Published online Nov 28, 2007. doi: 10.3748/wjg.v13.i44.5926
Revised: July 17, 2007
Accepted: October 19, 2007
Published online: November 28, 2007
AIM: To examine the expression of matrix metallo-proteinase-1 (MMP-1) and tumor necrosis factor-α (TNF-α) in the colon mucosa of patients with ulcerative colitis (UC).
METHODS: Reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry were used to examine the expression of MMP-1 and TNF-α at both mRNA and protein levels in the colon mucosa of patients with UC. Correlation between MMP-1 and TNF-α and their correlation with the severity of the disease were also analyzed statistically.
RESULTS: The expression of MMP-1 and TNF-α in the ulcerated and inflamed colon mucosa of patients with UC was significantly higher than that in the non-inflamed mucosa of normal controls at both mRNA and protein levels. Furthermore, the expression of MMP-1 and TNF-α in the ulcerated area was significantly higher than that in the inflamed area of patients with UC (0.9797 ± 0.1433 vs 0.6746 ± 0.0373, 0.8669 ± 0.0746 vs 0.5227 ± 0.0435, P < 0.05). There was no statistically significant difference in the non-inflamed area of normal controls. There was a significant correlation between MMP-1 and TNF-α expression (0.9797 ± 0.1433 vs 0.8669 ± 0.0746, P < 0.05), the correlating factor was 0.877. MMP-1 and TNF-α showed a significant correlation with the severity of the disease (0.0915 ± 0.0044 vs 0.0749 ± 0.0032 , 0.0932 ± 0.0019 vs 0.0724 ± 0.0043, P < 0.05), their correlating factors were 0.942 and 0.890, respectively.
CONCLUSION: Excessively expressed MMP-1 directly damages the colon mucosa by degrading extracellular matrix (ECM) in patients with UC. While damaging colon mucosa, excessively expressed TNF-α stimulates MMPs secreting cells to produce more MMP-1 and aggravates the mucosa damage. MMP-1 promotes secretion of TNF-α in a positive feedback manner to cause further injury in the colon mucosa. MMP-1 and TNF-α correlate well with the severity of the disease, and therefore, can be used clinically as biological markers to judge the severity of UC.
- Citation: Wang YD, Mao JW. Expression of matrix metalloproteinase-1 and tumor necrosis factor-α in ulcerative colitis. World J Gastroenterol 2007; 13(44): 5926-5932
- URL: https://www.wjgnet.com/1007-9327/full/v13/i44/5926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i44.5926
Ulcerative colitis (UC) is a chronic, non-specific inflammatory disease of the colon mucosa with an increasing morbidity due to life pattern changes in China. However, its etiology and pathogenesis are still unknown. Pathophysiologically, ulceration in the mucosal and submucosal areas of patients with UC is due to excessive degradation of extracellular matrix (ECM). In recent years, matrix metalloproteinases (MMPs) and some cytokines have been implicated in the development of a number of diseases, such as multiple sclerosis, rheumatic disease and UC[1-3]. In patients with UC, MMPs participate in tissue repair, vascularization and leucocyte chemotaxis in the ulcerated and inflamed colonic mucosa[3]. MMP-1 produced by cytokine-activated interstitial cells is one of the most important enzymes in degrading ECM[4]. Excessive expression of MMP-1 in the diseased colon mucosa of UC patients causes excessive hydrolysis of the ECM and ulceration[5,6]. It is also believed that imbalance between inflammatory and anti-inflammatory cytokines plays a central role in the development of UC[7]. For example, TNF-α, an important inflammatory cytokine produced by macrophages in the colon, takes part in the pathogenesis of UC[8] and can directly damage the colonic mucosal barrier, causing inflammatory changes in UC. Therefore, in this study we measured MMP-1 and TNF-α transcript and their proteins using reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry to explore their possible role and interrelationship in the pathogenesis of UC.
Thirty-six patients with UC confirmed by clinical manifestations, colonoscopy and biopsy were enrolled in this study. Among these patients, 15 were males and 21 were females with their age ranged from 22 to 72 years and averaged 44 years. Samples were taken from the ulcerated, inflamed and non-inflamed areas of the colon mucosa during colonoscopy. There were 4 patients with pan-colon lesions, 3 with hemi-colon lesions, 19 with recto-sigmoid lesions, and 10 with rectal lesions. Based on the clinical manifestations and colonoscopic findings, 8 patients were classified into mild type, 21 into moderate type, and 7 into severe type. Meanwhile, 20 normal subjects were chosen as normal controls, 12 of them were males and 8 were females with their age ranged from 22 to 56 years and averaged 34 years. Biopsy samples were immediately snap frozen in liquid nitrogen and stored at -80°C for RT-PCR. Biopsy samples were fixed in formalin, embedded in paraffin and cut into 4 μm-thick sections for immunohistochemistry.
Total RNA was extracted from the frozen samples using a RNA isolation kit (Invitrogen Company) following the manufacturer’s instructions. Five μL of the extracted RNA was run on 1% agarose gel electrophoresis to identify the extracted products.
RT-PCR was performed using the TaKaRa RNA PCR kit 3.0 (AMV) (supplied by Dalian Baosheng Biotechnology Company) following the manufacturer’s instructions. Primer sequences used are as follows: MMP-1 (sense: 5'-ATGCGAACAAATCCCTTCTACC-3', antisense: 5'-TTCCTCAGAAAGAGCAGCATCG-3'), TNF-α: (sense: 5'-CTGTAGCCCATGTTGTAGC-3', antisense: 5'-CAATGATCCCAAAGTAGACCT-3'). Primers for β-actin were used as the internal control (sense: 5'-CCTTCCTGGGCATGGAGTCCTG-3', antisense: 5'-GGAGCAATGATCTTGATCTTC-3'). Reverse transcription was carried out at 30°C for 10 min, at 42°C for 30 min, at 99°C for 5 min, and at 5°C for 5 min. PCR was performed as follows: initial denaturation at 94°C for 2 min, followed by 35 amplification cycles at 94°C for 30 s, at 53°C for 30 s, at 72°C for 1 min, extension at 72°C for 10 min. Five μL of PCR products was run on 2% agarose gel electrophoresis.
Sample sections were washed 3 times with PBS, 3 min each time after initial treatment. Primary antibodies, mouse anti-human MMP-1 monoclonal antibody and rabbit anti-human TNF-α polyclonal antibody (Beijing Zhongshan Biology Company) were added and incubated at room temperature for 1.5 h, washed again and incubated with peroxidase-conjugated secondary antibody for 15 min and washed again. A brown product was developed in diaminobenzidine (DAB) for 10 min.
A bio-imaging system (PALL Company, USA) was employed to analyze the density of the bands of PCR products. MMP-1 mRNA and TNF-α mRNA were semi-quantitatively expressed by the ratios between MMP-1, TNF-α and β-actin OD values. All values were expressed as mean ± SD.
Results of immunohistochemistry were considered positive when brown particles appeared in the cells after DAB staining. An image-pro-plus 4.5 microscopic image analyzing system was used to measure the density of the positive products. Five fields in each section were randomly selected to measure the total density and area. The mean density was determined by calculating the ratio between the total density and area in each section. A bigger ratio value indicates a greater expression of the corresponding proteins.
Student-Neuman-Keuls test was used to compare MMP-1 and TNF-α: mRNAs and their corresponding proteins in different colon samples and in different severity of the disease. Spearman correlation analysis was used to study the relationship between MMP-1, TNF-α and severity of the disease. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 11.5 for windows.
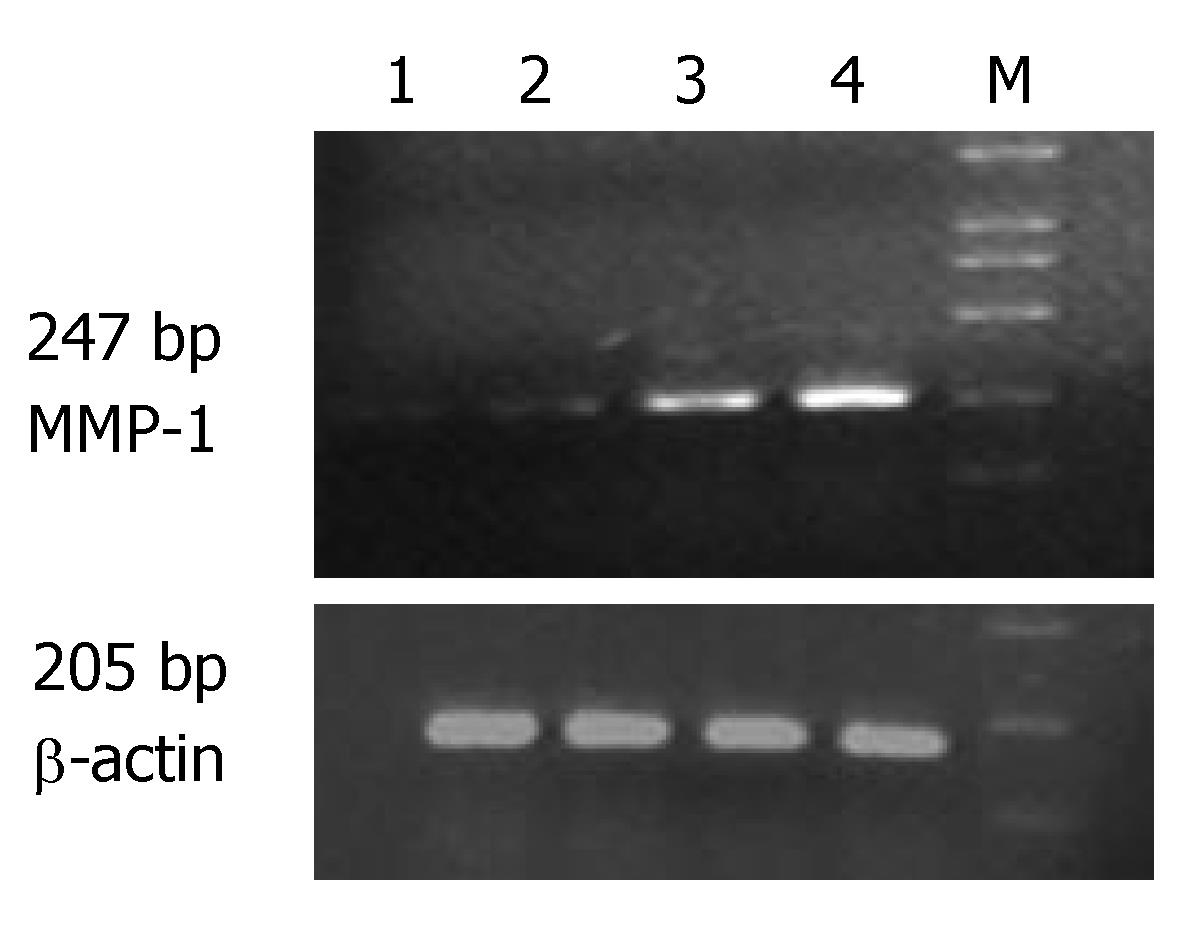
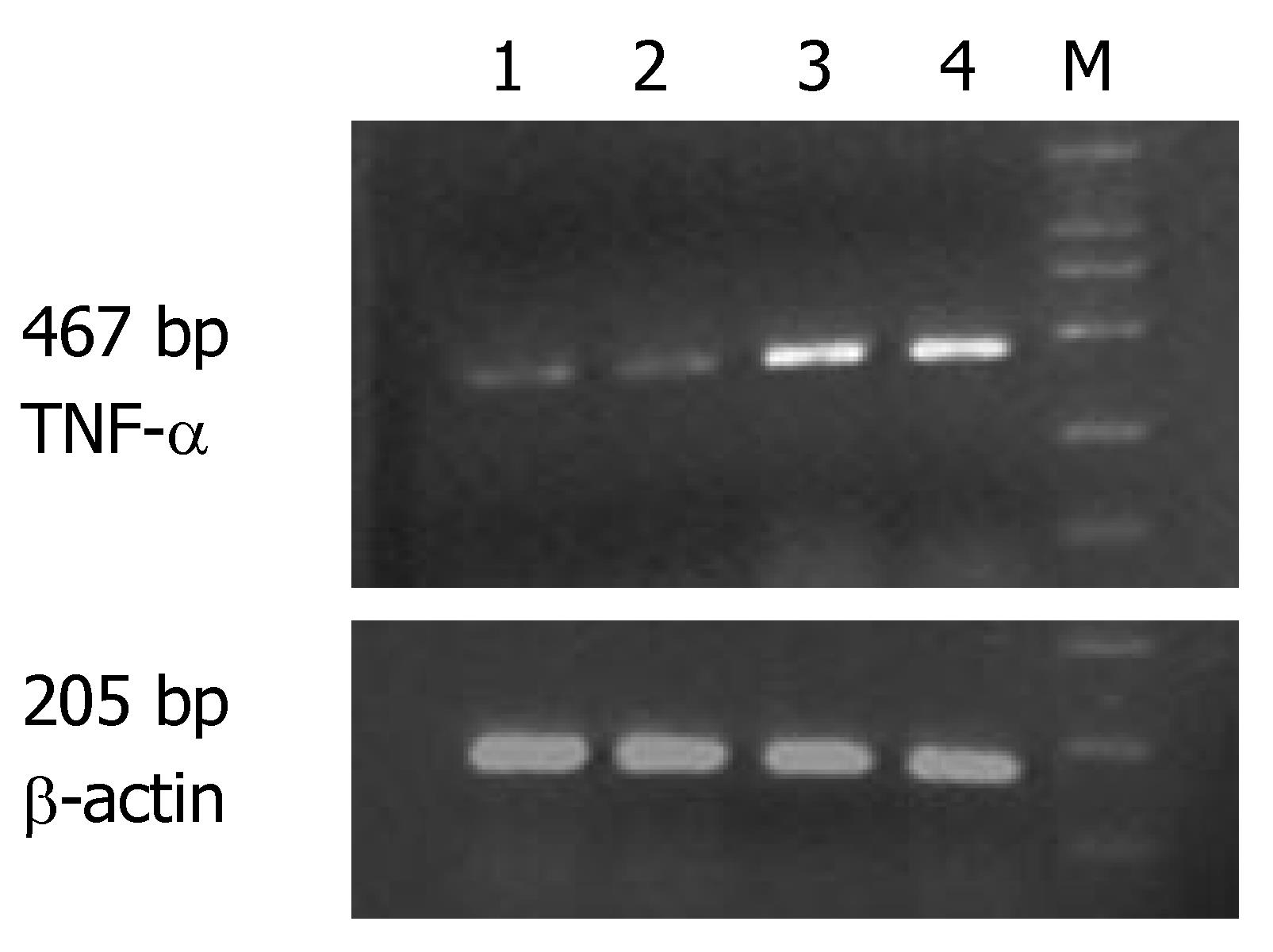
The expression of MMP-1 and TNF-α mRNA in the ulcerated area of colon was significantly higher than that in the inflamed colon area of patients with UC and non-inflamed colon area of normal controls (P < 0.05). The expression of MMP-1 and TNF-α mRNA in the inflamed colon area of patients with UC was also significantly higher than that in the non-inflamed colon area of normal controls (P < 0.05), but the extent was not as high as that in the ulcerated area. There was no statistically significant difference in non-inflamed colon area of normal controls (Table 1, Figures 1 and 2).
The expression of MMP-1 mRNA was significantly higher in different groups of patients than in normal controls (P < 0.05). Comparison among the three groups showed that the highest expression of MMP-1 and TNF-α mRNA was seen in the group of patients with severe UC followed by in groups of patients with mild and moderate UC (Table 2).
Correlation studies showed that the expression of MMP-1 mRNA was significantly correlated with that of TNF-α mRNA. The correlating factor was 0.877 (P < 0.01). The expression of MMP-1 and TNF-α mRNA was also significantly correlated with the severity of the disease. The correlating factor was 0.942 and 0.890, respectively (P < 0.01).
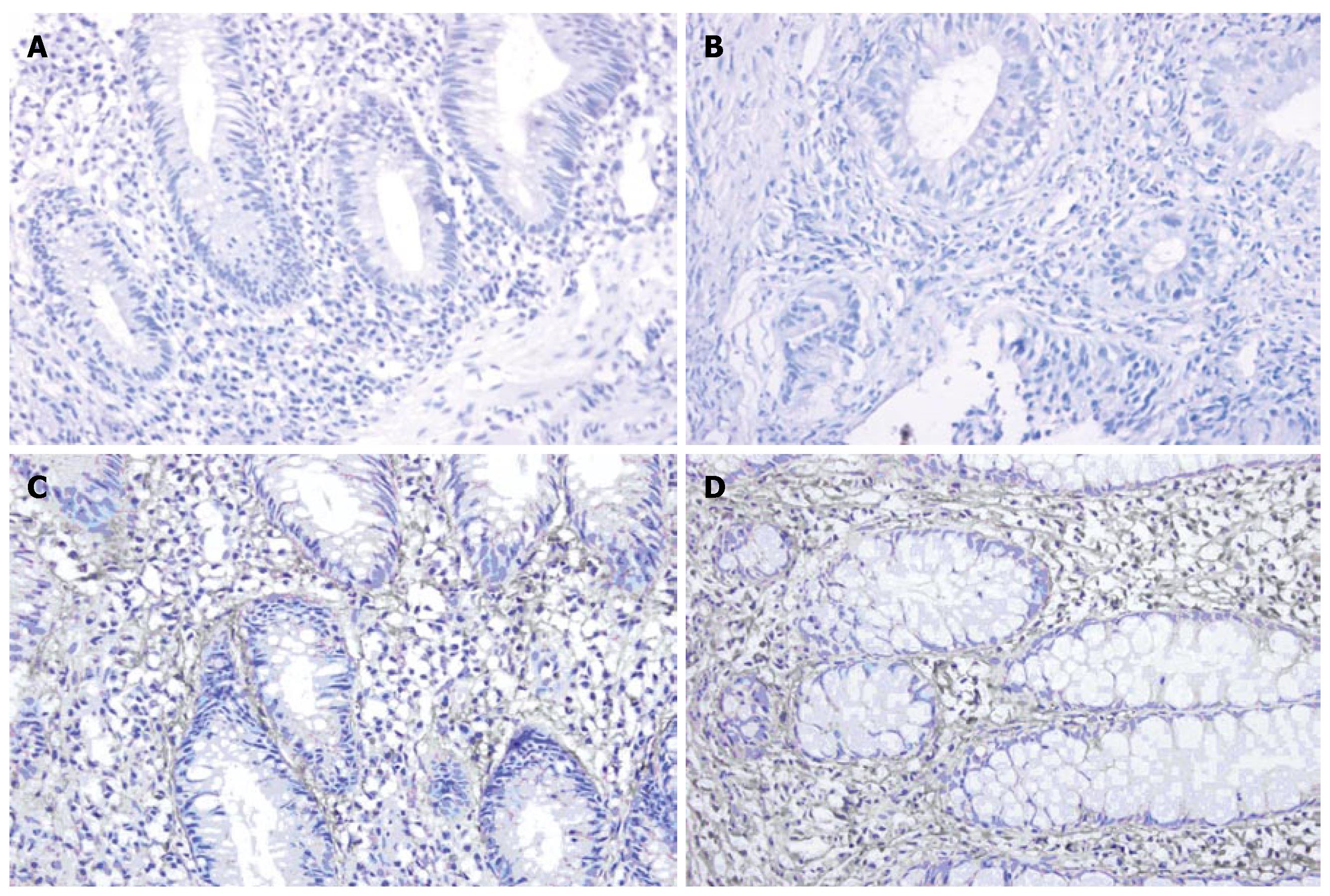
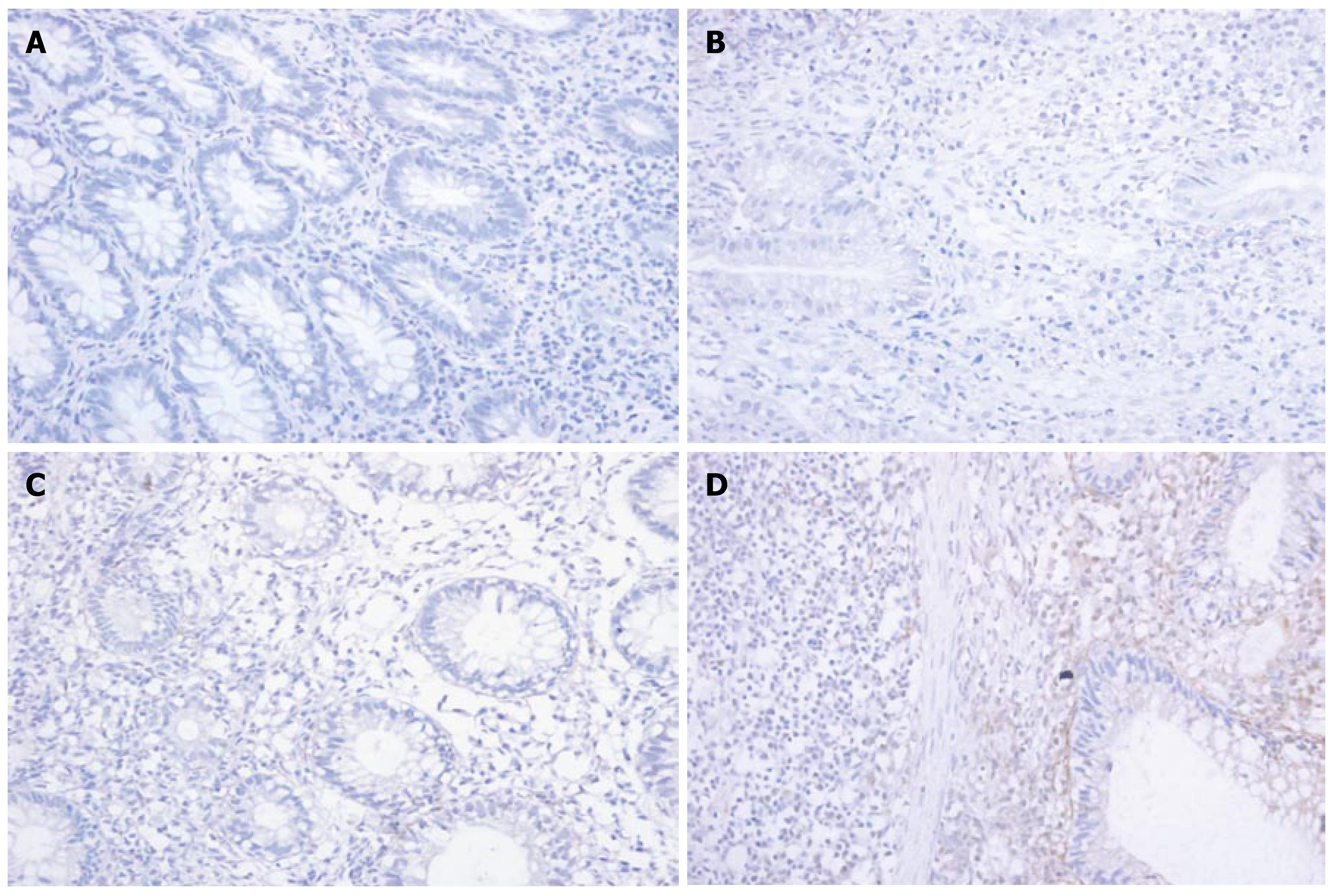
Immunohistochemistry showed that the expression of MMP-1 and TNF-α in different areas of colon was identical. The expression of MMP-1 and TNF-α in the ulcerated area was significantly higher than that in the inflamed colon area of UC patients and non-inflamed colon area of normal controls (P < 0.05). The expression of MMP-1 and TNF-α in the inflamed colon area of UC patients was also significantly higher than that in the non-inflamed colon area of normal controls (P < 0.05), but it was not as high as that in the ulcerated area. There was no statistically significant difference in the non-inflamed colon area of normal controls (Figure 3A-D and Figure 4A-D, Table 3).
Protein analysis showed that the expression of MMP-1 and TNF-α in patients with different severity of the disease was identical The expression of MMP-1 and TNF-α was significantly higher in different groups than that in normal controls (P < 0.05). Comparison among the three groups showed that the highest expression of MMP-1 and TNF-α was seen in the group of patients with severe UC followed by in groups with mild and moderate UC (Table 4).
Ulcerative colitis (UC) is a chronic, non-specific inflammatory disease with ulceration in the mucosal and submucosal areas of colon. Excessive degradation and insufficient synthesis of extracellular matrix (ECM) are the main pathophysiological events occurring in the process of ulceration. Since matrix metalloproteinases (MMPs) are the major hydrolytic enzymes that degrade ECM, the increased activity of MMPs is responsible for tissue damage of the colon in UC patients. It has been well accepted that inflammatory cytokines including TNF-α participate in the pathogenesis of UC[7]. The relationship between MMPs and inflammatory cytokines remains to be studied when both of them take part in the pathogenesis of UC.
MMPs are a group of zinc-dependent peptidases that degrade ECM. MMP-1, also known as interstitial collagenase, degrades mainly collagen types I, II, III, VI, IX and proteoglycan, and plays an important role in degrading ECM in UC patients. Using RT-PCR and immunohistochemistry, we found that at both transcription and protein levels, the expression of MMP-1 in ulcerated and inflamed colon area of patients with UC was significantly higher than that in non-inflamed colon area of normal controls. Furthermore, the expression of MMP-1 in ulcerated area was significantly higher than that in the inflamed area. In the present study, MMP-1 expression was closely correlated with the severity of the disease (correlating factor was 0.942, P < 0.05), indicating that MMP-1 is closely related to colon mucosa damage in UC patients[9,10]. Immunohistochemistry showed that MMP-1 was expressed mainly in the cytoplasm of mono-macrophages, which is consistent with the results reported by Von Lampec et al[11]. McKaig et al[12] also found that MMP-1 is expressed in damaged tissue vascular smooth muscle cells, indicating that MMP-1 may be related with formation of new blood vessels.
Our results showed that at transcription and protein levels, the expression of TNF-α in the ulcerated and inflamed area of UC patients was significantly higher than that in the non-inflamed area of normal controls. The expression of TNF-α was closely correlated with the severity of the disease (correlating factor was 0.890, P < 0.05), indicating that the more severe the disease, the higher the TNF-α expression. Immunohistochemistry revealed that the TNF-α positively stained cells were mainly mono-macrophages. Ishiguro[13] also reported that TNF-α expression in the diseased mucosa of colon in UC patients is significantly higher than that in the unaffected area of normal controls, suggesting that lipopolysaccharide produced by the intestinal flora may directly activate macrophages in the lamina propria, proliferating and producing a series of cytokines including TNF-α which damage the mucosa barrier of colon and produce typical inflammatory changes in UC. Apart from inflammatory cytokines, anti-inflammatory cytokines such as IL-10 also take part in the pathogenesis of UC. Gasche et al[14] reported that the expression of IL-10 mRNA is significantly decreased while Niessner et al[15] found that IL-10 mRNA is highly expressed in active UC, indicating that the expression of IL-10 mRNA is different in UC patients. Using in situ hybridization and immunohistochemical methods, Autschbach et al[16] showed that the number of IL-10 secreting monocytes and the mucosal expression of IL-10 are both significantly increased, but the expression of IL-10 in lanmina propria is relatively low, suggesting that IL-10 cannot effectively inhibit inflammatory cytokines such as TNF-α in lamina propria.
In the present study, MMP-1 was found to be closely correlated with TNF-α, indicating that there is a certain relationship between MMPs and cytokines. There is evidence that multiple cytokines may influence the expression of MMPs during inflammatory responses. Previous studies indicate that IL-1β and TNF-α are potent stimulators of MMP-1 and MMP-3[17,18]. They can regulate the secretion of MMP-1 and MMP-3 produced by mono-macrophages. Sylvia et al[19] found that the activity of T cells is correlated with the extent of colon mucosa damage, and that the colon mucosa injury is mediated by endogenously produced MMPs. Some authors believe that anti-inflammatory cytokines, such as IL-4 and IL-10, are able to inhibit the secretion of MMPs by monocytes[20-22]. Qiu et al[23] found that MMP-2 and MMP-9 combine with CD44 receptors on the cell membrane to form MMP-1/19-CD44 complex, making the inactivated TGF-β become its active form through hydrolysis and carry out its biological functions. Black et al[24] reported that MMPs activate TNF-α on cell membrane through hydrolysis to make it in an active state. MMPs may also block some cytokines, such as IL-6 and TGF-α to down-regulate their activities[25]. It is believed that MMPs not only appear in the down stream of inflammatory responses but also exert a positive feedback effect on cytokines. Therefore, they can be regarded as important “regulators” of inflammatory responses.
MMPs and cytokines play an important role in the process of UC. When infection, diet or other environmental factors act on hereditarily susceptible individuals, abnormal immune responses of the intestine may activate immune cells (such as T cells, lymphocytes and macrophages) to secrete a big amount of cytokines, inflammatory mediators and complements. These substances directly damage the colon mucosa, and induce interstitial cells (including smooth muscle cells, fibroblasts and mono-macrophages) to secrete MMPs. The increased MMPs degrade ECM in the colon mucosa, leading to mucosa damage and ulceration. While cytokines influence MMPs expression, and MMPs themselves are able to up-regulate cytokines through certain ways to cause further damage on the colon mucosa, MMPs can be inhibited by their inhibitors (MMPI) including their natural ones[26], revealing that MMPs have become one of the targets in anti-inflammatory treatment. MMPs inhibitors used in treatment of malignant tumors in clinical phase III trial[27] in America and Europe can also be used in the treatment of patients with UC[7], while anti-inflammatory or inflammatory cytokine inhibitors can be used to reduce MMPs expression so as to indirectly reduce tissue damage and ulceration in UC patients. For example, a TNF-α antagonist, Infliximab, has been proved effective against adult and children UC patients[28,29].
In conclusion, excessively expressed MMP-1 directly damages the colon mucosa by degrading ECM in UC patients. While damaging colon mucosa, excessively expressed TNF-α stimulates MMPs secreting cells to produce more MMP-1 and aggravates the mucosa damage. MMP-1 promotes secretion of TNF-α in a positive feedback manner to cause further injury in the mucosa of colon. MMP-1 and TNF-α can be used clinically as biological markers to judge the severity of UC.
Matrix metalloproteinases (MMPs), their tissue inhibitors (TIMPs) and inflammatory cytokines, e.g., tumor necrosis factor-α (TNF-α) participate in the development of ulcerative colitis (UC) which is a chronic, non-specific inflammatory disease of the colon mucosa with unknown etiology and pathogenesis. This study was to deal with the expression of MMP-1 and TNF-α transcript and their proteins in colonic mucosa of patients with UC and their interrelationships in the pathogenesis of UC.
Participation and functions of MMPs, TIMPs and inflammatory cytokines in the pathogenesis of UC have been extensively studied in recent years. Study in this field has become one of the hotspots at present. Previous studies have demonstrated that MMPs and some inflammatory cytokines, such as TNF-α, are responsible for the development of ulceration and inflammation in the colonic mucosa of UC patients. Based on these findings, treatment targeting these proteins, such as anti-TNF-α antibody and exogenous MMPs inhibitors has been designed and studied in animal models. Preliminary results of these studies have shown beneficial and promising effects. Further experimental and clinical studies are needed before certain conclusions can be reached.
The association between MMPs and inflammatory cytokines with UC has been studied previously. However, most of the studies focused on their functions on the development of UC. The relationship between MMPs and other cytokines and the activity of UC remains largely unexplored. This study has bridged this gap and may provide additional targets for therapeutic development.
Since some basic evidence provided for MMP-1, TNF-α and their relationships in the development of UC, therapeutic approaches targeting MMPs or TNF-α, can be implemented in future study and new methods for treating UC may be developed.
Matrix metalloproteinases (MMPs): MMPs are a group of zinc-dependent peptidases that degrade extracellular matrix (ECM). In this family, more than 20 MMPs have been identified. MMP-1, also known as interstitial collagenase, degrades mainly collagen type I, II, III, VI, IX, and proteoglycan, and plays an important role in degrading ECM and in leading to colonic mucosa damages in UC patients.
This is an informative study demonstrating the association between metalloproteinase (MMP) and tumor necrosis factor (TNF) with disease activity in individuals with ulcerative colitis. The association of TNF with UC is well known but the relationship of other cytokines with disease activity remains largely unexplored. This study is an attempt to bridge this gap and may provide additional targets for therapeutic development. The preliminary conclusion is justified and substantiated by the results obtained.
S- Editor Zhu LH L- Editor Wang XL E- Editor Li HY
| 1. | Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci USA. 1997;94:599-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 247] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Arihiro S, Ohtani H, Hiwatashi N, Torii A, Sorsa T, Nagura H. Vascular smooth muscle cells and pericytes express MMP-1, MMP-9, TIMP-1 and type I procollagen in inflammatory bowel disease. Histopathology. 2001;39:50-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582-1590. [PubMed] [Cited in This Article: ] |
| 5. | Wang YD, Yan PY. Expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in ulcerative colitis. World J Gastroenterol. 2006;12:6050-6053. [PubMed] [Cited in This Article: ] |
| 6. | Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000;47:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Xia B, Crusius J, Meuwissen S, Pe?a A. Inflammatory bowel disease: definition, epidemiology, etiologic aspects, and immunogenetic studies. World J Gastroenterol. 1998;4:446-458. [PubMed] [Cited in This Article: ] |
| 8. | Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533-539. [PubMed] [Cited in This Article: ] |
| 9. | Di Sebastiano P, di Mola FF, Artese L, Rossi C, Mascetta G, Pernthaler H, Innocenti P. Beneficial effects of Batimastat (BB-94), a matrix metalloproteinase inhibitor, in rat experimental colitis. Digestion. 2001;63:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000;47:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Gasche C, Bakos S, Dejaco C, Tillinger W, Zakeri S, Reinisch W. IL-10 secretion and sensitivity in normal human intestine and inflammatory bowel disease. J Clin Immunol. 2000;20:362-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995;101:428-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 292] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Autschbach F, Braunstein J, Helmke B, Zuna I, Schürmann G, Niemir ZI, Wallich R, Otto HF, Meuer SC. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | MacNaul KL, Chartrain N, Lark M, Tocci MJ, Hutchinson NI. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990;265:17238-17245. [PubMed] [Cited in This Article: ] |
| 18. | Frisch SM, Ruley HE. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987;262:16300-16304. [PubMed] [Cited in This Article: ] |
| 19. | Pender SL, Fell JM, Chamow SM, Ashkenazi A, MacDonald TT. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998;160:4098-4103. [PubMed] [Cited in This Article: ] |
| 20. | Quiding-Järbrink M, Smith DA, Bancroft GJ. Production of matrix metalloproteinases in response to mycobacterial infection. Infect Immun. 2001;69:5661-5670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304-2310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 291] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Mertz PM, DeWitt DL, Stetler-Stevenson WG, Wahl LM. Interleukin 10 suppression of monocyte prostaglandin H synthase-2. Mechanism of inhibition of prostaglandin-dependent matrix metalloproteinase production. J Biol Chem. 1994;269:21322-21329. [PubMed] [Cited in This Article: ] |
| 23. | Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163-176. [PubMed] [Cited in This Article: ] |
| 24. | Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2422] [Cited by in F6Publishing: 2361] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 25. | Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321:265-279. [PubMed] [Cited in This Article: ] |
| 26. | Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Aspects Med. 2005;26:379-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs. 1997;15:61-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 323] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Kohn A, Prantera C, Pera A, Cosintino R, Sostegni R, Daperno M. Infliximab in the treatment of severe ulcerative colitis: a follow-up study. Eur Rev Med Pharmacol Sci. 2004;8:235-237. [PubMed] [Cited in This Article: ] |
| 29. | Eidelwein AP, Cuffari C, Abadom V, Oliva-Hemker M. Infliximab efficacy in pediatric ulcerative colitis. Inflamm Bowel Dis. 2005;11:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |