Published online Mar 28, 2010. doi: 10.3748/wjg.v16.i12.1473
Revised: January 12, 2010
Accepted: January 19, 2010
Published online: March 28, 2010
AIM: To investigate whether Melatonin has synergistic effects with Doxorubicin in the growth-inhibition and apoptosis-induction of human hepatoma cell lines HepG2 and Bel-7402.
METHODS: The synergism of Melatonin and Doxorubicin inhibited the cell growth and induced cell apoptosis in human hepatoma cell lines HepG2 and Bel-7402. Cell viability was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Cell apoptosis was evaluated using TUNEL method and flow cytometry. Apoptosis-related protein Bax, Bcl-2 and caspase-3 expressions were measured by immunohistochemical staining.
RESULTS: Treatment with Melatonin (10-8-10-5 mol/L) alone had a dose-related inhibitory effect on cell proliferation but no cytotoxic effect on hepatoma cell lines HepG2 and Bel-7402. Interestingly, when combined with Doxorubicin, Melatonin significantly increased the effects of cell growth inhibition and cell apoptosis. Furthermore, TUNEL staining and flow cytometry revealed that cooperative apoptosis induction was associated with decreased expression of Bcl-2 as well as increased expression of Bax and Caspase3.
CONCLUSION: The synergism of Melatonin and Doxorubicin inhibits hepatoma cell growth and induces cell apoptosis.
- Citation: Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu WZ, Wang H. Melatonin and Doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol 2010; 16(12): 1473-1481
- URL: https://www.wjgnet.com/1007-9327/full/v16/i12/1473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i12.1473
Melatonin, a chief secretary product of the pineal gland, has diverse physiological functions. It plays a crucial role in regulating circadian rhythm, and is involved in immunomodulation, hematopoiesis, and antioxidative processes[1-5]. A large number of studies have demonstrated that Melatonin has important oncostatic properties[6,7]. It inhibits cell proliferation in several cancer cell lines including human B-lymphoma cells[8], human myeloid leukemia cells HL-60[9] and human neuroblastoma cancer cells[10]. Melatonin decreased the growth rates of tumors in vivo both in transplantable animal model and the animal model induced by the administration of carcinogens[11,12].
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the worldwide, and more than half a million new cases occur annually[13]. In some areas of Asia and the Middle East, HCC ranks as the first cause of death from cancer. The incidence of HCC is also increasing in Europe and the United States[14]. Chemotherapy is one of the common strategies in HCC treatment, especially for unresectable tumors. Conventional chemotherapeutic drugs such as Doxorubicin often have severe side effects that limit their efficacy. Combined therapy with multiple drugs or modalities is a common practice in the treatment of cancer, which can achieve better therapeutic effects than a single drug or modality, and can reduce the side effects and resistance to drugs as well. Thus it is imperative to develop new agents which can help enhance the drug effectiveness as well as reduce the toxicity.
The present study was designed to investigate the growth-inhibiting and apoptosis-inducing effects of Melatonin alone or combined with Doxorubicin in order to develop a new adjuvant therapy for HCC.
Human hepatoma cells HepG2 and Bel-7402 were purchased from Shanghai cell bank, Chinese Academy of Sciences, cultured in Dulbecco’s modified Eagles’s medium and RPMI-1640 medium respectively. Each was supplemented with 10% (v/v) heat-inactivated fetal bovine serum and antibiotics and incubated at 37°C in a humid atmosphere with 50 mL/L CO2.
The Doxorubicin injection was purchased from Shenzhen Main Luck Pharmceuticals Inc. (Shenzhen, China, CatNO0407E1, 10 mg/ampoule); Melatonin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT) and Rnase A was from Sigma (St Louis, MO, USA). DMEM and RPMI-1640 medium was from Gibco BRL Life Technologies Inc. (Grand Island, New York, USA); Rabbit polyclonal antibodies against human Bcl-2, Bax and caspase-3 were all purchased from Lab Vision Corporation (Fremont, California, USA) and the streptavidin-biotin-peroxidase (S-P) reagents kit was obtained from Fuzhou Maxim Biotech, Ltd. (Fuzhou, China). Cell Apoptosis PI Detection Kit was provided by Nanjing KeyGen Biotech Co. Ltd. (Nanjing, China). DeadEndTM Colorimetric TUNEL System was from Promega Biotech Co. Ltd. (Madison, WI, USA).
Cytotoxicity was measured using MTT assay. HepG2 and Bel-7402 cells in exponentially growth phase were cultured at a density of 1 × 104 cells/well in a 96-well plate. After treatment with various concentrations of drug for 48 h, MTT solution (5.0 mg/mL in phosphate-buffered saline) was added (20.0 μL/well), and the plates were incubated for another 4 h at 37°C. The purple formazan crystals were dissolved in 150.0 μL Dimethyl Sulfoxide DMSO per well. After 10 min, the plates were read on microplate reader (American Bio-Tek) at 490 nm. The cells without drugs were used as control. Assays were performed in three independent experiments. The percentage of cytotoxicity was calculated as follows: Cytotoxicity (%) = (1-A490 of experimental well)/A490 of control well. The median inhibitory concentration (IC50) (defined as the drug concentration at which cell growth was inhibited by 50%) was assessed from the dose-response curves.
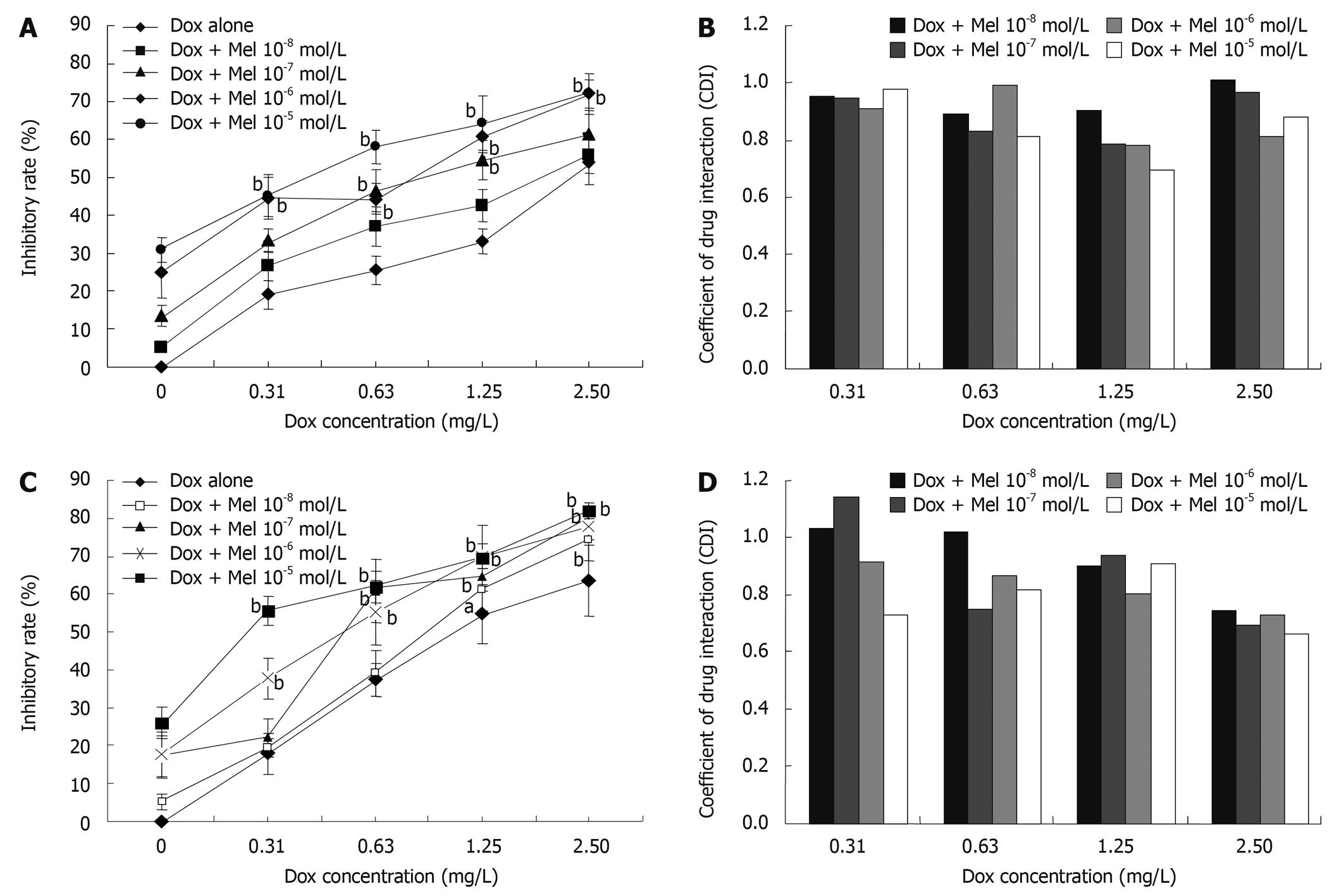
The coefficient of drug interaction (CDI) was used to analyze the synergistical inhibitory effect of drug combination. CDI is calculated as follows: CDI = AB/(A × B). According to the absorbance of each group, AB is the ratio of the combination groups to control group in A490; A or B is the ratio of the single agent groups to control group in A490. Thus CDI value less than, equal to or greater than 1 indicates that the drugs are synergistic, additive or antagonistic, respectively. CDI less than 0.7 indicates that the drugs are significantly synergistic.
The cells were cultured in 6-well plates containing cover slips overnight. After treatment with various concentrations of Melatonin or Doxorubicin or in combination for 48 h, the cover slips were washed twice with PBS and fixed in 4% paraformaldehyde solution for 25 min at room temperature. Apoptotic cells were detected by terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling (TUNEL) assay using DeadEnd™ Colorimetric TUNEL System Kit from Promega (Madison, WI), following the manufacturer’s instructions. The TUNEL assay results were quantitatively analyzed by biological image analysis system which consists of Nikon ECLIPSE 80i biology microscope, Nikon Digital Camera DXM 1200F, ACT-1 version 2.63 software (Japan).
Cells were seeded onto glass coverslips overnight. After incubation with various concentrations of the drugs for 48 h, the cover slips were washed twice with PBS and added 4% paraformaldehyde solution for 30 min at room temperature. Immunohistochemical staining for Bcl-2, Bax and caspase-3 was measured using the standard S-P method. The detailed manipulation was conducted according to the manufacturer’s instructions. As negative control, PBS was used instead of primary antibody, and other steps were followed in the same way. The immunocytochemical results were quantitatively analyzed by biological image analysis system which consists of Nikon ECLIPSE 80i biology microscope, Nikon Digital Camera DXM 1200F, ACT-1 version 2.63 software (Japan), and JEOA 801D morphological biological image analysis software version 6.0 (Jie Da Technologies. Inc., China). The average absorbance value was analyzed by 5 randomly selected optical fields under microscopy (× 400).
The cells were grown in 6-well plates and then treated with Melatonin and/or Doxorubicin at the desired concentrations. After exposure to drugs for 48 h, cells were trypsinized, washed twice with cold PBS and centrifuged. The cell pellet was resuspended in 1 mL cold PBS and fixed in 9 mL of 70% ethanol at -20°C for at least 12 h. Then cells were centrifuged and resuspended in 500 μL PBS, RNase A was added and the cells were incubated at 37°C for 30 min. Propidium iodide (PI) staining buffer was added in the dark at room temperature for 30 min (according to the procedure program of the Cell Apoptosis PI Detection Kit). A minimum of 1 × 106/mL cells for each group was analyzed using an EPICS XL-MCL model counter (Beckman Coulter, Fullerton, CA, USA).
Biostatistical analyses were done using the SPSS 11.5 software package. All experiments were repeated at least three times. The results of multiple experiments are given as the mean ± SE. Non-parametric Kruskal-Wallis test was used to detect differences among the different experimental groups. The Mann-Whitney U-test was subsequently used for statistical evaluation in two-group comparisons. Pearson correlation coefficient was used for continuous independent and dependent variables. A level of P < 0.05 was accepted as statistically significant.
HepG2 and Bel-7402 cells incubated with various concentrations of Melatonin alone for 48 h showed a dose-dependent reduction of cell proliferation. The inhibitory rate of Melatonin (10-8, 10-7, 10-6 and 10-5 mol/L) on HepG2 cells was 5.27% ± 1.53%, 13.53% ± 2.73%, 24.91% ± 6.60%, and 30.78% ± 3.28%, (r = 0.993, P < 0.01) and that on Bel-7402 cells was 5.35% ± 2.05%, 17.06% ± 5.51%, 17.73% ± 5.91%, and 26.09% ± 4.23%, respectively (r = 0.952, P < 0.05). The IC50 of Melatonin on HepG2 and Bel-7402 cells were 7.623 × 10-5 and 4.693 × 10-4, respectively.
To investigate the synergistic inhibitory effects of Melatonin and Doxorubicin, four different doses of Melatonin (10-8, 10-7, 10-6 and 10-5 mol/L) and four different concentrations of Doxorubicin (0.31, 0.63, 1.25 and 2.5 mg/L) were chosen and assessed. As depicted in Figure 1, Melatonin increased the cytotoxicity of Doxorubicin on HepG2 (Figure 1A and B) and Bel-7402 cells (Figure 1C and D). CDI was used to evaluate the nature of the interaction. When 10-5 mol/L Melatonin was combined with 1.25 mg/L Doxorubicin, the synergistic inhibitory effects is the strongest on HepG2 (Figure 1B). Doxorubicin and Melatonin had the strongest synergism on Bel-7402 when 10-5 mol/L Melatonin was combined with 2.5 mg/L Doxorubicin (Figure 1D).
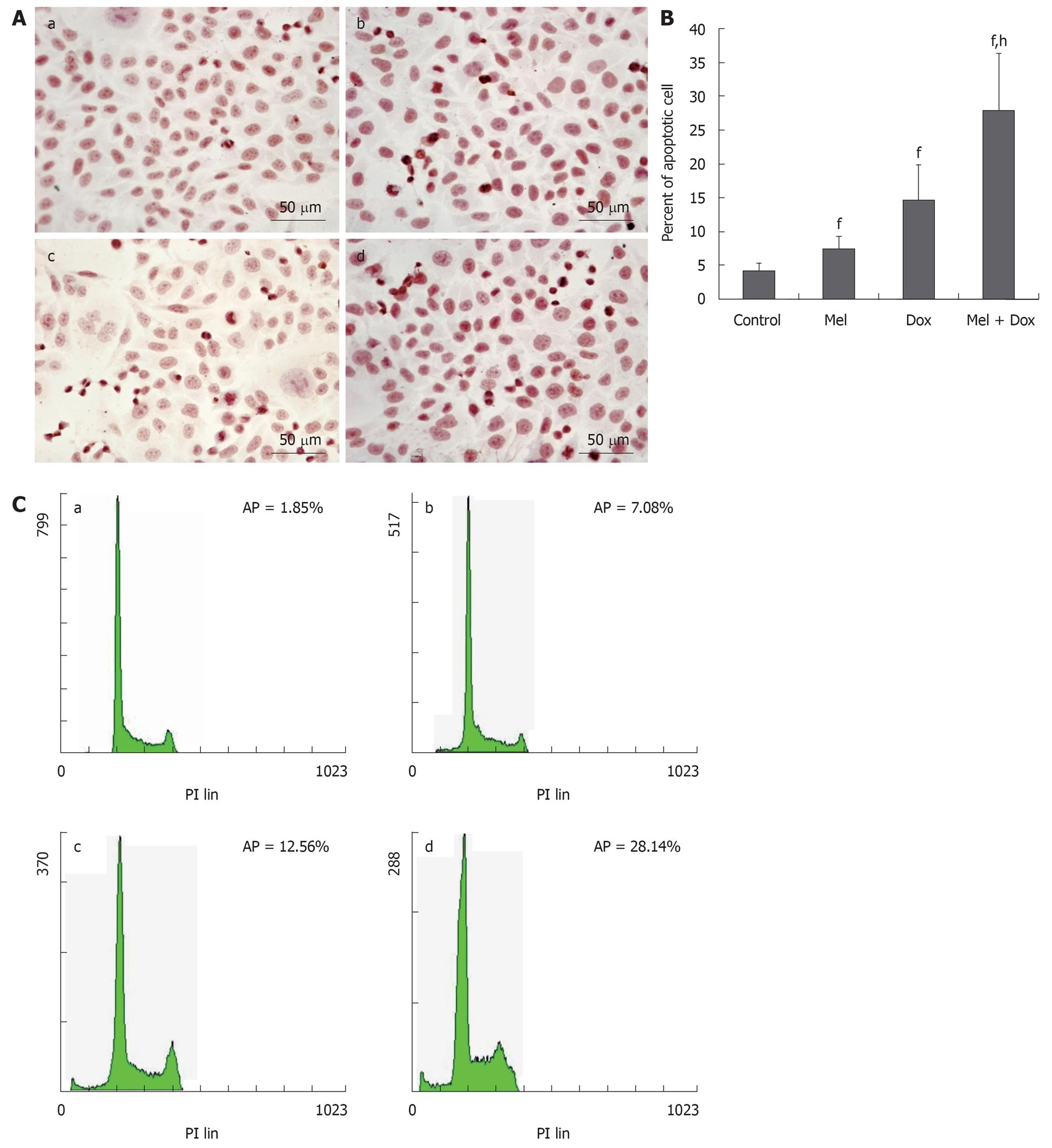
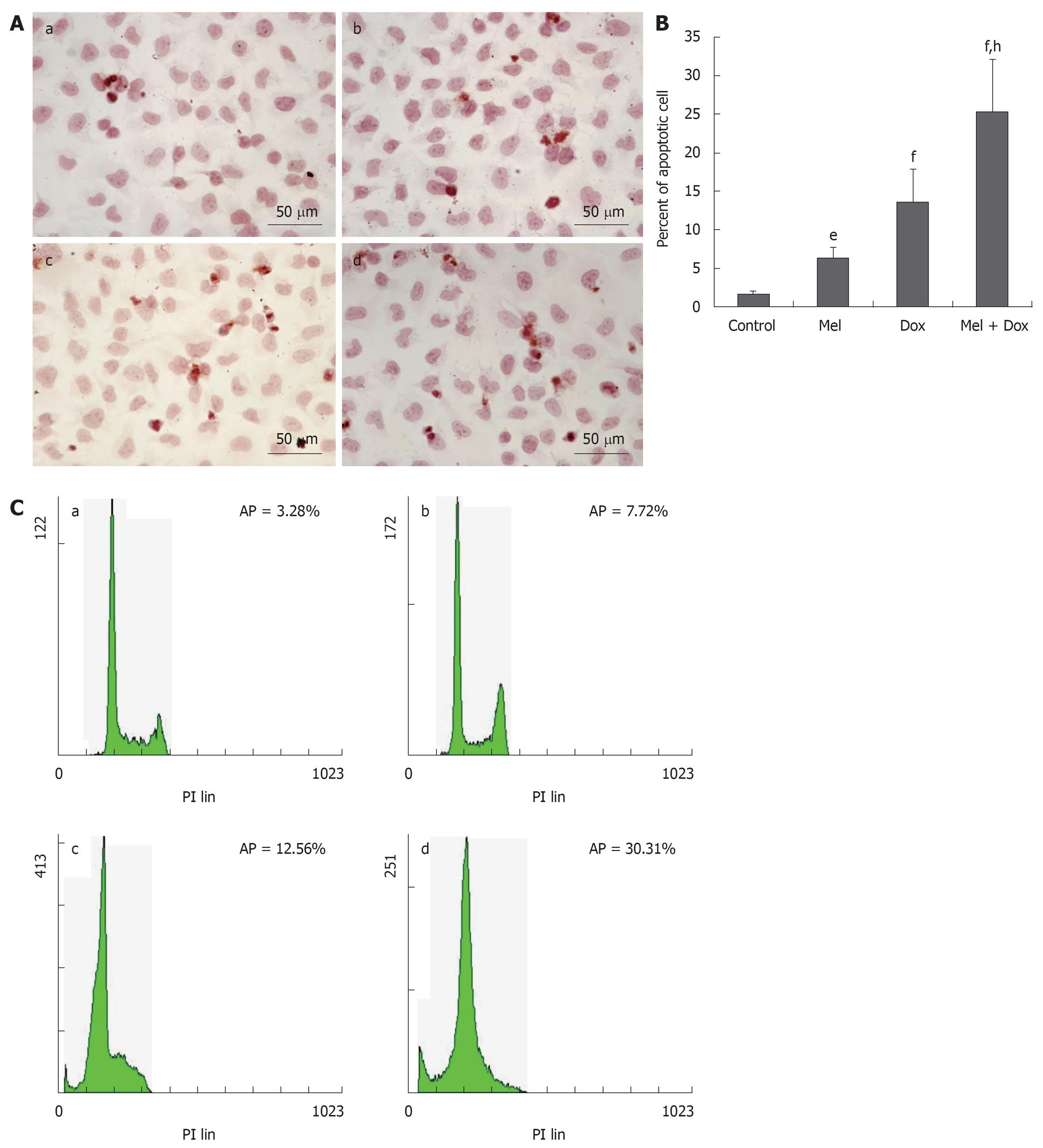
HepG2 and Bel-7402 cells were treated with different concentrations of Melatonin, Doxorubicin or a combination of both for 48 h, respectively, and apoptosis was assessed by TUNEL and FACS methods. We found that in the combination treatment group, there was a greater degree of induction of apoptosis than that caused by either agent alone. In HepG2 cells, the number of apoptosis cells in Melatonin (10-5 mol/L) or Doxorubicin (1.25 mg/L) alone treatment group was only slightly increased when compared with the cell control group. However, the apoptotic rate increased greatly when the cells were treated with combined Doxorubicin and Melatonin (Figure 2A and B). Similar results were found in Bel-7402 cells (Figure 3A and B). These results were confirmed by FCM assay. As shown in Figure 2C and Figure 3C, the sub-G1 peak, which appeared before the G1 phase that represents apoptotic cell population, was slightly increased in these two cell lines treated with Doxorubicin or Melatonin alone. The apoptotic peak was dramatically increased when the cells were exposed to combined Melatonin and Doxorubicin.
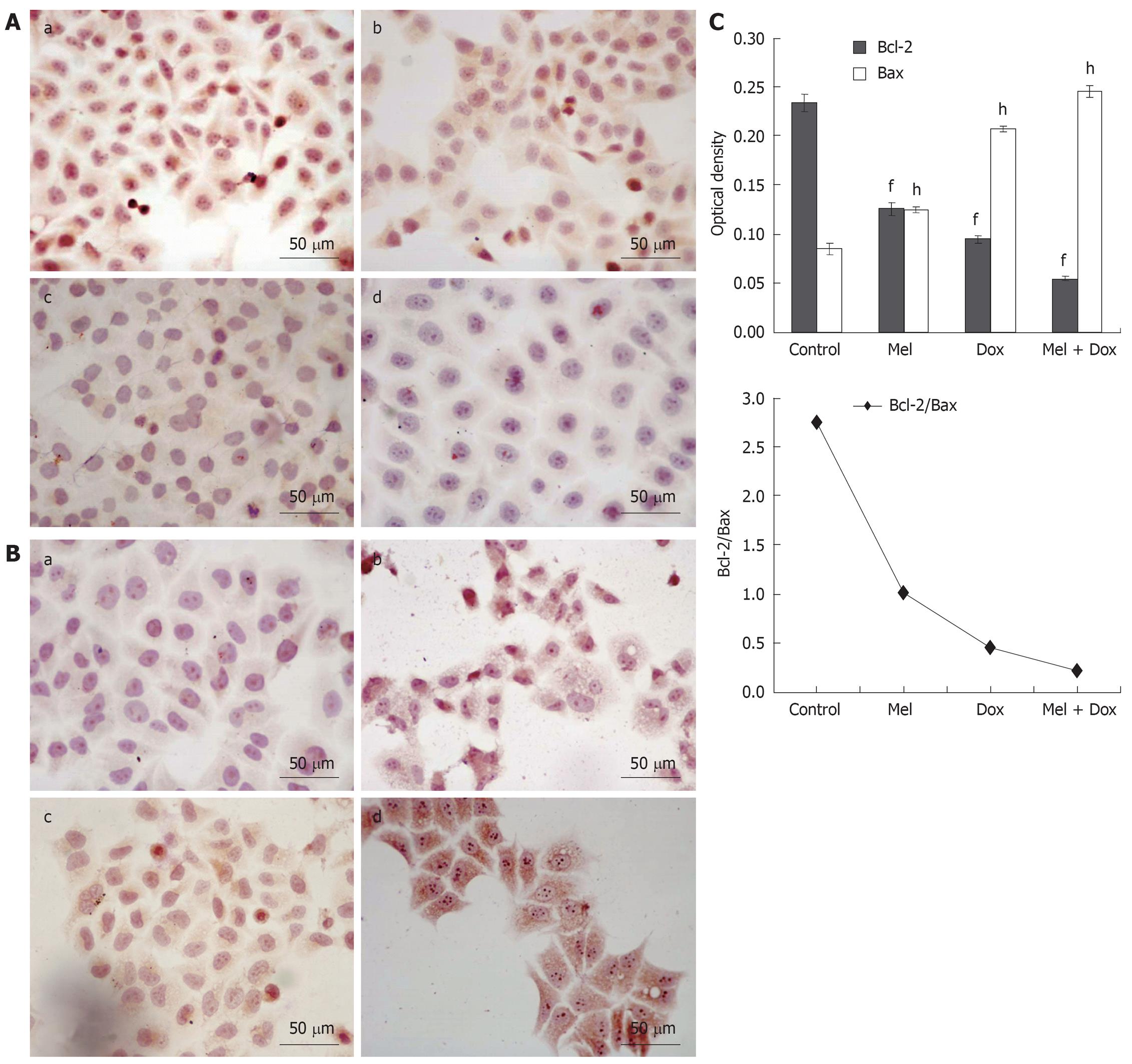
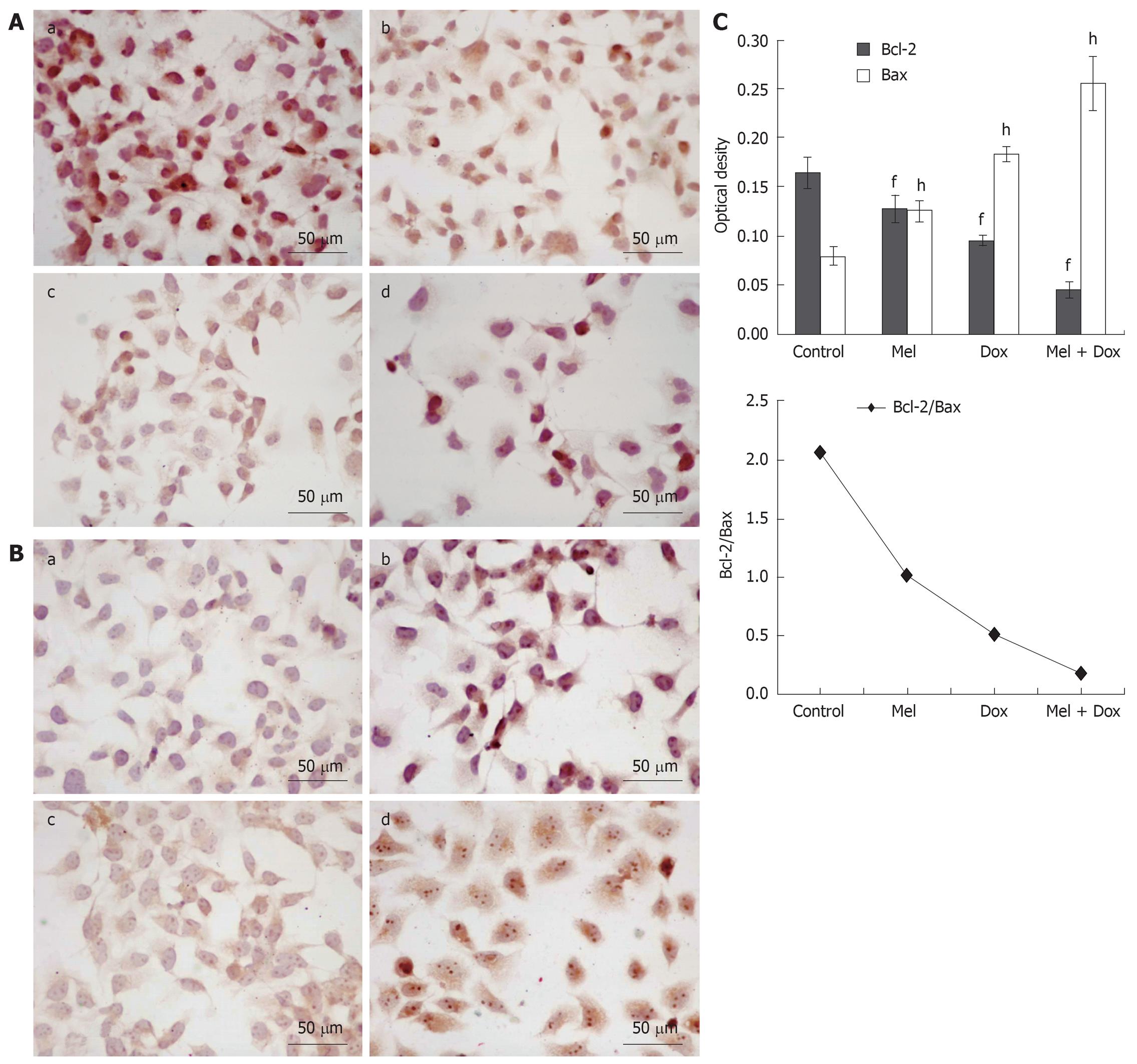
The standard positive Bcl-2 and Bax expressions were stained brown or yellow mainly in cytoplasm or membrane. After treatment for 48 h, the expression of Bcl-2 decreased (Figure 4A and Figure 5A) and in contrast, there was a significant increase of Bax expression, especially in the combination groups (Figure 4B and Figure 5B). The results were quantitatively analyzed by biological image analysis system and the ratio of Bcl-2/Bax decreased correspondingly (Figure 4C and Figure 5C).
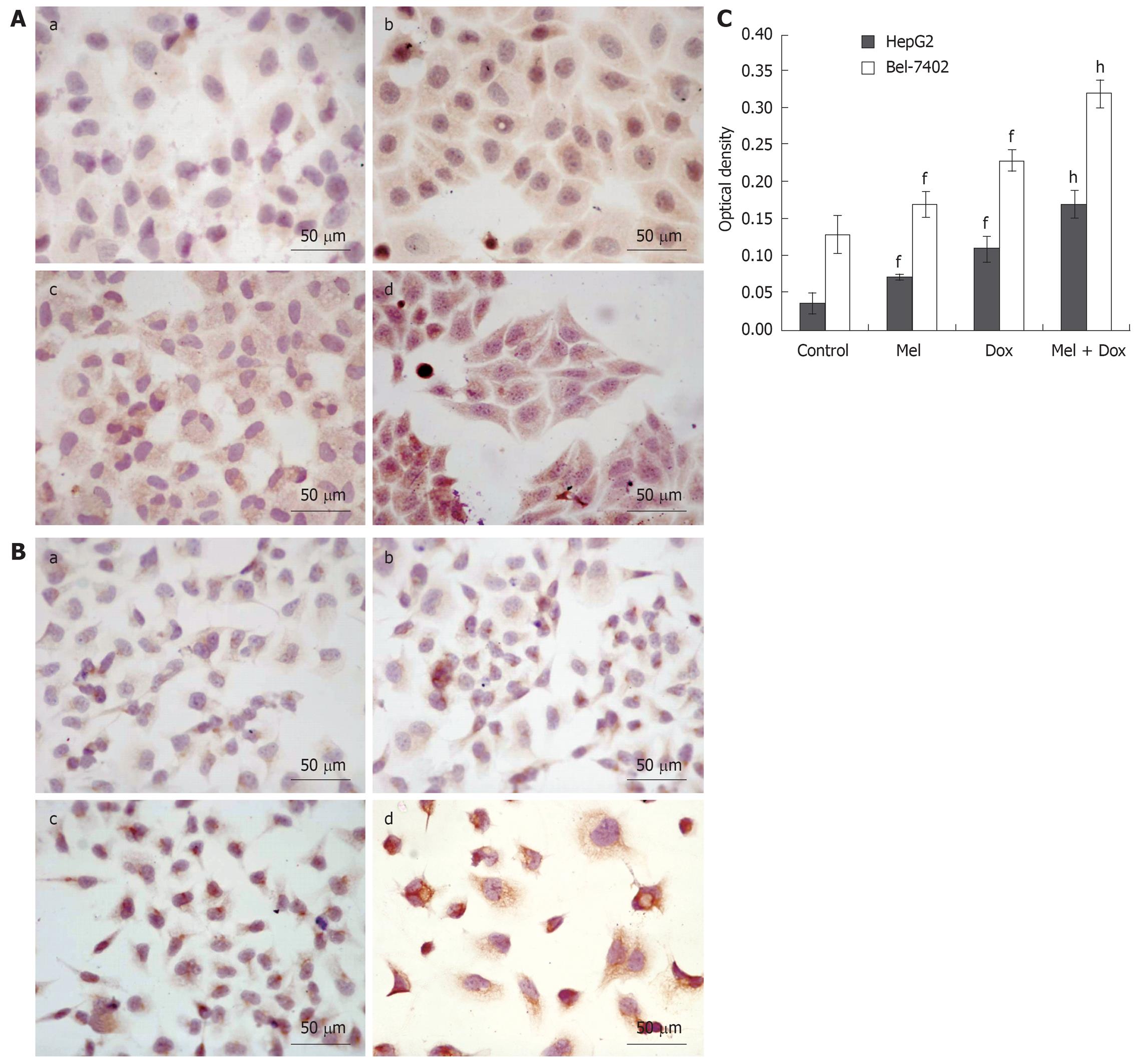
To determine whether caspase-3 plays a role in Melatonin and/or Doxorubicin mediated apoptosis of HepG2 and Bel-7402 cells, we assessed the activated caspase-3 protein level of the two cell lines before and after treatment with Melatonin and/or Doxorubicin using S-P method. The standard positive caspase-3 expressions were stained brown or yellow mainly in cytoplasm or nucleus. As shown in Figure 6, the expression of activated caspase-3 increased especially in the combination group after the two cell lines were exposed to drugs for 48 h.
Our results in the present study indicate that Melatonin inhibits growth of HepG2 and Bel-7402 cells in a dose-dependent manner. Meanwhile, combinations of Melatonin and Doxorubicin results in enhanced growth inhibitory effect and induction of cell apoptosis when compared with Melatonin or Doxorubicin used alone. These results suggest that chemotherapy combined with Melatonin may increase the therapeutic effect of anticancer drugs. Our results demonstrated that Melatonin had a synergetic effect with Doxorubicin in inhibiting the proliferation of non-small cell lung cancer (A-549)[15]. Moreover, combining with the study of Martin-Renedo et al[16], our observation extend the possibility that Melatonin may provide a potential approach for the development of agents for the treatment and prevention of HCC.
Although the exact mechanism of the cytotoxicity of Melotonin against tumor cells is not very clear, many potential mechanisms have been proposed for the growth inhibition by Melatonin on cultured cells and animal models. These mechanisms include the induction of apoptosis[17], the direct augmentation of natural killer (NK) cell activity[17], which increases immunosurveillance, as well as the stimulation of cytokine production, e.g. interleukin IL-2, IL-6, IL-12 and interferon IFN γ[18]. Apoptosis or programmed cell death is an essential physiological process that plays a critical role in development and tissue homeostasis. However, apoptosis is also involved in a wide range of pathological conditions[19]. Apoptotic defects are a common event in oncogenesis and contribute to drug resistance[20,21]. It is therefore interesting to search for ways to facilitate this apoptotic process after the use of chemotherapeutic drugs. Many studies have demonstrated that Melatonin could induce apoptosis in vitro and in vivo. To investigate the apoptosis-inducing effect of Melatonin as a single agent and combined Melatonin and Doxorubicin in hepatoma cells, the morphological changes and apoptotic rate were detected. The cells treated with the drugs showed the typical characteristics of apoptosis, which were more prominent in the combination group. Similarly, an apoptotic peak appeared before G1 phase when treated with Melatonin or Doxorubicin alone, and a significant synergistic effect on the induction of apoptosis was observed in the combination group.
Several factors contribute to apoptosis, but the key elements are categorized into two main families of proteins including caspase enzymes and Bcl-2 family[22]. Bcl-2 family is a set of cytoplasmic protein members that regulate apoptosis. The two main groups of this family, Bcl-2 and Bax proteins, are functionally opposed: Bcl-2 acts to inhibit apoptosis, whereas Bax counteracts this effect[23,24]. Caspases are crucial mediators of programmed cell death (apoptosis). Among them, caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins[23,25]. Thus, we studied bcl-2 and bax and caspase-3 protein in hepatoma cell lines. The current findings show that Melatonin and/or Doxorubicin increased the expression of Bax and caspase-3, decreased the expression of Bcl-2. Furthermore, a significant decrease in the ratio of Bcl-2/Bax was observed when Melatonin was treated in combination with Doxorubicin, which was correlated with the incidence of apoptosis.
In conclusion, these results indicate that Melatonin in combination with Doxorubicin has significantly synergistic growth-inhibitory and apoptosis-including effects on the human hepatoma cell lines HepG2 and Bel-7402, which may be related to down-regulation of Bcl-2, up-regulation of Bax, and activation of caspase-3. Melatonin is expected to be an adjuvant drug in HCC treatment in the future.
Hepatocellular carcinoma (HCC) is a major contributor to cancer incidence and mortality in the world. Currently, No effective treatment is available. Therefore, there is a critical need to develop effective chemotherapeutic strategies for hepatomas.
Melatonin, the chief secretary product of the pineal gland has shown anti-neoplastic activities in both cell lines and animal models. However, studies on the oncostatic effects of Melatonin in the treatment of hepatocarcinoma are limited. In this study, the authors demonstrate that Melatonin has synergistic effects with Doxorubicin on the growth-inhibition and apoptosis-induction of human hepatoma cell lines.
This is the first report on the anti-proliferation, induction of apoptosis by Melatonin and Doxorubicin synergistically in human hepatoma cell lines.
Melatonin might be useful as an adjuvant drug in hepatocarcinoma treatment.
Melatonin is a small lipophile molecule that is essentially secreted by the pineal gland and its synthesis shows a circadian pattern. Melatonin plays an important role in biological rhythm resynchronization, sleep induction, immunomodulation and tumor inhibition.
The study puts forward an interesting point in Melatonin-related chemotherapy and provides some experimental evidence that Melatonin can be of interest in treatment of hepatocellular carcinoma. This cell culture-based study focused on the molecular synergism of Melatonin and Doxorubicin in apoptosis of hepatoma cells. The main limit of the study is that cultured cell lines were exclusively used.
Peer reviewers: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany; Hong Joo Kim, MD, PRO, Department of Internal Medicine, Sungkyunkwan University Kangbuk Samsung Hospital, 108, Pyung-Dong, Jongro-Ku, Seoul 110-746, South Korea
S- Editor Wang JL L- Editor Ma JY E- Editor Ma WH
| 1. | Cardinali DP, Esquifino AI, Srinivasan V, Pandi-Perumal SR. Melatonin and the immune system in aging. Neuroimmunomodulation. 2008;15:272-278. [Cited in This Article: ] |
| 2. | Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189-200. [Cited in This Article: ] |
| 3. | Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43-50. [Cited in This Article: ] |
| 4. | Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci. 2007;52:11-28. [Cited in This Article: ] |
| 5. | Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259-264. [Cited in This Article: ] |
| 6. | Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 2008;7:189-203. [Cited in This Article: ] |
| 7. | Jung B, Ahmad N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66:9789-9793. [Cited in This Article: ] |
| 8. | Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, Di Primio R. Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J Pineal Res. 2005;39:425-431. [Cited in This Article: ] |
| 9. | Rubio S, Estévez F, Cabrera J, Reiter RJ, Loro J, Quintana J. Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL-60 cells. J Pineal Res. 2007;42:131-138. [Cited in This Article: ] |
| 10. | García-Santos G, Antolín I, Herrera F, Martín V, Rodriguez-Blanco J, del Pilar Carrera M, Rodriguez C. Melatonin induces apoptosis in human neuroblastoma cancer cells. J Pineal Res. 2006;41:130-135. [Cited in This Article: ] |
| 11. | Leon-Blanco MM, Guerrero JM, Reiter RJ, Calvo JR, Pozo D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204-211. [Cited in This Article: ] |
| 12. | Vesnushkin GM, Plotnikova NA, Semenchenko AI, Anisimov VN. Dose-dependent inhibitory effect of melatonin on carcinogenesis induced by benzo[a]pyrene in mice. J Exp Clin Cancer Res. 2006;25:507-513. [Cited in This Article: ] |
| 13. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [Cited in This Article: ] |
| 14. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [Cited in This Article: ] |
| 15. | Fic M, Podhorska-Okolow M, Dziegiel P, Gebarowska E, Wysocka T, Drag-Zalesinska M, Zabel M. Effect of melatonin on cytotoxicity of doxorubicin toward selected cell lines (human keratinocytes, lung cancer cell line A-549, laryngeal cancer cell line Hep-2). In Vivo. 2007;21:513-518. [Cited in This Article: ] |
| 16. | Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532-540. [Cited in This Article: ] |
| 17. | Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003;60:1407-1426. [Cited in This Article: ] |
| 18. | Pioli C, Caroleo MC, Nistico G, Doria G. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int J Immunopharmacol. 1993;15:463-468. [Cited in This Article: ] |
| 19. | Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57-70. [Cited in This Article: ] |
| 20. | Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807-810. [Cited in This Article: ] |
| 21. | Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539-542. [Cited in This Article: ] |
| 22. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [Cited in This Article: ] |
| 23. | Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614-620. [Cited in This Article: ] |
| 24. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [Cited in This Article: ] |
| 25. | Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99-104. [Cited in This Article: ] |