Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2355
Revised: February 23, 2010
Accepted: March 2, 2010
Published online: May 21, 2010
AIM: To investigate the effects of butyrate on interleukin (IL)-32α expression in epithelial cell lines.
METHODS: The human intestinal epithelial cell lines HT-29, SW480, and T84 were used. Intracellular IL-32α was determined by Western blotting analyses. IL-32α mRNA expression was analyzed by real-time polymerase chain reaction.
RESULTS: Acetate and propionate had no effects on IL-32α mRNA expression. Butyrate significantly enhanced IL-32α expression in all cell lines. Butyrate also up-regulated IL-1β-induced IL-32α mRNA expression. Butyrate did not modulate the activation of phosphatidylinositol 3-kinase (PI3K), a mediator of IL-32α expression. Like butyrate, trichostatin A, a histone deacetylase inhibitor, also enhanced IL-1β-induced IL-32α mRNA expression.
CONCLUSION: Butyrate stimulated IL-32α expression in epithelial cell lines. An epigenetic mechanism, such as histone hyperacetylation, might be involved in the action of butyrate on IL-32α expression.
- Citation: Kobori A, Bamba S, Imaeda H, Ban H, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Butyrate stimulates IL-32α expression in human intestinal epithelial cell lines. World J Gastroenterol 2010; 16(19): 2355-2361
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2355
Interleukin (IL)-32 was originally reported as natural killer (NK) transcript 4, and has been described as a cytokine produced mainly by T-lymphocytes, NK cells, epithelial cells, and blood monocytes[1-3]. The gene encoding IL-32 is located on human chromosome 16p13.3 and is organized into eight exons[4]. There are four splice variants, and IL-32α is reported as the most abundant transcript[5,6]. Recently, IL-32 was defined as a proinflammatory cytokine characterized by the stimulation of secretion of IL-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-8 via the activation of p38 mitogen-activated protein kinases (MAPKs), nuclear factor (NF)-κB and activating protein (AP)-1 signal transduction pathways[3,7]. Recently, we reported the overexpression of IL-32α in the inflamed mucosa of inflammatory bowel disease (IBD)[8]. In vitro experiments on human intestinal epithelial cell lines showed that IL-32α expression was induced by IL-1β, IFN-γ and TNF-α through the activation of Akt-phosphatidylinositol 3-kinase (PI3K)[8].
Dietary fiber (nonstarch polysaccharides) and resistant starch escape digestion in the upper gastrointestinal tract, and undergo anaerobic bacterial fermentation in the colon. This process produces short-chain fatty acids (SCFAs), predominantly acetate, propionate, and butyrate, as the major by-products[9,10]. The typical concentration of each SCFA has been reported as approximately 10-20 mmol/L[11]. SCFAs have been shown to have significant effects on the colonic epithelium both in vivo and in vitro. Butyrate serves as the primary energy source for the normal colonic epithelium[12], and stimulates the growth of the colonic mucosa[13]. However, in tumor cell lines, it induces apoptosis and inhibits cell growth[14]. Butyrate has also been reported to modulate gene transcription through the induction of histone hyperacetylation. Since butyrate inhibits histone deacetylation and induces histone hyperacetylation, it is categorized as a histone deacetylase (HDAC) inhibitor[15].
In the present study, we investigated the in vitro effects of the SCFAs on IL-32α expression in human intestinal epithelial cell lines.
Recombinant human IL-1β and TNF-α were purchased from R&D Systems (Minneapolis, MN, USA), and human IFN-γ was obtained from Pepro Tech (Rocky Hill, NJ, USA). SCFAs (sodium acetate, sodium propionate and sodium butyrate) were purchased from Sigma Chemical Co. (Dorset, UK). Biotinylated anti-human IL-32α antibodies were purchased from R&D Systems, and horseradish peroxidase (HRP)-conjugated streptavidin was purchased from Dako Japan (Kyoto, Japan). Antibodies against phosphorylated and total Akt were obtained from Cell Signaling Technology (Beverly, MA, USA). Trichostatin-A was purchased from Tocris Cookson (St. Louis, MO, USA).
The human intestinal epithelial cell lines HT-29[16], SW480[17], and T84[18] were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured as described previously[16-18].
Total RNA was isolated by the acid guanidinium thiocyanate-phenol-chloroform method, and was then reverse-transcribed into cDNA using a PrimeScript RT reagent kit (TAKARA-BIO, Shiga, Japan). The expression of human IL-32α mRNA was assessed by real-time polymerase chain reaction (PCR) analyses. Real-time PCR was performed using a LightCycler 2.0 system (Roche Applied Science, Tokyo, Japan). The oligonucleotide primers used in this study were specific for human IL-32α as follows: 5'-AGCTG- GAGGACGACTTCAAA [nucleotides 192-211, GenBank accession no. BC018782[19,20] and 3'-AGGTGGTGTCAGTATCTTCA (nucleotides 642-623)]. The PCR was conducted using a SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). β-actin was used as an endogenous control to normalize for differences in the amount of total RNA in each sample.
The intracellular expression of IL-32α protein was determined by Western blotting. Briefly, the cells were washed and lysed in sodium dodecyl sulphate (SDS) sample buffer containing 100 μmol/L orthovanadate. The lysates were homogenized, and the protein concentration was measured by the Bradford method. For Western blotting, 10 μg of protein from each sample was subjected to SDS-PAGE on a 4%-20% gradient gel under reducing conditions. The proteins were then electrophoretically transferred onto a nitrocellulose membrane, and the membrane was blocked with 5% skimmed milk. After washing with PBS containing 0.1% Tween-20 (PBST), the membrane was incubated with a biotinylated anti-human IL-32α antibody at 4°C overnight. Next, the membrane was reacted with HRP-conjugated streptavidin at room temperature for 1 h. The detection was performed using enhanced chemiluminescence (ECL) Western blotting systems (Amersham Biosciences).
Histone extraction and the detection of global histone H3 acetylation were performed using the Epiquik global histone H3 acetylation assay kit (Epigentek; Brooklyn, NY, USA). The histone protein content was detected by the Bradford method.
Statistical significance of the differences was determined using unpaired t test (Statview version 4.5). Differences resulting in P values less than 0.05 were considered to be statistically significant.
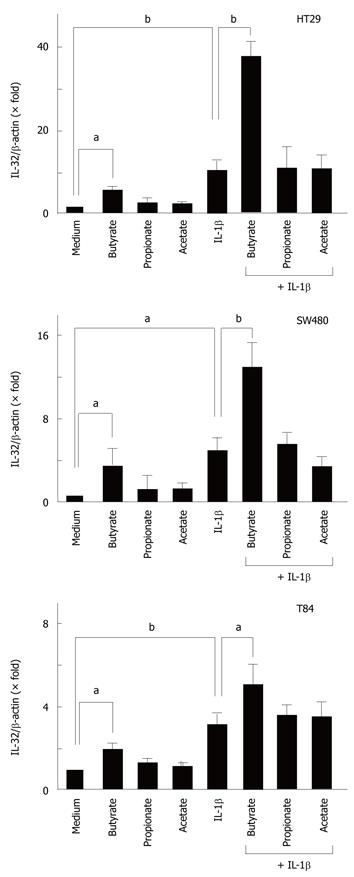
To evaluate the effects of SCFAs on IL-32α mRNA expression, intestinal epithelial cells (HT-29, Caco-2, T84 cells) were stimulated with 10 mmol/L of each SCFA (acetate, propionate, and butyrate) for 12 h, and then the expression of IL-32α mRNA was determined by real-time PCR. Acetate had no effect, but butyrate significantly increased IL-32α mRNA expression in all cell lines. Propionate increased the expression of IL-32α mRNA only in T84 cells, but in the other cell lines it had no effect (Figure 1).
Next, we investigated the effects of SCFAs on cytokine-induced IL-32α mRNA expression. We recently reported that IL-1β, IFN-γ and TNF-α stimulate IL-32α mRNA expression in intestinal epithelial cells[8], and the cells were stimulated with cytokines in the presence or absence of each SCFA (10 mmol/L). In all cell lines, butyrate by itself stimulated IL-32α mRNA expression, and enhanced the IL-1β-induced IL-32α mRNA expression. SCFAs did not modulate IFN-γ- and TNF-α-induced IL-32α mRNA expression in any of the cell lines (data not shown).
To confirm the effects of SCFAs on IL-32α protein expression, the cells were stimulated for 48 h with IL-1β and/or SCFAs. Intracellular IL-32α was detected as a 25 kDa protein[8,21]. The effects of the SCFAs were similar to those observed at the mRNA level. The combination of IL-1β and butyrate enhanced IL-32α protein expression additively (Figure 2).
To examine the effects of butyrate on IL-1β-induced IL-32α expression more precisely, HT-29 cells were incubated for 12 h with IL-1β (50 ng/mL) plus increasing concentrations of butyrate. The effects of butyrate on IL-1β-induced IL-32α secretion appeared at the concentration of 5.0 mmol/L, and the most significant difference was observed at 10 mmol/L (Figure 3).
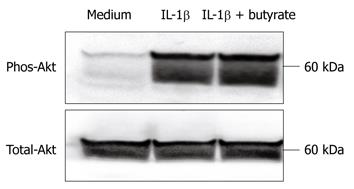
We tested the effects of butyrate on the IL-1β-induced phosphorylation of Akt, which plays a major role in IL-32α induction[8,19]. As shown in Figure 4, there were no effects of butyrate on IL-1β-induced Akt phosphorylation. These observations suggest that the modulation of Akt activity is not involved in the actions of butyrate on IL-1β-induced IL-32α expression.
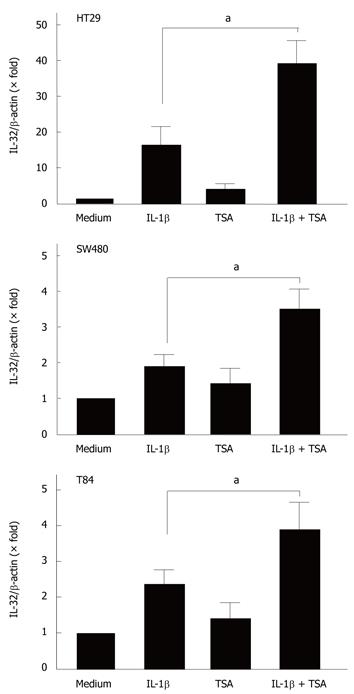
Previous studies have demonstrated that butyrate exerts its biological effects through the induction of histone hyperacetylation[15]. To examine the relationship between histone acetylation and the effects of butyrate on IL-32α mRNA expression, we tested the effects of trichostatin-A (TSA), a potent histone deacetylase inhibitor[22-24].
The cells were stimulated with cytokines in the presence or absence of TSA (5 μmol/L), and the expression of IL-32α mRNA was then analyzed by real-time PCR. As shown in Figure 5, TSA enhanced the IL-1β-induced IL-32α mRNA expression in all cell lines.
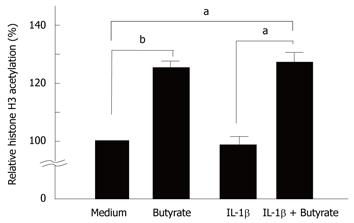
We tested the effects of butyrate and IL-1β on histone H3 hyperacetylation in HT-29 cells. As shown in Figure 6, butyrate induced histone H3 hyperacetylation in HT-29 cells (Figure 6).
Among SCFAs, the biological actions of butyrate have been well investigated. Butyrate is easily absorbed in the large intestine, and plays a role as the energy source for intestinal epithelial cells[13]. On the other hand, previous studies have demonstrated that butyrate blocks NF-κB activation in intestinal epithelial cells, and is thought to have an anti-inflammatory effect[25,26]. Based on this hypothesis, some clinical trials have been performed to test the therapeutic effects of butyrate enemas on ulcerative colitis lesions[27-29].
In this study, we examined the effects of SCFAs on IL-32α expression in intestinal epithelial cells. Initially, we hypothesized that SCFAs, in particular butyrate, might suppress IL-32α expression in these cells, since IL-32α expression has been reported to depend on NF-κB activation, and butyrate has been reported to suppress NF-κB activation. However, contrary to our initial expectation, butyrate stimulated IL-32α expression in these cells. This was characterized by two actions: butyrate by itself could stimulate IL-32α expression, and the synergistic effects of butyrate on cytokine-induced IL-32α expression was observed specifically in combination with IL-1β.
Recently, we reported that IL-32 expression mainly depends on the Akt-PI3K signaling pathway and NF-κB activation. As shown in Figure 6, butyrate did not affect Akt phosphorylation, indicating that the Akt-PI3K pathway was not involved in butyrate-mediated IL-32 expression. Furthermore, we have previously shown that butyrate inhibits IL-1β-induced NF-κB activation in HT-29 cells[26,30,31]. This suggests that the modulation of NF-κB activation is not involved in the effects of butyrate on IL-1β-induced IL-32 expression. This concept is supported by the finding that butyrate did not affect IL-32 expression by TNF-α, which is a potent stimulator of NF-κB activation[32,33]. Thus, the mode of action of butyrate on IL-32 expression in intestinal epithelial cells is mediated by different mechanisms from those reported previously.
It is generally accepted that butyrate leads to the development of reversible histone hyperacetylation via the inhibition of histone deacetylase activity[14,15]. Reversible histone hyperacetylation is now believed to play an important role in the regulation of chromatin structure[34,35]. Histone hyperacetylation leads to a more relaxed chromatin structure, and thus facilitates transcription factor access to the promoter regions of certain genes without directly initiating gene transcription[23,24]. Based on this knowledge, the mechanisms by which butyrate selectively induces the increase in IL-1β-induced IL-32 gene expression might be explained by an enhancement of transcription by histone hyperacetylation. This was supported by the effects of trichostatin A, a potent histone deacetylase inhibitor, on IL-32 expression in these cells. On the other hand, although the precise molecular mechanisms remain to be investigated, increased transcriptional activity by histone hyperacetylation might not account for the effects of TNF-α- and IFN-γ-on IL-32 expression. In order to clarify the precise mechanisms by which butyrate specifically stimulates IL-1β-stimulated IL-32 expression, further studies such as the effects of butyrate on signaling pathways to induce IL-32 expression are needed.
Clinical application of this study is limited because the role of IL-32α in inflammatory bowel disease (IBD) is still obscure. Netea et al[2] demonstrated recently that IL-32 augments the production of IL-1α, TNF-α, IL-6 and IL-8 by means of the nucleotide-binding oligomerization domain proteins (NOD1 and NOD2) through a caspase-1-dependent mechanism. NODs are a family of intracytoplasmic bacterial peptidoglycans which subsequently induce NF-κB activation. Mutations in NOD2 have been implicated in the pathogenesis of Crohn’s disease (CD). Recently, it has been shown that the NOD2 mutation in CD patients potentiates NF-κB activity and IL-1α processing. Thus, these findings suggest a pivotal role of IL-32 in the pathophysiology of IBD, and CD in particular. Our study also augments the proinflammatory aspect of butyrate.
In conclusion, we observed that butyrate stimulates IL-32α expression in intestinal epithelial cells. Since IL-32α is considered to be a proinflammatory cytokine, our finding might be a part of the proinflammatory picture of butyrate in mucosal inflammatory responses in the intestine. As the next step, the in vivo effects of butyrate on mucosal IL-32 expression should be investigated in the future.
Recently, interleukin (IL)-32 was defined as a proinflammatory cytokine characterized by the stimulation of secretion of IL-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-8. Dietary fiber (nonstarch polysaccharides) and resistant starch escape digestion in the upper gastrointestinal tract, and undergo anaerobic bacterial fermentation in the colon. This process produces short-chain fatty acids (SCFAs), predominantly acetate, propionate, and butyrate, as the major by-products.
The authors have previously reported that IL-32α is overexpressed in inflammatory bowel disease (IBD). IL-32α was overexpressed in the inflamed mucosa of IBD patients and was strongly induced by IL-1β, IFN-γ and TNF-α. However, the role of IL-32α is yet to be elucidated. In this study, they investigated the in vitro effects of butyrate on IL-32α expression in human intestinal epithelial cell lines.
The authors observed that butyrate stimulates IL-32α expression in intestinal epithelial cells. Since IL-32α is considered to be a proinflammatory cytokine, their finding might be a part of the proinflammatory picture of butyrate in mucosal inflammatory responses in the intestine.
Clinical application of this study is limited because the role of IL-32α in IBD is still obscure. A recent report demonstrated that IL-32 augments the production of IL-1α, TNF-α, IL-6 and IL-8 by means of the nucleotide-binding oligomerization domain proteins (NOD1 and NOD2) through a caspase-1-dependent mechanism. NODs are a family of intracytoplasmic bacterial peptidoglycans which subsequently induce NF-κB activation. Mutations in NOD2 have been implicated in the pathogenesis of Crohn’s disease (CD). Recently, it has been shown that the NOD2 mutation in CD patients potentiates NF-κB activity and IL-1α processing. Thus, these findings suggest a pivotal role of IL-32 in the pathophysiology of IBD, and CD in particular. This study also augments the proinflammatory aspect of butyrate.
IL-32 was originally reported as natural killer (NK) transcript 4, and has been described as a cytokine produced mainly by T-lymphocytes, NK cells, epithelial cells, and blood monocytes. The gene encoding IL-32 is located on human chromosome 16p13.3 and is organized into eight exons. There are four splice variants, and IL-32α is reported as the most abundant transcript. Recently, IL-32 was defined as a proinflammatory cytokine characterized by the stimulation of secretion of IL-1β, TNF-α, IL-6, and IL-8 via the activation of p38 mitogen-activated protein kinases, nuclear factor (NF)-κB and activating protein-1 signal transduction pathways. Recently, the authors reported the overexpression of IL-32α in the inflamed mucosa of IBD. In vitro experiments on human intestinal epithelial cell lines showed that IL-32α expression was induced by IL-1β, IFN-γ and TNF-α through the activation of Akt-phosphatidylinositol 3-kinase.
Kobori et al present an interesting study of a novel pro-inflammatory cytokine IL-32. They investigated the effects of butyrate on IL-32α expression in epithelial cell lines.
Peer reviewers: Giamila Fantuzzi, PhD, Associate Professor, Department of Kinesiology and Nutrition, University of Illinois at Chicago, 1919 W Taylor Street MC 517, Chicago, IL 60613, United States; Salvatore Auricchio, MD, PhD, Professor, Scientific Director of European Laboratory for the Investigation of Food-Induced Diseases, University Federico II, Via S. Pansini 5, I-80131 Naples, Italy; Dr. Ashraf Dahaba, MD, PhD, MSc, Professor, Department of Anaesthesiology, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, Yamamoto K. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. [Cited in This Article: ] |
| 2. | Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309-16314. [Cited in This Article: ] |
| 3. | Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131-142. [Cited in This Article: ] |
| 4. | Chen Q, Carroll HP, Gadina M. The newest interleukins: recent additions to the ever-growing cytokine family. Vitam Horm. 2006;74:207-228. [Cited in This Article: ] |
| 5. | Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci USA. 2006;103:3316-3321. [Cited in This Article: ] |
| 6. | Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65 Suppl 3:iii61-iii64. [Cited in This Article: ] |
| 7. | Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, Dinarello CA. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557-565. [Cited in This Article: ] |
| 8. | Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y, Andoh A. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480-486. [Cited in This Article: ] |
| 9. | Cummings JH, Macfarlane GT. Gastrointestinal effects of prebiotics. Br J Nutr. 2002;87 Suppl 2:S145-S151. [Cited in This Article: ] |
| 10. | Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763-779. [Cited in This Article: ] |
| 11. | Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031-1064. [Cited in This Article: ] |
| 12. | Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793-798. [Cited in This Article: ] |
| 13. | Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des. 2003;9:347-358. [Cited in This Article: ] |
| 14. | McBain JA, Eastman A, Nobel CS, Mueller GC. Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol. 1997;53:1357-1368. [Cited in This Article: ] |
| 15. | Monneret C. Histone deacetylase inhibitors. Eur J Med Chem. 2005;40:1-13. [Cited in This Article: ] |
| 16. | Zweibaum A, Pinto M, Chevalier G, Dussaulx E, Triadou N, Lacroix B, Haffen K, Brun JL, Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985;122:21-29. [Cited in This Article: ] |
| 17. | McInroy L, Määttä A. Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun. 2007;360:109-114. [Cited in This Article: ] |
| 18. | Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569-4574. [Cited in This Article: ] |
| 19. | Nishida A, Andoh A, Shioya M, Kim-Mitsuyama S, Takayanagi A, Fujiyama Y. Phosphatidylinositol 3-kinase/Akt signaling mediates interleukin-32alpha induction in human pancreatic periacinar myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2008;294:G831-G838. [Cited in This Article: ] |
| 20. | Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002;99:16899-16903. [Cited in This Article: ] |
| 21. | Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868-17876. [Cited in This Article: ] |
| 22. | Kawamura T, Andoh A, Nishida A, Shioya M, Yagi Y, Nishimura T, Hashimoto T, Tsujikawa T, Yasui H, Fujiyama Y. Inhibitory effects of short-chain fatty acids on matrix metalloproteinase secretion from human colonic subepithelial myofibroblasts. Dig Dis Sci. 2009;54:238-245. [Cited in This Article: ] |
| 23. | Rada-Iglesias A, Enroth S, Ameur A, Koch CM, Clelland GK, Respuela-Alonso P, Wilcox S, Dovey OM, Ellis PD, Langford CF. Butyrate mediates decrease of histone acetylation centered on transcription start sites and down-regulation of associated genes. Genome Res. 2007;17:708-719. [Cited in This Article: ] |
| 24. | McCue PA, Gubler ML, Sherman MI, Cohen BN. Sodium butyrate induces histone hyperacetylation and differentiation of murine embryonal carcinoma cells. J Cell Biol. 1984;98:602-608. [Cited in This Article: ] |
| 25. | Lührs H, Gerke T, Boxberger F, Backhaus K, Melcher R, Scheppach W, Menzel T. Butyrate inhibits interleukin-1-mediated nuclear factor-kappa B activation in human epithelial cells. Dig Dis Sci. 2001;46:1968-1973. [Cited in This Article: ] |
| 26. | Andoh A, Fujiyama Y, Hata K, Araki Y, Takaya H, Shimada M, Bamba T. Counter-regulatory effect of sodium butyrate on tumour necrosis factor-alpha (TNF-alpha)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. Clin Exp Immunol. 1999;118:23-29. [Cited in This Article: ] |
| 27. | Vernia P, Marcheggiano A, Caprilli R, Frieri G, Corrao G, Valpiani D, Di Paolo MC, Paoluzi P, Torsoli A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9:309-313. [Cited in This Article: ] |
| 28. | Vernia P, Cittadini M, Caprilli R, Torsoli A. Topical treatment of refractory distal ulcerative colitis with 5-ASA and sodium butyrate. Dig Dis Sci. 1995;40:305-307. [Cited in This Article: ] |
| 29. | Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51-56. [Cited in This Article: ] |
| 30. | Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. J Biol Chem. 2001;276:44641-44646. [Cited in This Article: ] |
| 31. | Andoh A, Fujiyama Y, Shimada M, Bamba T. Modulation of complement component (C3 and factor B) biosynthesis by a histone deacetylase inhibitor in human intestinal epithelial cells. Int J Mol Med. 2000;6:51-54. [Cited in This Article: ] |
| 32. | Erlejman AG, Jaggers G, Fraga CG, Oteiza PI. TNFalpha-induced NF-kappaB activation and cell oxidant production are modulated by hexameric procyanidins in Caco-2 cells. Arch Biochem Biophys. 2008;476:186-195. [Cited in This Article: ] |
| 33. | Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724-734. [Cited in This Article: ] |