crypt dropout and a marked neutrophil infiltration extending throughout the mucosa and submucosal layers (Figure 5B) and by a 20-fold increase in microscopic damage score over the non-inflamed control animals (Figure 3, panel B); crypt abscesses and depletion of goblet cells were observed in some regions of colonic mucosa. A 3-fold increase in MPO activity was found (Figure 3, panel C) compared with healthy rats. Moreover, in DSS-treated animals, we detected neutrophil infiltration (red cells, Figure 6B) with respect to controls (Figure 6A), extending throughout the mucosa and submucosa. GST activity slightly decreased in inflamed animals as compared with healthy controls, although statistical significance was not achieved (1.40 ± 0.14 vs 1.27 ± 0.11).
Six-day treatment with lipophilic IAC (30 mg/kg, orally and ip) reduced intestinal inflammation and damage: indeed, treated rats had neither bloody diarrhea nor perianal injury and an improvement was observed in gross findings (clinical signs and symptoms of colitis) such as weight loss (Figure 2). Total macroscopic score was significantly improved by drug treatment (Figure 3, panel A). DSS-induced colon shrinkage and weight loss were significantly improved by IAC treatment (lipophilic) as compared to inflamed rats, and these features were similar to healthy controls (Figure 4). Moreover, lipophilic IAC (orally and ip) significantly decreased DSS-induced microscopic damage (Figure 3, panel B and Figure 5D), down-regulated MPO activity (Figure 3, panel C) and also minimized DSS-induced neutrophil infiltration within the colonic wall (Figure 6D).
In contrast, hydrophilic IAC 30 mg/kg orally was able to decrease only microscopic damage score, but substantially failed to protect the colon from DSS-induced damage (Figures 2-4, 5C and 6C).
GST activity was not significantly affected either by hydrophilic IAC po (1.27 ± 0.10 vs 1.27 ± 0.11 in DSS 5%) or lipophilic IAC po (1.28 ± 0.07 vs 1.27 ± 0.11 in DSS 5%) or ip (1.00 ± 0.07 vs 1.27 ± 0.11 in DSS 5%, P = 0.07).
This study shows that the lipophilic form of IAC at the dose of 30 mg/kg significantly ameliorates damage and inflammation in DSS-induced colitis, an experimental model of UC, and is far better than its hydrophilic form, since it positively affected all the parameters under scrutiny. This different activity on inflammation can be ascribed to the differing ability of the two distinct forms of IAC (hydrophilic vs lipophilic) to distribute in cell membranes and intra-/extra-cellular compartments; hydrophilic IAC may have a particular profile of absorption and distribution, leading to a lower concentration within areas of major damage.
Different experimental models can be used for pathophysiological studies and, because of their similarity to human IBD (UC and CD), they represent useful tools to test the therapeutic potential of new drugs. These models allow the study of early events and interactions among different components of IBD and the identification of immunologic processes and genes determining susceptibility to inflammatory disorders. Although there is no unique model of IBD and the various experimental models differ in their pathophysiology, each of them can be useful to gain insight into the multi-factorial nature of IBD[10].
In DSS-induced colitis, as in UC, only the mucosa is affected by inflammation; early damage includes shortening and dropout of crypts in the left colon, particularly over lymphoid aggregates, progressing to focal ulceration, mononuclear cell and neutrophil infiltration.
Reactive oxygen, nitrogen and carbon species are produced and released during the acute phase of inflammation, resulting in epithelial damage with consequent activation of innate immune responses by luminal bacterial components and eventual activation of Th1 and later Th1/Th2 responses during chronic colitis[2,11].
In our study, 5-d treatment with 5% DSS induced weight loss and bloody diarrhea and caused a substantial degree of inflammation and tissue injury in the rat colon, which was edematous, erythematous and characterized by mucosal ulceration. Moreover, inflammation was associated with polymorphonuclear colonic infiltrate (histology, MPO activity and naphthol AS-D chloroacetate esterase assay). Indeed, within the bowel wall of IBD patients and of animals with experimental colitis, a massive infiltration of polymorphonuclear and mononuclear leukocytes, which may produce large amounts of free radicals, is commonly observed[1,9,21,22].
During DSS-induced colitis, we also studied GST activity. GST is a detoxification enzyme catalyzing the conjugation of reactive electrophiles with the thiol glutathione, providing cellular protection from highly reactive electrophiles[23]. A significant decrease in the GST activity was reported in patients with family history of colon cancer and polyps[24]; moreover, GST level changes have been observed both in IBD patients[25] and in some experimental models of colitis[23,26]. The only marginal (not statistically significant) decrease in GST activity observed by us on day 5 after DSS administration is actually in line with data obtained by Clapper et al[23], who showed cyclical changes in GST activity during acute DSS colitis, with a significant decrease in enzyme activity on days 2 and 7, while on day 5 they reported only marginally decreased GST activity, exactly as we did.
Treatment with lipophilic IAC (30 mg/kg, orally and ip) significantly ameliorated colonic damage and inflammation induced by DSS, decreased MPO activity and also minimized DSS-induced neutrophil infiltration within the colonic wall. These observations extend and corroborate our previous report that IAC is effective in DNBS-induced colitis[9], a quite different model where DNBS causes transmural immunological activation and inflammation resembling CD. In our hands, lipophilic IAC was slightly more effective ip than orally, probably because it undergoes protonation at low gastric pH after oral administration and is more quickly excreted from the gut (the protonated form has higher polarity). For clinical use, it may be necessary to protect IAC from low gastric pH using a specific formulation.
Thus, lipophilic IAC is protective both in DNBS and DSS experimental models of inflammation and has a wide spectrum of activity; its lipophilic form (ip) significantly reduced DAI (30%), macroscopic and microscopic damage (70% and 45%, respectively) and decreased MPO activity (58%) in DSS-induced colitis. Likewise, in DNBS-induced colitis, treatment with lipophilic IAC can decrease macroscopic and microscopic damage (55% and 46%, respectively), reduce MPO and tumor necrosis factor-α tissue levels (80% and 30%, respectively) and lipidic peroxidation (40%).
Notably, in both the DSS and DNBS models[9], IAC is able to significantly decrease MPO activity and neutrophil infiltration within the bowel wall. In both experimental models, as in several human inflammatory diseases such as rheumatoid arthritis and IBD, infiltrating neutrophils are the major candidate for the production of reactive oxygen radicals[27]. Oxidative damage may represent a pathogenic factor in IBD because intestinal inflammation is accompanied by increased production of reactive oxygen and nitrogen species and an imbalanced antioxidant response[1,28,29]. Indeed, free radical production is a key mechanism for the appearance and the maintenance of colonic inflammation in experimental models of colitis[7,30,31].
Thus, we can hypothesize that IAC exerts its protective effects by reducing inflammatory neutrophil infiltrate and by scavenging reactive oxygen species produced by infiltrating cells which during inflammation contribute to the recruitment and activation of further neutrophils, and by counteracting this self-sustaining cycle of oxidant production, which propagates inflammation and tissue damage. This hypothesis of reduced oxidative damage by IAC is not contrary to its lack of activity on GST levels because, in our hands, GST levels were only marginally affected by DSS-induced inflammation. It has been shown that treatment with antioxidant compounds and radical scavengers exerts a protective effect in several models of intestinal inflammation[9,32,33]: Cuzzocrea et al[7,34] demonstrated that treatment with antioxidants such as tempol and M40403, a superoxide dismutase mimetic, ameliorated TNBS/DNBS experimental colitis, probably by limiting leukocyte recruitment. However, cyclic nitroxides such as tempol are very persistent in water or organic solutions, but when used in vivo or in a biological sample are reduced to the parent hydroxylamine by several enzymatic processes mainly involving ascorbate or glutathione. IAC is more stable in physiological solutions and possesses a stronger antioxidant capability than that of the aforementioned cyclic nitroxides; it is easily distributed through cell membranes and intra-/extra-cellular compartments, thus it can directly react with oxidant molecules within the cell, where free radicals are produced[35]. In DNBS-induced colitis, IAC seems to display higher activity than tempol, at least in reducing MPO activity and neutrophil infiltration[7]. Recently, the lipophilic form of IAC was used in different experimental disease models all characterized by oxidative stress (e.g. nonobese mouse diabetes model[15] and a rat model of transient middle cerebral artery occlusion[36]), as well as in vitro[37], with positive results.
Notably, IAC is a low molecular weight radical scavenger which can rapidly react with most carbon-, nitrogen- and oxygen-centered radicals of biological interest, including peroxyl, superoxide, and peroxynitrite radicals[13]. Its antioxidant activity is a direct effect of the molecule itself; its activity is due to hydroxylic hydrogen transfer to peroxyl radicals, which generates the corresponding nitroxide, unable to propagate the autoxidation chain. Its peculiar physico-chemical properties affect its partition properties across cell membranes and both intra- and extra-cellular compartments: the free form is highly lipophilic (the calculated logP is 4.01) and easily crosses the cell membrane, allowing distribution to any compartment where the production of free radicals occurs, but it is also in equilibrium with the protonated form, which administered to a biological system is completely water-soluble and distributes in the extra-cellular compartments[13].
In conclusion, our data show that treatment with the lipophilic (but not the hydrophilic) form of the radical scavenger IAC, at the dose of 30 mg/kg, ameliorates DSS-induced colitis in rats. These results, taken together with our previous data showing a protective effect of lipophilic IAC in DNBS-induced colitis, provide further evidence of the involvement of reactive oxygen species in inflammation and support the concept that antioxidant therapy may have an important role in treatment of IBD.
Ulcerative colitis (UC) and Crohn’s disease are chronic inflammatory bowel diseases (IBDs) of unclear etiology: environmental and genetic factors regulating mucosal immune response, mucosal barrier function and response to intestinal microflora are all thought to contribute to the pathogenesis of these diseases, characterized by mucosal inflammatory infiltrates, intestinal barrier function alteration and erosive loss of mucosa and submucosa. Indeed, the intestinal mucosa of patients with IBD is characterized by radical species overproduction and imbalance of the most important antioxidants leading to oxidative damage.
Over the last two decades, the incidence and the prevalence of IBD seem to have increased; IBDs cause a large number of hospitalizations for the patients affected. Many drugs are used to treat IBD, given for a variety of reasons: to suppress inflammation in patients with active disease, to prevent flare-ups in those with inactive disease, to control symptoms such as pain or diarrhea or to replace or supplement essential nutrients which are poorly absorbed because of extensive disease or surgery. However, the etiology of IBD is still unknown and new therapeutic options are needed, as available drugs are still unsatisfactory to treat and heal IBD.
It is generally hypothesized that oxidative stress is a potential etiological and/or triggering factor for IBD, because the detrimental effects of reactive oxygen molecules have been well established in the inflammation process. Antioxidant compounds and free radical scavengers have been shown to improve colitis in several experimental models suggesting an important role of reactive oxygen species in intestinal inflammation. This study clearly shows the effect of an antioxidant molecule in an experimental model of colitis which resembles human UC, indicating a potential clinical application for this class of compounds in treating IBD.
The protective effect shown by antioxidant therapy in this experimental model of colitis indicates a potential clinical application for this class of compounds in treating IBD. However, several clinical studies have been largely disappointing, probably due to the inability of the antioxidant to reach sufficient concentrations at the inflammation site. Lipophilic bis(1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl)decandioate (IAC) is easily distributed through cell membranes and intra-/extra-cellular compartments and directly reacts with oxidant molecules within the cell, where free radicals are produced. For clinical use, it may be necessary to protect IAC from low gastric pH using a specific formulation in order to improve its bioavailability.
The study is well done with adequate supporting documentation, controls and references. The difference in activity between the lipophilic and hydrophilic forms of IAC in suppressing dextran sodium sulphate-induced colitis is a novel and important finding.
Peer reviewers: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan; Dr. Christoph Reichel, Priv.-Doz., Head of the Gastroenterological Rehabilitation Center Bad Brückenau, Clinic Hartwald, German Pension Insurance Federal Office, Schlüchterner Str. 4, 97769 Bad Brückenau, Germany; Jay Pravda, MD, Inflammatory Disease Research Center, Gainesville, Florida, FL 32614-2181, United States
S- Editor Wang JL L- Editor Logan S E- Editor Zheng XM
| 1. | Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28-36. [Cited in This Article: ] |
| 2. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [Cited in This Article: ] |
| 3. | Naito Y, Takagi T, Yoshikawa T. Molecular fingerprints of neutrophil-dependent oxidative stress in inflammatory bowel disease. J Gastroenterol. 2007;42:787-798. [Cited in This Article: ] |
| 4. | Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015-2021. [Cited in This Article: ] |
| 5. | Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13:169-181. [Cited in This Article: ] |
| 6. | Simmonds NJ, Rampton DS. Inflammatory bowel disease--a radical view. Gut. 1993;34:865-868. [Cited in This Article: ] |
| 7. | Cuzzocrea S, McDonald MC, Mazzon E, Dugo L, Lepore V, Fonti MT, Ciccolo A, Terranova ML, Caputi AP, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol. 2000;406:127-137. [Cited in This Article: ] |
| 8. | Ishihara T, Tanaka K, Tasaka Y, Namba T, Suzuki J, Ishihara T, Okamoto S, Hibi T, Takenaga M, Igarashi R. Therapeutic effect of lecithinized superoxide dismutase against colitis. J Pharmacol Exp Ther. 2009;328:152-164. [Cited in This Article: ] |
| 9. | Vasina V, Broccoli M, Ursino MG, Bellot SF, Soleti A, Paolini M, De Ponti F. Effects of the non-peptidyl low molecular weight radical scavenger IAC in DNBS-induced colitis in rats. Eur J Pharmacol. 2009;614:137-145. [Cited in This Article: ] |
| 10. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. [Cited in This Article: ] |
| 11. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [Cited in This Article: ] |
| 12. | Kim HS, Berstad A. Experimental colitis in animal models. Scand J Gastroenterol. 1992;27:529-537. [Cited in This Article: ] |
| 13. | Valgimigli L, Pedulli GF, Paolini M. Measurement of oxidative stress by EPR radical-probe technique. Free Radic Biol Med. 2001;31:708-716. [Cited in This Article: ] |
| 14. | Verma-Gandhu M, Verdu EF, Bercik P, Blennerhassett PA, Al-Mutawaly N, Ghia JE, Collins SM. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56:358-364. [Cited in This Article: ] |
| 15. | Novelli M, D'Aleo V, Lupi R, Paolini M, Soleti A, Marchetti P, Masiello P. Reduction of oxidative stress by a new low-molecular-weight antioxidant improves metabolic alterations in a nonobese mouse diabetes model. Pancreas. 2007;35:e10-e17. [Cited in This Article: ] |
| 16. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [Cited in This Article: ] |
| 17. | Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240-248. [Cited in This Article: ] |
| 18. | Diaz-Granados N, Howe K, Lu J, McKay DM. Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am J Pathol. 2000;156:2169-2177. [Cited in This Article: ] |
| 19. | Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25:115-123. [Cited in This Article: ] |
| 20. | Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-7139. [Cited in This Article: ] |
| 21. | Kitahora T, Suzuki K, Asakura H, Yoshida T, Suematsu M, Watanabe M, Aiso S, Tsuchiya M. Active oxygen species generated by monocytes and polymorphonuclear cells in Crohn's disease. Dig Dis Sci. 1988;33:951-955. [Cited in This Article: ] |
| 22. | Damiani CR, Benetton CA, Stoffel C, Bardini KC, Cardoso VH, Di Giunta G, Pinho RA, Dal-Pizzol F, Streck EL. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol. 2007;22:1846-1851. [Cited in This Article: ] |
| 23. | Clapper ML, Adrian RH, Pfeiffer GR, Kido K, Everley L, Cooper HS, Murthy S. Depletion of colonic detoxication enzyme activity in mice with dextran sulphate sodium-induced colitis. Aliment Pharmacol Ther. 1999;13:389-396. [Cited in This Article: ] |
| 24. | Szarka CE, Pfeiffer GR, Hum ST, Everley LC, Balshem AM, Moore DF, Litwin S, Goosenberg EB, Frucht H, Engstrom PF. Glutathione S-transferase activity and glutathione S-transferase mu expression in subjects with risk for colorectal cancer. Cancer Res. 1995;55:2789-2793. [Cited in This Article: ] |
| 25. | Duncan H, Swan C, Green J, Jones P, Brannigan K, Alldersea J, Fryer AA, Strange RC. Susceptibility to ulcerative colitis and Crohn's disease: interactions between glutathione S-transferase GSTM1 and GSTT1 genotypes. Clin Chim Acta. 1995;240:53-61. [Cited in This Article: ] |
| 26. | Nieto N, Torres MI, Fernández MI, Girón MD, Ríos A, Suárez MD, Gil A. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. 2000;45:1820-1827. [Cited in This Article: ] |
| 27. | Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S-15S. [Cited in This Article: ] |
| 28. | Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859-861. [Cited in This Article: ] |
| 29. | Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Verspaget HW. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 2003;201:17-27. [Cited in This Article: ] |
| 30. | Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990;31:786-790. [Cited in This Article: ] |
| 31. | Seguí J, Gil F, Gironella M, Alvarez M, Gimeno M, Coronel P, Closa D, Piqué JM, Panés J. Down-regulation of endothelial adhesion molecules and leukocyte adhesion by treatment with superoxide dismutase is beneficial in chronic immune experimental colitis. Inflamm Bowel Dis. 2005;11:872-882. [Cited in This Article: ] |
| 32. | Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297-304. [Cited in This Article: ] |
| 33. | Kruidenier L, van Meeteren ME, Kuiper I, Jaarsma D, Lamers CB, Zijlstra FJ, Verspaget HW. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med. 2003;34:753-765. [Cited in This Article: ] |
| 34. | Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Riley DP, Salvemini D. Protective effects of M40403, a superoxide dismutase mimetic, in a rodent model of colitis. Eur J Pharmacol. 2001;432:79-89. [Cited in This Article: ] |
| 35. | Valgimigli L, Valgimigli M, Gaiani S, Pedulli GF, Bolondi L. Measurement of oxidative stress in human liver by EPR spin-probe technique. Free Radic Res. 2000;33:167-178. [Cited in This Article: ] |
| 36. | Nurmi A, Miettinen TK, Puoliväli J, Pussinen R, Soleti A, Bagate K, Riccardino F, Grundy RI, Yrjänheikki J, Canistro D. Neuroprotective properties of the non-peptidyl radical scavenger IAC in rats following transient focal cerebral ischemia. Brain Res. 2008;1207:174-181. [Cited in This Article: ] |
| 37. | D'Aleo V, Del Guerra S, Martano M, Bonamassa B, Canistro D, Soleti A, Valgimigli L, Paolini M, Filipponi F, Boggi U. The non-peptidyl low molecular weight radical scavenger IAC protects human pancreatic islets from lipotoxicity. Mol Cell Endocrinol. 2009;309:63-66. [Cited in This Article: ] |