Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1649
Revised: December 31, 2009
Accepted: January 7, 2010
Published online: April 7, 2010
AIM: To investigate the role of perioperative chemoradiotherapy (CRT) in the treatment of locally advanced thoracic esophageal squamous cell carcinoma (ESCC).
METHODS: Using preoperative computed tomography (CT)-based staging criteria, 238 patients with ESCC (stage II-III) were enrolled in this prospective study between January 1997 and June 2004. With informed consent, patients were randomized into 3 groups: preoperative CRT (80 cases), postoperative CRT (78 cases) and surgery alone (S) (80 cases). The 1-, 3-, 5- and 10-year survival were followed up. Progression-free survival (PFS) was chosen as the primary endpoint by treatment arm measured from study entry until documented progression of disease or death from any cause. The secondary endpoint was overall survival (OS) determined as the time (in months) between the date of therapy and the date of death. Other objectives were surgical and adjuvant therapy complications.
RESULTS: With median follow-up of 45 mo for all the enrolled patients, significant differences in the 1-, 3-, 5-, 10-year OS (91.3%, 63.5%, 43.5%, 24.5% vs 91%, 62.8%, 42.3%, 24.4% vs 87.5%, 51.3%, 33.8%, 12.5%, P = 0.0176) and PFS (89.3%, 61.3%, 37.5%, 18.1% vs 89.1%, 61.1%, 37.2%, 17.8% vs 84.5%, 49.3%, 25.9%, 6.2%, P = 0.0151) were detected among the 3 arms. There were no significant differences in OS and PFS between the preoperative CRT and postoperative CRT arm (P > 0.05). For the patients who had radical resection, significant differences in median PFS (48 mo vs 61 mo vs 39.5 mo, P = 0.0331) and median OS (56.5 mo vs 72 mo vs 41.5 mo, P = 0.0153) were detected among the 3 arms, but there were no significant differences in OS and PFS between the preoperative CRT and postoperative CRT arm (P > 0.05). The local recurrence rates in the preoperative CRT, postoperative CRT group and S group were 11.3%, 14.1% and 35%, respectively (P < 0.05). No significant differences were detected among the 3 groups when comparing complications but tended to be in favor of the postoperative CRT and S groups (P > 0.05). Toxicities of CRT in the preoperative or postoperative CRT arms were mostly moderate, and could be quickly alleviated by adequate therapy.
CONCLUSION: Rational application of preoperative or postoperative CRT can provide a benefit in PFS and OS in patients with locally advanced ESCC.
- Citation: Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010; 16(13): 1649-1654
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1649
Esophageal squamous cell carcinoma (ESCC) is one of the common malignancies and the seventh leading cause of cancer-related deaths in the world[1]. The 5-year survival rate is only 15%-20% in patients with locally advanced esophageal cancer[2]. Treatment failure mainly results from recurrence or metastasis. A standardized comprehensive treatment is still under development. Radiotherapy can control local-regional esophageal cancer and chemotherapy has both local and systemic antineoplastic activity. With increasing enthusiasm for multidisciplinary treatment modalities to improve outcome, a commonly employed treatment approach for esophageal cancer is chemoradiotherapy (CRT) in addition to surgical resection[3]. Preoperative CRT had been applied to patients with esophageal carcinoma in an effort to reduce the relapse rate and improve survival. Many studies have demonstrated the effectiveness of neoadjuvant CRT[4-9]. In contrast to data available on preoperative CRT, although a number of trials compared adjuvant chemotherapy or radiotherapy with surgery alone, there are almost no randomized trials comparing adjuvant CRT with surgery alone. The only exception is a trial that compared surgery alone with surgery and adjuvant CRT for patients with resectable cancers of the stomach and gastroesophageal junction[10]. Although the results suggest that resection followed by concurrent CRT should be considered for patients with adenocarcinoma of the stomach and the gastroesophageal junction, the question remains whether postoperative CRT can improve overall survival in patients with ESCC.
To our knowledge, as yet no study is available to determine the best sequencing of CRT and surgery. To resolve this deficiency, the present prospective study was conducted in our center from January 1997 to June 2004.
Two hundred and seventy one patients with ESCC were diagnosed by endoscopic biopsy and histopathology between January 1997 and June 2004 in our center. According to the preoperative computed tomography (CT) staging criteria, 238 patients with stage II-III thoracic ESCC were enrolled in this prospective study. The CT staging criteria were as follows: Stage I, the tumor was limited to the esophageal lumen or the thickness of the esophageal wall varied between 3 and 5 mm; Stage II, the thickness exceeded 5 mm but there was no invasion of the mediastinum or distant metastasis; Stage III, the tumor invaded the adjacent mediastinal structure; and Stage IV, there was distant metastasis. All patients gave informed consent prior to their inclusion in the study. The form had been reviewed by the appropriate ethics committee and had been developed and was administered in accordance with the ethical standards laid down in an appropriate version of the 1964 Declaration of Helsinki. The enrolled patients were randomized into 3 groups: preoperative CRT, postoperative CRT, and surgery alone (S). The randomization method was based on random numerals produced by computer. Characteristics of the preoperative patients are shown in Table 1.
| Pre-CRT (n = 80) | Post-CRT (n = 78) | S (n = 80) | Statistics | ||
| χ2 | P | ||||
| Sex (M:F) | 52:28 | 48:30 | 50:30 | 3.1326 | 0.209 |
| Age (yr) | 1.2375 | 0.975 | |||
| 40- | 12 | 13 | 11 | ||
| 50- | 24 | 25 | 24 | ||
| 60- | 28 | 29 | 30 | ||
| 70- | 16 | 11 | 15 | ||
| Location | 0.1920 | 0.996 | |||
| Upper | 11 | 10 | 12 | ||
| Middle | 45 | 45 | 44 | ||
| Lower | 24 | 23 | 24 | ||
| CT staging | 0.1165 | 0.943 | |||
| II | 35 | 33 | 36 | ||
| III | 45 | 45 | 44 | ||
The surgical procedure used in this study was either a radical resection, which involved an esophagectomy through a left or right thoracotomy with 2-field lymphadenectomy, or palliative resection or esophageal bypass. All the patients who underwent palliative resection or esophageal bypass had taken traditional Chinese medicine themselves.
For patients in the preoperative arm, surgical resection was to be performed 4-6 wk after induction of CRT. The patients treated with postoperative CRT underwent surgery and received CRT 4-6 wk later. For patients treated with preoperative CRT, radiation was delivered in a total dose of 40 Gy (20 fractions at 2 Gy per fraction) in anteroposterior fields including esophageal tumors and enlarged lymph nodes, with a 4-5 cm proximal and distal margin and a 1-2 cm radial margin. For 30 out of 78 patients treated with postoperative CRT, radiation was delivered in daily fractions of 2 Gy to a total dose of 40 Gy over 4 wk by using the same double field technique as the preoperative CRT group, and the anteroposterior fields of the following 48 patients were extended from the sixth cervical vertebrae to the first lumbar vertebrae, including the origin of esophagus and lymph drainage that encompassed the supraclavicular regions and left gastric lymph nodes. Then a 10 Gy boost was delivered through parallel opposed lateral or oblique portals for limitation of spinal cord radiation dose. Radiotherapy was carried out by linear accelerators with 6 MV photons; treatment ports were designed to include enlarged regional nodes based on CT evaluation and endoscopic ultrasound.
For chemotherapy, 2 cycles were administered on days 1-3 and days 22-24 of radiotherapy. A paclitaxel (PTX) + cisplatin (DDP) regimen was used, including PTX (135 mg/m2 per day) administered as a short-term infusion on day 1 of each cycle, while DDP (20 mg/m2 per day) was delivered as a continuous infusion over 24 h on days 1-3 of each cycle. The patients received the antiemetics granisetron and metoclopramide before and after the cisplatin infusion. The dose of chemotherapy in the second cycle was adjusted according to hematological toxicities.
Any perioperative complications were observed and recorded. During treatment, the patients were monitored weekly with a physical examination and blood chemistry evaluation. Clinical evaluation was carried out by endoscopy, endoscopic ultrasonography, and CT scans. All results of the examination were collected and evaluated by the oncology experts. The 1-, 3-, 5- and 10-year survival were followed up.
Clinical follow-up after completion of treatment was based on periodic visits (every 3 mo during the first 2 years, every 6 mo after 2 years). The follow-up time for survivors ranged from 5 to 124 mo (median 45 mo).
STATA 10.0 for Windows (StataCorp, College Station, Texas 77845, USA) was used for statistical analysis. The differences in age group, sex, tumor location, and tumor staging, complications, and cause of death among groups were compared by the χ2 test or Fisher’s Exact test. The survival among groups were described by Kaplan-Meier curves and analyzed by the log-rank test. P values less than 0.05 were considered statistically significant. Progression free survival (PFS) was chosen as the primary endpoint by treatment arm measured from study entry until documented progression of disease or death from any cause. The secondary endpoint was overall survival (OS) determined as the time (in months) between the date of therapy and the date of death. Other factors determined were surgical and adjuvant therapy complications.
By June 30 2009, 228 out of all cases were followed up by means of telephone or outpatient service, and 10 cases were lost. There were 3.8% treatment-related deaths in preoperative CRT but no treatment-related deaths in the postoperative CRT and S group, and 51.3% of patients died from the local tumor recurrence and distant metastasis.
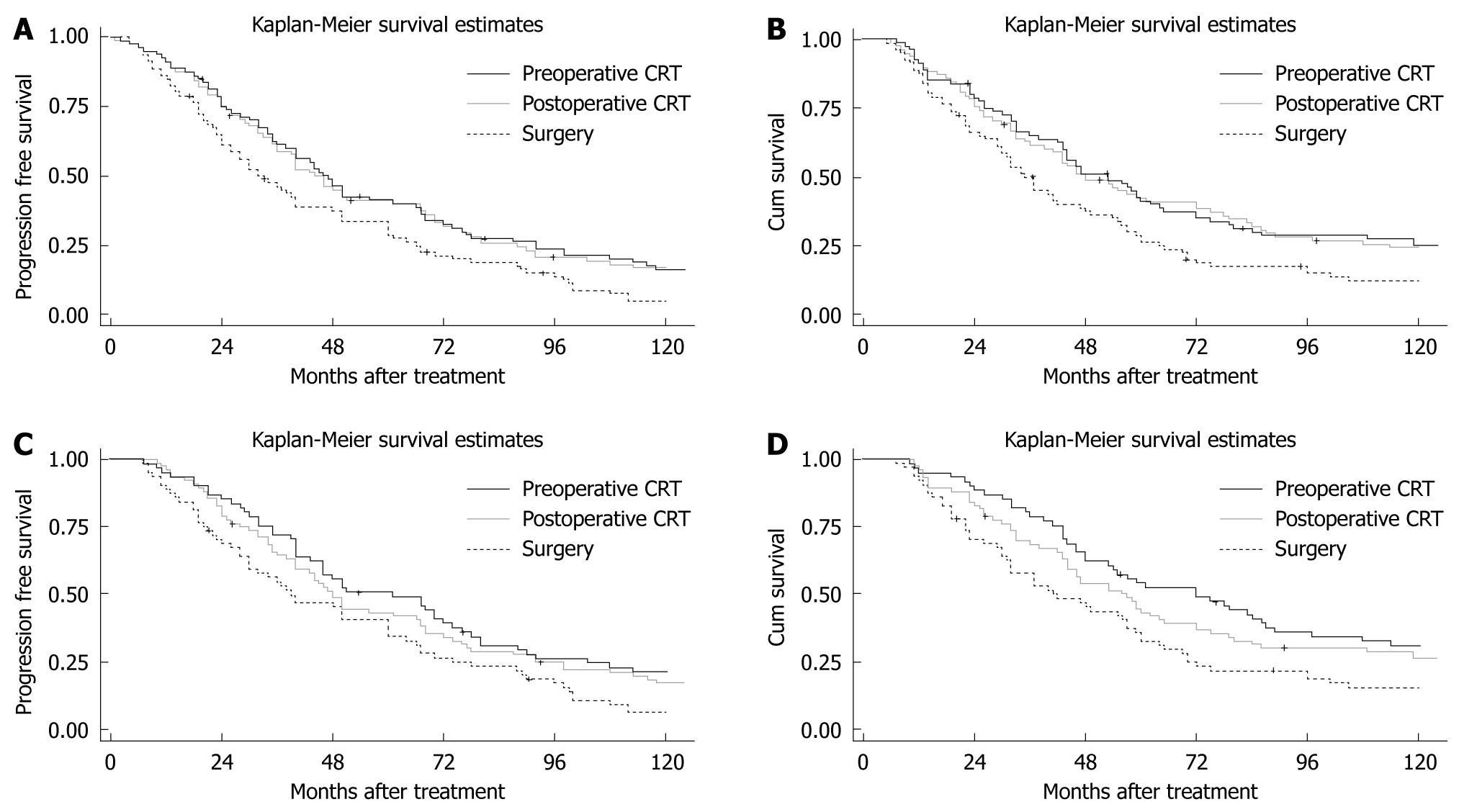
Median PFS was 46.5 mo (95% CI: 37.4-66.3) in the preoperative CRT group, 45 mo (95% CI: 35.8-67) in the postoperative CRT group, and 32.5 mo (95% CI: 25.4-42.3) in the S group. The 1-year PFS in preoperative CRT, postoperative CRT and S groups was 89.3%, 89.1% and 84.5%, respectively, with no significant differences (χ2 = 0.64, P = 0.4123). However, the 3-, 5- and 10-year PFS of the preoperative CRT (61.3%, 37.5%, 18.1%, respectively) and postoperative CRT groups (61.1%, 37.2%, 17.8%, respectively) were significantly different (χ2 = 4.16, P = 0.0319; χ2 = 4.14, P = 0.0321; χ2 = 5.38, P = 0.0203; respectively) from those of the S group (49.3%, 25.9%, 6.2%, respectively). There was no significant difference in PFS between the neoadjuvant and adjuvant therapy groups (χ2 = 0.14, P = 0.7060) (Figure 1A). Median OS was 53 mo (95% CI: 43-64.3) in the preoperative CRT group, 48 mo (95% CI: 38.5-72.5) in the postoperative CRT group, and 36 mo (95% CI: 29.7-47.3) in the S group. The 1-year OS in the preoperative CRT, postoperative CRT and S groups was 91.3%, 91% and 87.5%, respectively, which were not significantly different (χ2 = 0.72, P = 0.3970). However, the 3-, 5- and 10-year survival rates of the preoperative CRT (63.5%, 43.5%, 24.5%, respectively) and postoperative CRT groups (62.8%, 42.3%, 24.4%, respectively) were significantly different (χ2 = 3.98, P = 0.0453; χ2 = 4.76, P = 0.0402; χ2 = 4.27, P = 0.0389; respectively) from those of the S group (51.3%, 33.8%, 12.5%, respectively). There was no significant difference in OS between the neoadjuvant and adjuvant therapy groups (χ2 = 0.46, P = 0.4978) (Figure 1B).
For the patients who experienced radical resection, median PFS of the preoperative CRT group, postoperative CRT group and S group was 48 mo (95% CI: 40-67.0), 61 mo (95% CI: 44.0-75.4), and 39.5 mo (95% CI: 30-60), respectively. A significant difference in PFS was detected among the 3 arms (χ2 = 6.82, P = 0.0331), however, there was no significant difference in PFS between neoadjuvant and adjuvant therapy groups (χ2 = 0.22, P = 0.6416) (Figure 1C). There was a significant difference in OS among the 3 arms (χ2 = 8.36, P = 0.0153). The median OS of the preoperative CRT group, postoperative CRT group and S group was 56.5 mo (95% CI: 44-72), 72 mo (95% CI: 49.6-88.4), and 41.5 mo (95% CI: 31.6-57.7), respectively. However, there was no significant difference in OS between neoadjuvant and adjuvant therapy groups (χ2 = 0.16, P = 0.6873) (Figure 1D).
The local recurrence rates of the preoperative CRT group, postoperative CRT group and S group were 11.3%, 14.1% and 35% respectively, and showed a statistically significant difference (P = 0.011). There was no significant difference among the 3 groups when comparing perioperative complications but there was a tendency in favor of the postoperative CRT and S groups (P = 0.179).
For the 158 patients receiving CRT, incidence of grade 3 or greater leucopenia, thrombocytopenia, anemia and vomiting were 11.4% (18/158), 6.3% (10/158), 1.3% (2/158) and 6.3% (10/158), respectively. Postoperative pathologic staging and perioperative complications, and the causes of death are presented in Table 2.
| Pre-CRT (n = 80) | Post-CRT (n = 78) | S (n = 80) | n = 238 | Statistics | ||
| χ2 | P | |||||
| Resection | - | 0.0111 | ||||
| R | 76 (97.4) | 61 (78.2) | 64 (80) | 201 (84.5) | ||
| P | 4 (2.6) | 13 (16.7) | 13 (16.3) | 30 (12.6) | ||
| EB | 0 (0) | 4 (5.1) | 3 (3.8) | 7 (2.9) | ||
| Stage | - | 0.0001 | ||||
| I | 4 (2.6) | 0 (0) | 0 (0) | 4 (1.7) | ||
| IIa | 22 (27.5) | 13 (16.7) | 12 (15.4) | 47 (19.7) | ||
| IIb | 29 (36.3) | 17 (21.8) | 19 (23.8) | 65 (27.3) | ||
| III | 25 (31.3) | 48 (61.5) | 49 (61.3) | 122 (51.3) | ||
| Complications | - | 0.1791 | ||||
| Hemorrhage during | ||||||
| Surgery (> 300 mL) | 8 (10.0) | 2 (2.6) | 2 (2.5) | 12 (5.0) | ||
| Stomal leakage | 1 (1.3) | 0 (0) | 0 (0) | 1 (0.4) | ||
| Stomal stricture | 2 (2.5) | 3 (3.8) | 1 (1.3) | 6 (2.5) | ||
| Reflux esophagitis | 13 (16.3) | 13 (16.7) | 15 (18.8) | 41 (17.2) | ||
| Acute lung injury | 3 (3.8) | 0 (0) | 0 (0) | 3 (1.3) | ||
| Death | - | 0.0111 | ||||
| Local recurrence | 9 (11.3) | 11 (14.1) | 28 (35) | 48 (20.2) | ||
| Distant metastasis | 20 (25) | 23 (29.5) | 31 (38.8) | 74 (31.1) | ||
| Treatment related | 3 (3.8) | 0 (0) | 0 (0) | 3 (1.3) | ||
| Not related | 1 (1.3) | 5 (6.4) | 1 (1.3) | 7 (2.9) | ||
Patients with resectable ESCC should receive multimodal treatment to prolong OS[11,12]. There is still controversy about how to improve prognosis and how to reduce local recurrence and distant metastasis. Both chemotherapy and radiotherapy may be active against different tumor cell populations, the chemotherapy may be effective against micrometastases while radiation counteracts spatial metastases. In the Western world, preoperative chemotherapy and CRT can increase OS by 4.4% and 6.4%, respectively, however, treatment-related mortality increases by 1.7% with neoadjuvant chemotherapy and by 3.4% with CRT, compared with surgery alone[13]. There were a few nonrandomized trials on postoperative CRT in treating esophageal carcinoma[14-19], however, the results had discrepancies. Regretfully, the most optimal sequence of CRT in relation to surgical resection is unclear. Thus our study is a unique randomized controlled study to evaluate the outcome of preoperative CRT in patients with local advanced thoracic ESCC, and it includes a long-term follow-up.
The present study showed a benefit in PFS and OS with neoadjuvant or adjuvant therapy. Meanwhile, our study indicated that preoperative CRT reduced the rate of local recurrence, and the survival benefit resulted from improved local cancer control brought about by the adjuvant arm. Although there was no significant difference found in 1-year PFS and OS, both preoperative CRT and postoperative CRT showed a significant advantage in longtime PFS and OS. A meta-analysis had suggested that preoperative CRT may improve survival and locoregional control but was associated with higher toxicity and increased mortality[3]. Our results also confirm this point. Rice and coworkers found that 31 patients treated with postoperative adjuvant CRT had improved survival, in a retrospective review[20]. Together with our present study, it further confirms that postoperative CRT may be an alternative option especially for locally advanced thoracic ESCC. Moreover, the results indicated that postoperative CRT may have almost the same long-term of efficacy as preoperative CRT, although the latter showed a nonsignificant trend of higher survival compared to the former.
To throw further light on the benefit in PFS and OS with neoadjuvant or adjuvant therapy for ESCC, we subsequently analyzed the data in patients who had undergone radical resection. The results also showed the same advantages as the above when including the palliative resection and esophageal bypass patients. The results also showed no significant difference in survival rates when comparing the preoperative CRT and postoperative CRT arms. It further clarifies that adjuvant CRT can provide almost the same long-term of efficacy as neoadjuvant CRT.
In addition, there was no suggestion that treatment-related mortality was increased by the use of postoperative CRT, which was accomplished with manageable toxicity. Most patients had less than grade 2 hematological toxicities. However, our results showed an increase in postoperative deaths and a trend for relatively higher perioperative complications in the neoadjuvant CRT arm. The involved reasons could be as follows: surgeons may undertake a challenging esophagectomy resulting in surgical difficulty and postoperative complications when performed after neoadjuvant CRT. For example, radiation might contribute to the failure of an anastomotic leak and postoperative acute lung injury. The results also showed that preoperative CRT can facilitate complete resection by downstaging tumors when compared to postoperative CRT and surgery alone. Thus, whether or not the survival benefit of neoadjuvant CRT can be negated by an increase in postoperative deaths should of concern. In addition, loss to follow-up was low (4.2%), with only 3 patients receiving preoperative CRT and 3 patients receiving postoperative CRT and 4 patients receiving surgery alone, thus our study is robust.
In conclusion, long-term survival is maximized by the use of CRT followed by surgery for locally advanced esophageal cancer. However, patients are more likely to develop toxicity. As therapies improve, it is likely that the toxicity may be reduced and neoadjuvant CRT may provide a more marked benefit in esophageal cancer. Meanwhile, postoperative CRT can also be safely administered and considered as the multimodal treatment of choice for locally advanced ESCC. In appropriately selected patients, either pre- or postoperative CRT is a viable strategy. Further comparison of pre- and postoperative CRT in treating esophageal cancer is required for verification through multicenter and large sample randomized clinical trials.
Esophagectomy is a standard treatment for resectable esophageal carcinoma but relatively few patients are cured. Combined neoadjuvant chemoradiotherapy (CRT) or adjuvant CRT with surgery may improve survival but there is concern about treatment morbidity and the best sequencing of CRT and surgery.
This study investigated the overall survival and profession-free survival data (up to 10-year survival).
Some studies have demonstrated the effectiveness of neoadjuvant CRT and there are almost no randomized trials comparing adjuvant CRT with surgery alone. As yet no study is available to determine the best sequencing of CRT and surgery. The most optimal sequence of CRT in relation to surgical resection is unclear. This study was a unique randomized controlled study to evaluate the outcome of preoperative and postoperative CRT in patients with local advanced thoracic esophageal squamous cell carcinoma, and it includes a long-term follow-up.
The authors present the results of a randomized prospective study which examined the efficacy and safety of 3 treatment regimens in esophageal squamous cell carcinoma stage II and III. The study is of interest to readers of the journal. The discussion is satisfactory and is limited to the topic. Tables depicted essential data and are well constructed.
Peer reviewers: Marco Giuseppe Patti, MD, Professor of Surgery, Director, Center for Esophageal Diseases, University of Chicago Pritzker School of Medicine, 5841 S. Maryland Avenue, MC 5095, Room G 201, Chicago, IL 60637, United States; Luis Grande, Professor, Department of Surgery, Hospital del Mar, Passeig Marítim 25-29, Barcelona 08003, Spain
S- Editor Wang JL L- Editor Cant MR E- Editor Lin YP
| 1. | Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:477-481. [Cited in This Article: ] |
| 2. | Graham AJ, Shrive FM, Ghali WA, Manns BJ, Grondin SC, Finley RJ, Clifton J. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg. 2007;83:1257-1264. [Cited in This Article: ] |
| 3. | Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol. 2009;15:4962-4968. [Cited in This Article: ] |
| 4. | Ruol A, Portale G, Castoro C, Merigliano S, Cagol M, Cavallin F, Chiarion Sileni V, Corti L, Rampado S, Costantini M. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2007;14:3243-3250. [Cited in This Article: ] |
| 5. | Zemanova M, Petruzelka L, Pazdro A, Kralova D, Smejkal M, Pazdrova G, Honova H. Prospective non-randomized study of preoperative concurrent platinum plus 5-fluorouracil-based chemoradiotherapy with or without paclitaxel in esophageal cancer patients: long-term follow-up. Dis Esophagus. 2009;Epub ahead of print. [Cited in This Article: ] |
| 6. | Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Yokomakura N, Ishigami S, Owaki T, Aikou T. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus. 2006;19:468-472. [Cited in This Article: ] |
| 7. | Greer SE, Goodney PP, Sutton JE, Birkmeyer JD. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137:172-177. [Cited in This Article: ] |
| 8. | Dixit S, Tilston M, Peter WM. Risk stratification for recurrence in patients with esophageal and junctional carcinoma treated with neoadjuvant chemotherapy and surgery. Med Oncol. 2009;Epub ahead of print. [Cited in This Article: ] |
| 9. | Bonnetain F, Bouché O, Michel P, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Paillot B, Arveux P. A comparative longitudinal quality of life study using the Spitzer quality of life index in a randomized multicenter phase III trial (FFCD 9102): chemoradiation followed by surgery compared with chemoradiation alone in locally advanced squamous resectable thoracic esophageal cancer. Ann Oncol. 2006;17:827-834. [Cited in This Article: ] |
| 10. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [Cited in This Article: ] |
| 11. | Di Fiore F, Lecleire S, Rigal O, Galais MP, Ben Soussan E, David I, Paillot B, Jacob JH, Michel P. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185-4190. [Cited in This Article: ] |
| 12. | Triboulet JP, Mariette C. [Oesophageal squamous cell carcinoma stade III. State of surgery after radiochemotherapy (RCT)]. Cancer Radiother. 2006;10:456-461. [Cited in This Article: ] |
| 13. | Iyer R, Wilkinson N, Demmy T, Javle M. Controversies in the multimodality management of locally advanced esophageal cancer: evidence-based review of surgery alone and combined-modality therapy. Ann Surg Oncol. 2004;11:665-673. [Cited in This Article: ] |
| 14. | Bédard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423-2430. [Cited in This Article: ] |
| 15. | Kurtzman SM, Whittington R, Vaughn D, Rosato EF, Haller DG. Post-operative chemosensitized radiation with modulated 5-fluorouracil (5-FU) following resection of adenocarcinoma of the esophagus and esophagogastric (EG) junction. Int J Radiat Oncol Biol Phys. 1995;32:266. [Cited in This Article: ] |
| 16. | Kang HJ, Ebie N, Murthy AK, Galinsky DL, Tsekeris P, Griem K. Surgery followed by concomitant accel¬erated fractionation irradiation, cisplatin, and 5-FU for esophageal carcinoma. Proc Am Soc Clin Oncol. 1992;11:167. [Cited in This Article: ] |
| 17. | Ebie N, Kang HJ, Millikan K, Murthy AK, Griem K, Hartsell W, Recine DC, Doolas A, Taylor S 4th. Integration of surgery in multimodality therapy for esophageal cancer. Am J Clin Oncol. 1997;20:11-15. [Cited in This Article: ] |
| 18. | Taylor SG, Bonomi PD, Kiel KD, Slayton RE, Wolter J. Failure of simultaneous cisplatin/5FU infusion chemotherapy and radiation to improve control of esophageal cancer. Proc Am Soc Clin Oncol. 1986;5:88. [Cited in This Article: ] |
| 19. | Saito T, Shigemitsu Y, Kinoshita T, Shimoda K, Abe T, Nakamura A, Chikuba K, Kobayashi M. Cisplatin, vindesine, pepleomycin and concurrent radiation therapy following esophagectomy with lymph adenectomy for patients with an esophageal carcinoma. Oncology. 1993;50:293-297. [Cited in This Article: ] |
| 20. | Rice TW, Adelstein DJ, Chidel MA, Rybicki LA, DeCamp MM, Murthy SC, Blackstone EH. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590-1596. [Cited in This Article: ] |