Published online Feb 28, 2005. doi: 10.3748/wjg.v11.i8.1225
Revised: September 10, 2004
Accepted: October 7, 2004
Published online: February 28, 2005
AIM: To investigate the effect of fermented soy milk on human ecosystem in the intestinal tract by way of examining the population of different microorganisms isolated from fecal samples.
METHODS: A crossover experimental design was applied. Twenty-eight healthy adults completed this experiment. Each subject consumed 250 mL, twice a day between meals, of either fermented soy milk or regular soy milk first for 2 wk, then switched to the other drink after 2 wk. Fecal samples were collected from all subjects every week starting from the second week to the end of the experiment. The microorganisms analyzed were Bifidobacterium spp., Lactobacillus spp., Clostridium perfringens, coliform organisms, and total anaerobic organisms.
RESULTS: In the period of fermented soy milk consumption, the populations of Bifidobacterium spp. and Lactobacillus spp. increased (P<0.05) as well as the ratios of Bifidobacterium spp. and Lactobacillus spp. to Clostridium perfringens (P<0.05). The population of coliform organisms decreased (P<0.05) when subjects were in the period of fermented soy milk consumption.
CONCLUSION: Intake of fermented soy milk significantly improved the ecosystem of the intestinal tract in the body by increasing the amount of probiotics.
- Citation: Cheng IC, Shang HF, Lin TF, Wang TH, Lin HS, Lin SH. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J Gastroenterol 2005; 11(8): 1225-1227
- URL: https://www.wjgnet.com/1007-9327/full/v11/i8/1225.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i8.1225
Probiotics are bacteria with health-benefits that live in the intestinal tract. Probiotics reduce lactose-intolerance symptoms, increase the resistance of the intestines to diseases, inhibit cancer cells from proliferating, modulate the concentration of plasma cholesterol, improve digestive functions, and stimulate the immune system[1,2]. On the other hand, prebiotics are the food ingredients that can be utilized by or can enhance the growth of probiotics. Some commonly mentioned prebiotics are lactose, fructooligosaccharides, and galactooligosaccharides[3-6]. Soybeans and soy products have been well known for their health benefits. In soybeans, oligosaccharides were also proven to be prebiotics[7]. The combination of probiotics and prebiotics is called synbiotics[1]. Fermented soy milk, according to previous statements, can be considered as a synbiotic product. Thus, our objective was to investigate the effect of fermented soy milk on the ecosystem in the intestinal tract of human subjects.
This study was approved by the Human Ethics Committee of Taipei Medical University (Taipei, Taiwan). Subjects were recruited mostly from the campus of Taipei Medical University and had no acute or chronic diseases, gastrointestinal problems, or a recent history of taking antibiotics. Before executing this study, written informed consents were acquired from all subjects. Totally 36 subjects participated at the beginning. Subjects were advised to maintain their normal life style during the experiment.
A crossover design was used in this study. Subjects were randomly assigned to two groups, A and B. In group A, subjects consumed fermented soy milk first, and then switched to regular soy milk, while regular soy milk was consumed first in group B. The total experimental time was 9 wk. A 2-wk adjustment period was carried out, followed by consumption of experimental drinks for 2 wk. Before switching to the other experiment drink, there was another 2-wk period for washout. After all subjects had completed consuming the two kinds of experimental drink, there was a 1-wk washout period before the experiment formally ended. The drink consisted of 250 mL each time given 30 min after a meal, twice a day (500 mL/d). A 3-d (Sunday, Monday and Tuesday) dietary record was completed by subjects every week during the experiment.
About 1 g of fecal samples from each subject was collected in wk 2, 3, 4, 6, 7, 8, and 9 for microorganism analyses. Samples were stored at -20 °C for less than 24 h before analysis. For the analyses, 0.5 g of the inner part of a fecal sample (to retrieve anaerobic material) was mixed well with 15 mL of an anaerobic solution (Table 1), followed by serial dilutions to acquire different concentrations (10-1 to 10-8). Certain microorganisms were isolated from fecal samples using the media and methods developed by Molly et al[8]. The bacteria, media and incubation times are listed in Table 2. Starting from the lowest concentration, 50 μL of the solution was then inoculated on different media using the spread plate method. For incubating Clostridium perfringens, 1 mL of solutions with suitable concentrations, determined by the result of a pre-experiment, was mixed well with TSC medium (without egg-yolk) using the pour plate method, followed by mixing with the regular TSC medium. After the liquid medium solidified, the plate was placed in an anaerobic chamber.
| Chemicals | Weight |
| KH2PO4 | 4.5 g |
| Na2HPO4 | 6.0 g |
| L-cysteine HCl·H2O | 0.5 g |
| Tween 20 | 1 g |
| Galtin | 2 g |
| Distilled water | 1 L |
| Medium | Microorganism | Color of the colony | Incubation conditions |
| Bifidobacteria iodoacetate medium25 (BIM-25) | Bifidobacterium spp. | Green | 48 h, 35-37 °C |
| Lactobacillus anaerobic MRS with bromocresol green (MRS-modified) | Lactobacillus spp. | Reddish orange | 48 h, 35-37 °C |
| Tryptose-sulfite-D-cycloserine agar (TSC) | Clostridium perfringens | White with black center | 24 h, 35-37 °C |
| Endo agar plates (Endo) | Coliform organisms | Metallic red | 24 h, 35-37 °C |
| CDC anaerobe blood agar plates (CDC) | Total anaerobic organisms | White | 48 h, 35-37 °C |
When counting colonies, plates with 30-300 colonies were included. The number of bacteria was presented as log CFU/g of wet weight of feces. The calculation formulae are listed as follows and are based on the FDA Bacteriological Analytical Manual: Bifidobacterium spp., Lactobacillus spp., coliform organisms, and total anaerobic organisms: CFU/plate ×20 (50 μL/plate)×dilution factor×15 mL/sample (g); and Clostridium perfringens: CFU/plate×dilution factor×15 mL/sample (g).
All data are presented as the mean±SD. One-way analysis of variance (ANOVA), unpaired and paired t-tests were performed using SAS version 8.1. P<0.05 was the level of significance.
Before the experiment started, 8 people withdrew from the study due to personal reasons. Thus, totally 28 people participated and completed two experimental periods. As shown in Table 3, there were no significant changes in weight, height, or BMI between before and after the study.
| Group | n | Age (yr) | Wk 0 | Wk 8 | ||||
| Weight (kg) | Height (cm) | BMI | Weight (kg) | Height (cm) | BMI | |||
| A | 14 | 23.1±1.1 | 56.4±10.6 | 162.0±7.9 | 21.3±2.3 | 56.1±10.2 | 162.0±7.9 | 21.2±2.2 |
| B | 14 | 22.6±1.8 | 55.9±8.3 | 162.6±8.2 | 21.1±1.5 | 56.1±9.5 | 163.1±8.8 | 21.1±3.5 |
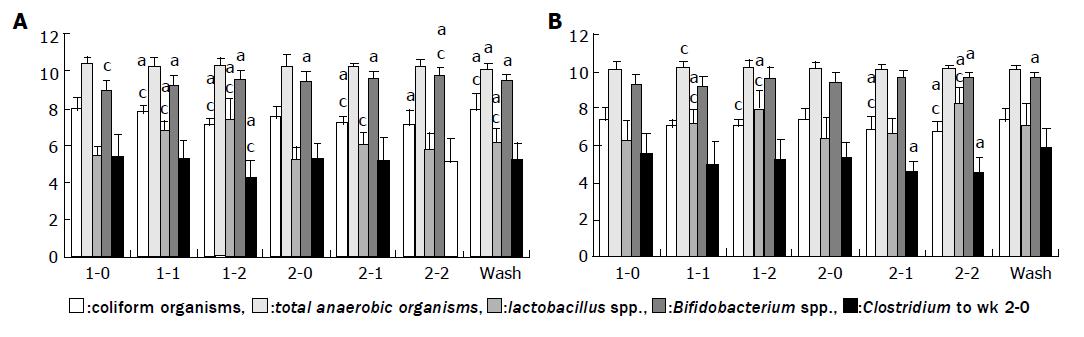
In this group (Figure 1A), we found that during the first period of fermented soy milk consumption, the populations of coliform organisms and Clostridium perfringens significantly decreased (P<0.05). In the same period, the populations of both Lactobacillus spp. and Bifidobacterium spp. increased (P<0.05). The ratios of Lactobacillus spp. and Bifidobacterium spp. to Clostridium perfringens also increased in the first period. In the second period of regular soy milk consumption, the populations of coliform organisms and Clostridium perfringens and the ratios of Lactobacillus spp. and Bifidobacterium spp. to Clostridium perfringens did not change.
In this group (Figure 1B), in the first and second periods, the population of Lactobacillus spp. significantly increased (P<0.05). In the second period, the populations of coliform organisms and Clostridium perfringens significantly decreased (P<0.05), while the population of Bifidobacterium spp. significantly increased (P<0.05). At the end of the first and second periods, the ratios of Lactobacillus spp. and Bifidobacterium spp. to Clostridium perfringens had significantly increased (P<0.05). The population of total anaerobic bacteria did not change in either group.
The population of microorganisms in the intestine is in a balanced phase[9]. When the number of probiotics increases, the number of harmful bacteria decreases. As seen in the results, we found that when subjects were in the period of fermented soy milk consumption, their intestinal ecosystem tended to be improved by an increase in the populations of the so-called “good bacteria”. The effect could be maintained even 3 wk after cessation of fermented soy milk consumption. On the other hand, results showed that regular soy milk also had some effect on increasing the population of Lactobacillus spp. This may have been because soybeans contain certain types of oligosaccharides such as raffinose and stachyose that can be utilized by Lactobacillus spp. as energy sources[10,11]; this reduces the beany odor and gas production in the intestines[12]. It was found that, by culturing different probiotics in soy milk, the amounts of raffinose and stachyose that caused reduction of gas production in the stomach while the amounts of sucrose, glucose, galactose, acetic acid, and free amino acids increased[13]. The increase in probiotics lowers the risk of GI tract dysfunction from bacterial invasion and hence, maintains one of the major functions of GI tract, the barrier function[14]. Beside the effects of consuming fermented soy milk determined in this experiment, soy products are well known for their health benefits. It has been found that the intake of soy products reduces the risk of various cancers, lowers the levels of blood lipid and cholesterol, and prevents oxidation of VLDL and LDL, and hence lowering the risk of cardiovascular diseases[15-17].
In conclusion, consumption of fermented soy milk is beneficial to the ecosystem of the intestinal tract by increasing the populations of probiotics and reducing the populations of unwanted bacteria. In addition, fermented soy milk may also provide other exclusive ingredients such as isoflavone and saponin that do not exist in dairy products.
| 1. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [PubMed] [Cited in This Article: ] |
| 2. | Gonzalez SN, Cardozo R, Apella MC, Oliver G. Biotherapeutic role of fermented milk. Biotherapy. 1994;8:129-134. [PubMed] [Cited in This Article: ] |
| 3. | Buddington RK, Williams CH, Chen SC, Witherly SA. Dietary supplement of neosugar alters the fecal flora and decreases activities of some reductive enzymes in human subjects. Am J Clin Nutr. 1996;63:709-716. [PubMed] [Cited in This Article: ] |
| 4. | Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1062] [Cited by in F6Publishing: 872] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 5. | Rowland IR, Tanaka R. The effects of transgalactosylated oligosaccharides on gut flora metabolism in rats associated with a human faecal microflora. J Appl Bacteriol. 1993;74:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | MacGillivray PC, Finlay HVL, Binns TB. Use of lactulose to create a preponderance of Lactobacilli in the intestine of bottle-fed infants. Scott Med J. 1959;4:182-189. [PubMed] [Cited in This Article: ] |
| 7. | Chow J. Probiotics and prebiotics: A brief overview. J Ren Nutr. 2002;12:76-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Molly K, Vande Woestyne M, De Smet I, Verstraete W. Validation of the simulator of the human intestinal microbial ecosystem (SHIME) reactor using microorganism-associated activities. Microb Ecol Health Dis. 1994;7:191-200. [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Todar K. The bacterial flora of humans. Todar's Online Textbook of Bacteriology. 2002. Available from: http: //textbookofbacteriology.net/normalflora.html. [Cited in This Article: ] |
| 10. | Hang YD, Jackson H. Preparation of soybean cheese using lactic starterorganisms I. General characteristics of the finished cheese. Food Technol. 1967;21:1967-1995. [Cited in This Article: ] |
| 11. | Yamanaka Y, Okumura S, Mitsugi K, Hasagawa Y. Process for preparing a sour milk beverage or yoghurt. Food Sci Technol Abstr. 1969;1:1308-1310. [Cited in This Article: ] |
| 12. | Thananunkul D, Tanaka M, Chichester CO, Lee TC. Degradation of raffinose and stachyose in soybean milk by α-galactosidase from Mortierella vinacea. Entrapment of α-galactosidase within polyacrylamide gel. J Food Sci. 1976;41:173-175. [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wang YC, Yu RC, Yang HY, Chou CC. Sugar and acid contents in soymilk fermented with lactic acid bacteria alone or simultaneously with bifidobacteria. Food Microbiol. 2003;20:333-338. [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Ding LA, Li JS. Intestinal failure: pathophysiological elements and clinical diseases. World J Gastroenterol. 2004;10:930-933. [PubMed] [Cited in This Article: ] |
| 15. | Samman S, Khosla P, Carroll KK. Intermediate density lipoprotein-apolipoprotein B turnover in rabbits fed semipurified diets containing casein or soy protein. Ann Nutr Metab. 1990;34:98-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Vahouny GV, Adamson I, Chalcarz W, Satchithanandam S, Muesing R, Klurfeld DM, Tepper SA, Sanghvi A, Kritchevsky D. Effects of casein and soy protein on hepatic and serum lipids and lipoprotein lipid distributions in the rat. Atherosclerosis. 1985;56:127-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |