Published online Jun 7, 2006. doi: 10.3748/wjg.v12.i21.3410
Revised: August 28, 2005
Accepted: November 18, 2005
Published online: June 7, 2006
AIM: To investigate the influence of experimental obstructive jaundice and exogenous bombesin (BBS) and neurotensin (NT) administration on the expression of the tight junction (TJ)-protein claudin-4 in intestinal epithelium of rats.
METHODS: Forty male Wistar rats were randomly divided into five groups: I = controls, II = sham operated, III = bile duct ligation (BDL), IV = BDL+BBS (30 μg/kg per d), V = BDL+NT (300 μg/kg per d). At the end of the experiment on d 10, endotoxin was measured in portal and aortic blood. Tissue sections of the terminal ileum were examined histologically and immunohistochemically for evaluation of claudin-4 expression in intestinal epithelium.
RESULTS: Obstructive jaundice led to intestinal barrier failure demonstrated by significant portal and aortic endotoxemia. Claudin-4 expression was significantly increased in the upper third of the villi in jaundiced rats and an upregulation of its lateral distribution was noted. Administration of BBS or NT restored claudin-4 expression to the control state and significantly reduced portal and aortic endotoxemia.
CONCLUSION: Experimental obstructive jaundice increases claudin-4 expression in intestinal epithelium, which may be a key factor contributing to the disruption of the mucosal barrier. Gut regulatory peptides BBS and NT can prevent this alteration and reduce portal and systemic endotoxemia.
- Citation: Assimakopoulos SF, Vagianos CE, Charonis AS, Alexandris IH, Spiliopoulou I, Thomopoulos KC, Nikolopoulou VN, Scopa CD. Experimental obstructive jaundice alters claudin-4 expression in intestinal mucosa: Effect of bombesin and neurotensin. World J Gastroenterol 2006; 12(21): 3410-3415
- URL: https://www.wjgnet.com/1007-9327/full/v12/i21/3410.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i21.3410
Patients with obstructive jaundice, especially when exposed to the additional stress of an invasive diagnostic or therapeutic procedure, are prone to septic complications and renal dysfunction contributing to high morbidity and mortality rates[1]. Systemic endotoxemia appears to play a key role in the development of these complications[2]. Experimental and clinical studies have shown that obstructive jaundice increases intestinal permeability permitting the escape of endotoxin from the gut lumen[3,4]; however, little is known of the molecular events leading to intestinal permeability alterations.
Tight junctions (TJs) are located at the apical part of lateral membranes of polarized epithelial and endothelial cells forming a barrier that regulates the permeability of ions, macromolecules and cells through the paracellular pathway[5,6]. Up to now, three groups of macromolecules are considered as bona fide integral components of the tight junction: occludin, claudins and junctional adhesion molecule[6]. We have recently demonstrated using immunohistochemistry that intestinal mucosal barrier dysfunction in obstructive jaundice is associated with regional loss of occludin expression in the intestinal epithelium, observed mainly in the upper part of the villi[7]. Our findings were recently confirmed by other investigators using immunoblotting[8].
Claudins comprise a multigene family consisting of 24 members believed to be major functional elements of TJ[9]. TJ strands are copolymers of heterogeneous claudin species and occludin, and heterogeneous claudin species constitute the backbone of TJ strands in situ[10]. The localization and contribution to barrier function of each member of the claudin family vary among tissues. Claudin-4 is highly expressed in rat intestine[11], with a preferential localization in the epithelium overlying the tips of intestinal villi and the follicle associated epithelium (FAE) of gut associated lymphoid tissue (GALT)[12]. We focused on this molecule because in obstructive jaundice loss of occludin expression was observed mainly at the upper part of the villi where claudin-4 is preferentially expressed.
Regulatory peptides bombesin (BBS) and neurotensin (NT), with a wide spectrum of biological actions on gastrointestinal tissues (influencing intestinal growth and adaptation, intestinal motility, blood flow, secretion, nutrient absorption and immune response), exert a protective role in preserving gut barrier integrity after various injurious insults[13-18]. Beyond their potent enterotrophic action we have shown that BBS and NT fully restored intestinal occludin expression in bile duct ligated rats, thus preventing endotoxin translocation. It suggests a role of these peptides as molecular modulators of TJs[7].
The present study was undertaken to investigate the effect of experimental obstructive jaundice on intestinal claudin-4 expression and examine the potential effect of BBS and NT on this parameter of intestinal mucosal barrier.
Forty male albino Wistar rats, weighing 250-320 g, were used in the study. They were housed in stainless-steel cages, three rats per cage, under controlled temperature (23°C) and humidity conditions, with 12-h dark/light cycles, and maintained on standard laboratory diet with tap water ad libitum throughout the experiment, except for an overnight fast before surgery.
The experiments were carried out according to the guidelines set forth by the Ethics Committee of Patras University Hospital, Patras, Greece.
Animals were randomly divided into five groups: I (n = 8), controls; II (n = 8), sham operated; III (n = 8), bile duct ligation (BDL); IV (n = 8), BDL and BBS treatment and V (n = 8), BDL and NT administration. All surgical procedures were performed under strict sterile conditions, and light ether anesthesia. Rats from groups II, III, IV and V underwent laparotomy on d 0. Via a 1 cm upper midline incision, the gastroduodenal ligament was isolated and the common bile duct was mobilized. In groups III, IV and V, the common bile duct was further doubly ligated in its middle third with a 4-0 silk suture and was transected between the two ligatures. The abdominal incision was closed in two layers with chromic 4-0 cat gut and 4-0 silk.
For the 10 subsequent days, the animals of groups IV and V were treated daily with BBS (10 μg/kg, subcutaneously, three times a day) and NT (300 μg/kg, intraperitoneally, once a day) respectively, while the animals of groups I, II, and III were divided to receive daily either three subcutaneous or one intraperitoneal injection of 0.5 mL normal saline. All injections were given after topical application of an antiseptic solution of povidone iodine 10%. Previous studies have shown that the way of saline administration does not affect the results[7]. On the 10th d, all animals were operated (group I) or reoperated (groups II, III, IV and V). Samples were obtained according to the experimental protocol, after which the rats were sacrificed by exsanguination.
Bombesin: A stock solution of BBS (Sigma Chemical Co, St. Louis, Missouri, USA) was prepared by first dissolving the amount of peptide needed for the study in 1 mL sterile water containing 0.1% (w/v) bovine serum albumin and then diluted with normal saline containing 1% (w/v) bovine serum albumin, so that the amount of BBS needed for each injection to be contained in a volume of 0.1 mL. The solution was divided into equal aliquots of 0.1 mL which were stored in plastic tubes at -20°C. At the time of administration, in order to prolong absorption, each aliquot was mixed with 0.4 mL of a solution 8% (w/v) hydrolyzed gelatin (Sigma Chemical Co, St. Louis, Missouri, USA). A final volume of 0.5 mL, containing 10 μg BBS/kg body weight, was injected subcutaneously three times daily. Selection of dose and route of administration was based on previous reports[7].
Neurotensin: A stock solution of NT (Sigma Chemical Co, St. Louis, Missouri, USA) was prepared by first dissolving the amount of peptide needed for the study in 1 mL sterile water containing 0.1% (w/v) bovine serum albumin and then diluted with normal saline containing 0.1% (w/v) bovine serum albumin, so that the amount of BBS needed for each injection to be contained in a volume of 0.1 mL. The solution was divided into equal aliquots of 0.1 mL which were stored in glass vials at -20 °C.At the time of administration, each aliquot was further diluted with 0.4 mL sterile saline to a final volume of 0.5 mL and was given intraperitoneally through a bolus injection containing 300 μg NT/kg body weight. Selection of dose and route of administration was based on previous reports[7].
For the determination of total bilirubin, 0.5 mL of blood was collected from all animals, by transecting the tip of their tail. Then a laparotomy was performed and in all groups, the portal vein and the abdominal aorta were punctured and samples of 1 and 2 mL of blood, respectively, were obtained for estimation of endotoxin. Bilirubin concentrations were assayed using a standard biochemical technique and expressed in mg/dl. Endotoxin concentration was determined by the quantitative chromogenic Limulus Amebocyte Lysate test (QCL-1000, BioWhittaker, Walkersville, USA) and expressed in EU/mL. Samples were processed according to the manufacturer’s instructions. By this test it is possible to measure concentrations of endotoxin ≥ 0.01 EU/mL.
In tissue sections of the terminal ileum from all rats a standard immunohistochemical technique was applied to detect claudin-4. Deparaffinization, rehydration and microwave antigen retrieval were performed in TrilogyTM solution (Cell Marque, Hot Springs, AK). Then slides were incubated for 1 h at room temperature with a commercially available mouse monoclonal anti-claudin-4 antibody (1:40, ZYMED Laboratories, San Francisco, CA), followed by antigen-antibody detection using the DAKO ChemMate EnVision detection kit (DAKO A/S, Glostrup, Denmark). Colour was developed with diaminobenzidine (Sigma Fast DAB tablets, D-4293, St. Louis, Mo) and counterstained with Harris hematoxylin. All procedures took place at room temperature and between steps sections were washed in TBS. In negative control slides, the primary antibody was substituted by mouse serum. Twenty randomly selected adjacent perpendicularly sectioned villi from each case (n = 8 cases per group) were evaluated. The frequency of claudin-4 immunohistochemical expression in the upper third of the villi was recorded by dividing the number of claudin-4 positive enterocytes by the total number of enterocytes in the upper third of each villous.
The results are expressed as mean (SD). Comparisons among multiple groups were performed using the one-way ANOVA, followed by Dunnette’s T3 post hoc test. Variance equality was tested by Levene statistical analysis. Differences were considered significant when P < 0.05.
All animals survived and were in good health throughout the experiment. Bile duct ligated rats were clinically jaundiced within three days. At reoperation on d 10 it was found that the ligation and division of the common bile duct in groups III, IV and V had been successful in all cases and resulted in dilatation of the common bile duct remnant proximal to the ligature without signs of bile leakage.
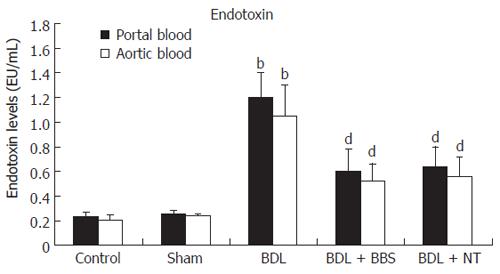
Bile duct ligation led to significantly raised total bilirubin levels in groups III, IV and V compared with control and sham groups (P < 0.001). Figure 1 demonstrates endotoxin values, measured in blood collected from portal vein and aorta. Jaundiced animals (group III) presented significantly elevated endotoxin concentrations in portal and aortic blood compared with groups I and II (P < 0.001). When ligation of common bile duct was followed by either BBS or NT treatment, endotoxin values were significantly reduced both in portal vein (P < 0.001, respectively) and aorta (P < 0.001, respectively).
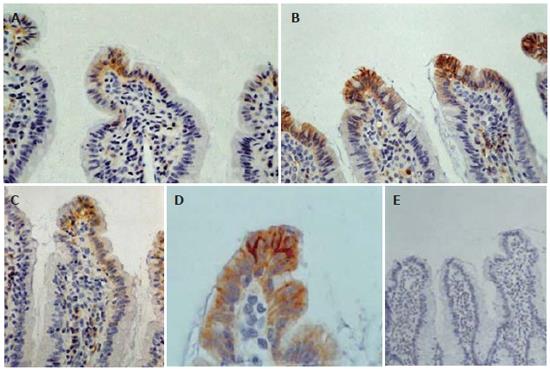
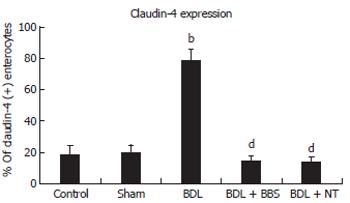
Overall, the ileal architecture remained intact and epithelial continuity was retained, in all specimens studied, while no significant pathology was observed. Obvious alterations of claudin-4 immunohistochemical expression in the intestinal epithelium were observed among experimental groups. In control and sham operated rats claudin-4 was expressed in a few villous surface epithelial cells (Figure 2A), while occasionally unstained villi were observed. Claudin-4 was detected as membranous immunostaining, stronger on the basolateral sides than on the apical sides, with weak cytoplasmic staining (Figure 2A). In jaundiced rats, claudin-4 was expressed in most epithelial cells lining throughout the upper third of the villi, and this staining was observed in every villous (Figure 2B). Also the lateral expression of claudin-4 was upregulated in jaundiced rats (Figure 2D). In BBS or NT-treated rats claudin-4 expression was restored to the control state (Figure 2C). The frequency of claudin-4-positive cells in the upper third of the villi was significantly increased in jaundiced rats as compared with control and sham operated (P < 0.001), whereas it was reduced in rats administered BBS or NT (P < 0.001 as compared to BDL) (Figure 3).
Increased intestinal permeability has been postulated to be a key factor contributing to bacterial and endotoxin translocation and the pathogenesis of the so called “gut derived sepsis”[19]. In obstructive jaundice, increased intestinal permeability has been demonstrated not only in animal models but in patients as well and has been positively correlated with severity of biliary obstruction[3,4]. The present study, using an experimental model of biliary obstruction for 10 d in rats, demonstrated significant portal and aortic endotoxemia, a clinically important marker of ineffective intestinal barrier, accompanied by altered immunohistochemical expression of the TJ-protein claudin-4 in intestinal epithelium.
The molecular mechanisms responsible for permeability alterations in obstructive jaundice have not been adequately investigated until now. Previously we had shown for the first time that intestinal mucosal barrier dysfunction in obstructive jaundice is associated with regional loss of expression of the TJ-protein occludin in the intestinal epithelium, observed mainly at the upper part of the villi[7]. Our immunohistochemical observations were confirmed with immunoblotting method by other investigators, who additionally showed that obstructive jaundice led to decreased mucosal expression of the TJ-associated protein zonulin-1 as well[8]. The present study offered further insight into TJs alterations in the intestinal mucosa in obstructive jaundice, demonstrating upregulation of claudin-4 expression in the upper part of the villi.
The results obtained by the present study may initially seem paradoxical and a question raised is how increased expression of a TJ-protein may lead to increased permeability. Claudins are the only known variable elements in TJs and different expression, combination and mixing ratios of various members of the claudin family are essential in the regulation of barrier properties of TJs[20]. Overexpression of some claudins is often associated with the expected reduction of permeability, e.g. claudin-1 overexpression in Madin-Darby canine kidney (MDCK) cells increases transepithelial resistance[21]. On the other hand, increased blood-brain barrier permeability in inflammatory pain is associated with significant increase of claudin-3 and -5 expression, while occludin expression is decreased[22]. Regarding the functional role of claudin-4 in the intestinal epithelium, it may be associated with loosening of intercellular junctions and opening of the paracellular route, as indicated by findings of previous studies[12]. For instance, it has been shown that there is a close association between the locations of claudin-4 and TUNEL-positive apoptotic cells in intestinal villi and the FAE of GALT, suggesting that claudin-4 may be involved in the process of peeling off epithelial sheets of apoptotic enterocytes. In addition, claudin-4 is preferentially expressed at the apices of intestinal villi and the FAE, which represent important antigen-sampling sites. Given that both apoptosis and antigen capturing are associated with looseness of intercellular junctions, Tamagawa et al suggested that claudin-4 expression contributes to the formation of relatively loose intercellular junctions[12]. The present study provides support to the above proposal. Furthermore, taking into consideration our recent findings of decreased intestinal occludin expression in obstructive jaundice[7] and the results of the present study showing increased intestinal claudin-4 expression, one could hypothesize the existence of a novel “switch mechanism” by which claudin-4 may replace occludin and lead to increased permeability of tight junctions. The key role of claudin-4 and occludin in obstructive jaundice-associated intestinal permeability alterations is further evidenced by improvement of gut mucosal barrier after restoration of their expression by regulatory peptides administration.
Which is the critical factor inducing these alterations in obstructive jaundice The present study cannot answer this question due to the interplay of several factors in the intact organism. However, a recent in vitro study has shown that bile is crucial for maintenance of intestinal TJs integrity[8]. Beyond absence of intraluminal bile other factors such as systemic endotoxemia, cytokinemia and intestinal oxidative stress may contribute to intestinal barrier failure in obstructive jaundice[5,23-25]. Oxidative stress, attracting a growing research interest, has been recognized as an important modulator of TJs. It has been shown that oxidative stress increases epithelial permeability through modulation of the assembly, localization, expression and function of the molecular components of the TJ structural complex[24]. We have recently shown that experimental obstructive jaundice induces intestinal oxidative stress, demonstrated by increased lipid peroxidation, protein oxidation and oxidation of non-protein and protein thiols[25]. Therefore, oxidative stress may be implicated in altered occludin and claudin-4 expression in the intestinal mucosa of jaundiced rats.
The present study also showed that when jaundiced rats were treated with either BBS or NT, claudin-4 expression in the intestinal epithelium was restored to the control state and endotoxin levels in portal and aortic blood were significantly reduced. These regulatory peptides may have restored claudin-4 expression via a direct receptor-mediated mechanism. Animal studies provide evidence for the presence of BBS and NT receptors in intestinal epithelial cells[26,27]. Binding to peptides’ specific G protein-coupled receptors activates multiple intracellular signals including protein phosphorylation pathways, accumulation of cyclic-adenosine monophosphate and mobilization of intracellular Ca++[28], which may account for the maintenance of structurally and functionally effective TJs[6,20]. A potential molecular mechanism mediating the effect of BBS and NT on claudin-4 expression is activation of protein kinase C (PKC). PKC plays a central role in transducing neuropeptides’ signals[28] and recent studies have shown that PKC activity is required for normal claudin assembly and the integrity of the intestinal epithelial barrier[29]. Also the antiapoptotic effect of BBS and NT on the intestinal epithelium of jaundiced rats[25] may be related to their modulating action on the expression of claudin-4, since there is evidence that intestinal apoptosis and claudin-4 expression may be interconnected[12]. Additionally, indirect mechanisms such as reduction of systemic endotoxemia, improvement of energy supply to enterocytes through splachnic vasodilatation[30,31] and reduction of intestinal oxidative stress[25] may contribute to the regulatory action of BBS and NT on the TJ structure and function.
In conclusion, our results show that enhanced claudin-4 expression in the upper part of the intestinal villi represents an important molecular mechanism for increased intestinal mucosal permeability in obstructive jaundice. Gut regulatory peptides BBS and NT, acting as molecular modulators of TJs, totally restored this alteration thus preventing endotoxin translocation from the gut lumen. These findings offer further insight into the pathophysiology of gut barrier failure in obstructive jaundice and suggest the potential therapeutic efficacy of regulatory peptides. However, further investigations to elucidate the details of the functional mechanisms and possible side effects of BBS and NT are needed before their clinical application.
S- Editor Pan BR L- Editor Zhu LH E- Editor Liu WF
| 1. | Pain JA, Cahill CJ, Bailey ME. Perioperative complications in obstructive jaundice: therapeutic considerations. Br J Surg. 1985;72:942-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Clements WD, Parks R, Erwin P, Halliday MI, Barr J, Rowlands BJ. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut. 1996;39:587-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Welsh FK, Ramsden CW, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998;227:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Madara JL. Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137:1273-1281. [PubMed] [Cited in This Article: ] |
| 6. | Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467-G475. [PubMed] [Cited in This Article: ] |
| 7. | Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004;198:748-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005;31:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96:511-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 829] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 10. | Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 561] [Cited by in F6Publishing: 542] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 411] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Tamagawa H, Takahashi I, Furuse M, Yoshitake-Kitano Y, Tsukita S, Ito T, Matsuda H, Kiyono H. Characteristics of claudin expression in follicle-associated epithelium of Peyer's patches: preferential localization of claudin-4 at the apex of the dome region. Lab Invest. 2003;83:1045-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Chu KU, Evers BM, Ishizuka J, Townsend CM Jr, Thompson JC. Role of bombesin on gut mucosal growth. Ann Surg. 1995;222:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Zarzaur BL, Wu Y, Fukatsu K, Johnson CD, Kudsk KA. The neuropeptide bombesin improves IgA-mediated mucosal immunity with preservation of gut interleukin-4 in total parenteral nutrition-fed mice. Surgery. 2002;131:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Alican I, Unlüer EE, Yeğen C, Yeğen BC. Bombesin improves burn-induced intestinal injury in the rat. Peptides. 2000;21:1265-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Chung DH, Evers BM, Shimoda I, Townsend CM Jr, Rajaraman S, Thompson JC. Effect of neurotensin on gut mucosal growth in rats with jejunal and ileal Thiry-Vella fistulas. Gastroenterology. 1992;103:1254-1259. [PubMed] [Cited in This Article: ] |
| 17. | Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg. 2003;186:253-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Vagianos C, Karatzas T, Scopa CD, Panagopoulos C, Tsoni I, Spiliopoulou I, Kalfarentzos F. Neurotensin reduces microbial translocation and improves intestinal mucosa integrity after abdominal radiation. Eur Surg Res. 1992;24:77-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 320] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci. 2000;113 Pt 19:3387-3398. [PubMed] [Cited in This Article: ] |
| 22. | Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738-H743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. [PubMed] [Cited in This Article: ] |
| 24. | Rao RK, Basuroy S, Rao VU, Karnaky Jr KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 318] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159-167. [PubMed] [Cited in This Article: ] |
| 26. | Seybold VS, Parsons AM, Aanonsen LM, Brown DR. Characterization and autoradiographic localization of gastrin releasing peptide receptors in the porcine gut. Peptides. 1990;11:779-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Seybold VS, Treder BG, Aanonsen LM, Parsons A, Brown DR. Neurotensin binding sites in porcine jejunum: biochemical characterization and intramural localization. Synapse. 1990;6:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol. 1998;177:507-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 29. | Banan A, Zhang LJ, Shaikh M, Fields JZ, Choudhary S, Forsyth CB, Farhadi A, Keshavarzian A. theta Isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: a novel mechanism for regulation of permeability. J Pharmacol Exp Ther. 2005;313:962-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Heuser M, Pfaar O, Gralla O, Gröne HJ, Nustede R, Post S. Impact of gastrin-releasing peptide on intestinal microcirculation after ischemia-reperfusion in rats. Digestion. 2000;61:172-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Harper SL, Barrowman JA, Kvietys PR, Granger DN. Effect of neurotensin on intestinal capillary permeability and blood flow. Am J Physiol. 1984;247:G161-G166. [PubMed] [Cited in This Article: ] |